Abstract
The primary identified function of complement receptor 1 (CR1/CD35) on primate erythrocytes is to bind complement-tagged inflammatory particles including microbes and immune complexes. When erythrocytes circulate through liver and spleen, sinusoidal phagocytes remove CR1-adherent particles and erythrocytes return to the circulation. This process of immune adherence clearance is important for host defense and prevention of autoimmunity. CR1 was previously described as clustered in the human erythrocyte membrane, which was thought to be necessary for binding complement-opsonized particles. In contrast, we demonstrate that on erythrocytes CR1 is not clustered, but dispersed, and able to bind complement-tagged particles. When fresh erythrocytes are solubilized by nonionic detergent, CR1 partitions to the cytoskeleton fraction. Using a PDZ-peptide array, CR1's cytoplasmic tail, which contains 2 PDZ-motifs, binds PDZ domains 2, 3, and 5 of Fas-associated phosphatase 1 (FAP-1), a scaffolding protein. We show that FAP-1, not previously recognized as an erythroid protein, is expressed on circulating erythrocytes. CR1 and FAP-1 coimmunoprecipitate, which confirms their molecular association. Disperse CR1 on erythrocytes may be advantageous for capturing immune-complexes, while ligation-induced CR1 clustering may prevent ingestion of the erythrocyte during the immune-complex transfer to the macrophages by keeping the opsonic stimulus localized thus preventing phagocyosis.
Introduction
Evolution of a closed circulatory system necessitated an efficient means of clearing the intravascular space of inflammatory particles, for example, microbes and immune complexes. To accomplish this task, vertebrates first tag the particles with complement opsonins, predominately C3b, then immobilize the particles by ligation to a cell expressing a complement receptor: process known as immune adherence.1 Nonprimate vertebrates use platelets and adherent factor H as the complement receptor to capture circulating opsonized particles.2,3 Once platelets bearing their cargo reach the liver or spleen, resident phagocytes remove the entire platelet-particle complex.2,3 In contrast, primates use erythrocytes and complement receptor 1 (CR1 or CD35) to ligate complement-tagged inflammatory particles to their membranes.4,5 When primate erythrocytes with immune-adherent particles move through the liver and spleen, sinusoidal macrophages remove only the immune-adherent particles and allow erythrocytes to return to the circulation.6-8
Previous studies have shown that CR1 on human erythrocytes is constitutively expressed in large clusters, which were thought to be important for promoting multivalent ligand binding.9,10 These results were based on indirect immunofluorescence methods or binding studies.9-11 In addition, 2 different electron microscopy methods that used a 2-step erythrocyte staining with anti-CR1 Ab and immunogold-labeled secondary Ab reported either 2-15 or 30-75 immunogold particles per CR1 cluster.9,12 We demonstrated previously that storage and ex vivo manipulation of erythrocytes induced CR1 clustering as well as an apparent decrease in the total number of CR1 molecules on erythrocytes.13 A similar phenomena had been noted when the GPI-anchored nonhuman primate erythrocyte CR1-like molecules were ligated by immune-complexes: the apparent number of CR1 molecules decreased and then returned to baseline after 24 hours.6 Because erythrocytes cannot up or down regulate membrane proteins, these effects were likely due to tight aggregation of CR1 molecules in clusters, which hindered antibody binding.
In this study we demonstrate that the constitutive distribution of CR1 on circulating erythrocytes is not natively in large clusters, but rather CR1 is dispersed in human erythrocyte membrane. We also show that dispersed CR1 is capable of efficiently binding and retaining complement-tagged particles. Therefore clustering of CR1 is a ligand-induced secondary event that likely plays a role in transporting and/or transferring immune-adherent particles to the phagocytes. CR1's cytoplasmic domain contains 2 PDZ (postsynaptic density protein, Drosophila disc large tumor suppressor and zonula occludens-1) motifs that potentially bind PDZ domains. PDZ motifs are small (3-6 amino acids) protein: protein interaction modules found at the carboxyl-terminal ends of certain transmembrane proteins. They are involved in binding of larger PDZ domains (80-100 amino acids), which are commonly found in scaffolding proteins that direct the assembly of signaling complexes under the plasma membrane (reviewed in Xu et al14 and Brone et al15 ). We found that a CR1 cytoplasmic domain peptide (CR1-tail peptide) bound PDZ domains 2, 3, and 5 of Fas-associated phosphatase 1 (FAP-1), a scaffolding protein with tyrosine phosphatase activity. In addition to its 5 PDZ domains and a KIND domain (kinase noncatalytic C-lobe domain), FAP-1 also has a FERM (four.one [4.1], ezrin, radixin, and moesin) domain, which is likely responsible for linking FAP-1 to the cytoskeleton.16 Although FAP-1 has been recognized as a scaffolding molecule in diverse tissues,17 this is the first report of FAP-1 as a human erythroid protein.
Methods
Antibodies and reagents
Antibodies (Abs) were obtained as follows: rabbit anti–FAP-1 peptide #B12060 antiserum (Stratagene, La Jolla, CA), mouse monoclonal anti–FAP-1, clone 359313 from R&D Systems (Minneapolis, MN), rabbit anti–FAP-1 (hPTP1E) peptide antiserum was a gift of Prof Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden)17 ; nonimmune IgG1, (BD Biosciences, San Jose, CA); anti-CR1 mAbs: 1F11 (a gift of Henry Marsh, PhD, Avant Immunotherapeutics, Needham, MA), YZ-1 and 2B1113,18 ; rabbit polyclonal anti-CR1 IgG19 ; anti-CD59 (Abcam, Cambridge, MA) secondary antibodies: AlexaFluor-488 goat anti–mouse IgG, AlexaFluor-594 goat anti–rabbit IgG, and AlexaFluor-594 goat anti–mouse IgG (Invitrogen, Carlsbad, CA); horseradish peroxidase (HRP)-goat anti–mouse IgG, HRP-donkey anti–goat IgG, and HRP-donkey anti–rabbit IgG (Jackson ImmunoResearch, West Grove, PA). A peptide representing the 43-amino-acid cytoplasmic domain of CR1 (CR1-tail peptide) was synthesized with an additional cysteine on its amino terminus and purified by HPLC (Biopolymers Lab, Harvard Medical School, Boston, MA). Rabbit anti–Cyto-CR1 (BWH-60) was raised against the Cyto-CR1 synthetic peptide. BW59, an antibody that did not react with CR1-tail peptide, was used as negative control.
Reagents were obtained as follows: protease inhibitor cocktail (Roche, Nutley, NJ); phosphatase inhibitor cocktail, C12E8 detergent (Sigma-Aldrich, St Louis, MO); paraformaldehyde and glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA); and IgG-free BSA (Jackson ImmunoResearch, West Grove, PA). Complement-tagged beads (C3b-beads) were made by the sequential incubations of 19-nm polystyrene microspheres (Invitrogen) with 5% AlexaFluor-488 BSA (Invitrogen) in HBSS++ for 60 minutes at room temperature, 25 μg/mL rabbit anti–BSA IgG at 37°C for 45 minutes, and 20% fresh human serum at 37°C for 15 minutes. Beads were washed in HBSS++ after each step and sonicated before use.
Fresh and fixed erythrocytes
Blood was drawn from healthy adult volunteers in accordance with the guidelines of the Institutional Review Committee of Beth Israel Deaconess Medical Center and after informed consent was obtained in accordance with the Declaration of Helsinki. Small volumes of fresh blood (<50 μL) obtained by finger prick were diluted 20-fold in Hanks balanced salt solution with Ca++ and Mg++ (HBSS++; Invitrogen) and washed twice before use. Larger volumes (5-20 mL) of blood, which were obtained by venipuncture, were diluted 10-fold in HBSS++ and centrifuged at 1300g for 5 minutes at room temperature. Upper layers containing leukocytes were removed along with the top 10% of erythrocytes. Erythrocytes were then washed 3 times by dilution with HBSS++ and centrifugation. Erythrocytes were resuspended in 0.5% IgG-free bovine serum albumin (BSA)–HBSS++ and used at a hematocrit of 5%. For microscopy and flow cytometry experiments, fresh erythrocytes were fixed in dimethylsuberimidate (50 mM; Sigma-Aldrich) in sodium borate buffer (100 mM, pH 9.5) and 0.05% glutaraldehyde for 2 hours, washed with 0.5% IgG-free BSA-HBSS++ and incubated for an additional 1 hour with glycine (0.1 M) to quench autofluorescence and nonspecific binding due to fixation.
Fluorescence and electron microscopy
Fresh or fixed erythrocytes were incubated with 10 μg/mL anti-CR1 mAb 1F11 for 15 minutes in HBSS++ with 0.5% BSA (staining buffer), washed, and stained with AlexaFluor-594 goat anti–mouse Ab for an additional 10 minutes. For colocalization, all erythrocytes were fixed either before or after CR1 crosslinking with primary and secondary Ab, followed by permeabilization in 0.5% Triton X-100 buffer and incubation with anti–FAP-1 antiserum17 for 20 minutes. Permeabilized erythrocytes were then washed and incubated with AlexaFluor-488 goat anti–rabbit Ab for 30 minutes at room temperature. After staining, cells were washed twice, mounted in fluorescence mounting media (DakoCytomation, Carpinteria, CA), and imaged using an Olympus BX62 fitted with a cooled Hamamatsu Orca AG camera. The microscope, filters, and camera were controlled by iVision v. 4.0.9 (Biovision Technoogies, Exton, PA). For colocalization studies the microscope was fitted with Texas Red and GFP-green filters (Zero-Shift; Semrock, Rochester, NY) that provide exact registration between the channels. Acquired images were further processed using the colocalization module of Volocity 4.2 (Improvision, Waltham, MA). For analysis of the intensity profiles on fixed and fresh erythrocytes, both green and red channels of each image were analyzed with iVision 4.0.9 and the values generated exported to Prism 4.0 (GraphPad, La Jolla, CA) for statistical analysis (Spearman rank correlation).
For immunogold colocalization by electron microscopy (EM), freshly purified erythrocytes were incubated at room temperature for 10 minutes with 5 μg/mL of anti-CR1 mouse mAb (YZ-1) in staining buffer, washed twice, and incubated with 18 nm gold-labeled goat anti–mouse IgG (Jackson ImmunoResearch) for another 30 minutes at room temperature. Cells were washed twice and fixed for 1 hour in 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) containing 4% sucrose. The pellet was washed in cacodylate buffer and postfixed in 1% osmium tetroxide. Postfixation, cells were processed for thin section transmission EM using standard protocols and sections were stained with FAP-1 followed by 10 nm gold-labeled goat anti–rabbit Ig (Jackson ImmunoResearch). All steps after glutaraldehyde fixation were done in the Microscopy and Histology Core Facilities at Beth Israel Deaconess Medical Center.
Flow cytometry
Fresh or fixed erythrocytes were incubated for 15 minutes with Ab (as noted in the figure legends) in staining buffer at 4°C, followed by 2 washes and incubation for 15 minutes with AlexaFluor 488–labeled secondary Ab specific for each particular primary Ab at a dilution recommended by the manufacturer. For analyzing FAP-1 expression, fixed erythrocytes were permeabilized and treated with control or relevant Ab as described for microscopy. After incubation with secondary Ab, erythrocytes were washed once and analyzed in a FACScan (BD Biosciences). For analysis of C3b-bead binding, beads prepared as described above were incubated for 60 minutes at room temperature with 2 × 106 fresh or fixed erythrocytes, washed once, and analyzed. In all the experiments at least 10 000 events were recorded and analyzed (CellQuest Pro version 4.0.1 software; BD Biosciences).
Immunoblotting and coimmunoprecipitation
Erythrocyte pellets (5 μL) were lysed in 90 μL of 1× reducing-loading buffer and boiled for 4 minutes. Samples were run on 10% Bis-Tris gels (Invitrogen), transferred to nitrocellulose paper (Pierce, Rockford, IL), and blocked with 6% nonfat dry milk in Tris buffer with Tween 0.1% for 1 hour at room temperature. Membranes were incubated with anti–FAP-1 Ab, as noted in figure legends, for 30 minutes at room temperature, washed, and incubated with HRP-conjugated appropriate secondary Ab for an extra 30 minutes. Nitrocellulose membranes (Pierce) were washed extensively in Tris buffer with Tween 0.1% and developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce) and exposed to XAR film (Kodak, Rochester, NY).
To separate membrane (soluble) and cytoskeletal (insoluble) fractions, erythrocytes were processed as described.20 Briefly, 50 μL packed erythrocytes were lysed in 5 mL ice-cold ghosting buffer containing 5 mM sodium phosphate pH 8.2, 2 mM MgCl2, 3 mM EGTA, and protease inhibitor cocktail for 30 minutes. Ghosts were washed free of hemoglobin and then mixed 1:1 with ice-cold buffer containing 0.5% C12E8 for 5 to 10 minutes with continuous rocking. The resulting lysate was layered over 10 mL of 35% sucrose in the same buffer and centrifuged 100 minutes at 20 000g at 4°C. The pellet (cytoskeletal fraction) and the fraction above the sucrose cushion (membrane fraction) were each mixed with an equal volume of 2× reducing-loading buffer and boiled for 3 minutes. Proteins were further separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described above.
For coimmunoprecipitation studies, fresh erythrocytes or anti-CR1 ligated-erythrocytes were washed twice in HBSS++ and lysed in 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 5 mM EDTA, 0.1% Nonidet P-40 with added protease inhibitor cocktail for 30 minutes on ice. Lysates were centrifuged for 20 minutes at 15 000g, and the supernatants precleared for 12 hours with Sepharose beads (Pierce). The precleared supernatants from fresh erythrocytes were incubated (4 hours) with protein A + G beads (Sigma) coupled with anti-CR1 mAb (YZ-1). In contrast, lysates from erythrocytes that had CR1 preligated with YZ-1 and goat anti–mouse antibody were reacted with goat anti–mouse IgG coupled-protein A + G beads. All beads were washed in lysing buffer (with 0.01% Nonidet P-40 instead of 0.1%) 4 times for a total of 30 minutes, boiled 5 minutes in nonreducing loading buffer (Invitrogen), and electrophoresed and immunoblotted for FAP-1 as described above. HEK cells (gift of the William C. Aird Laboratory, Beth Israel Deaconess Medical Center) were used as a positive control for FAP-1 signal.
PDZ domain array
Four different PDZ protein arrays membranes (PDZ I-IV, Panomics, Fremont, CA) were probed overnight with CR1 tail peptide (100 ng/mL, recombinant peptide corresponding to the cytoplasmic tail of CR1) and developed with rabbit anti-CR1 tail peptide (BW60) Ab at a dilution of 1:800. Membranes were extensively washed and incubated with HRP-conjugated goat anti–rabbit Ab per manufacturer's instructions. Membranes were developed as was done for immunoblots. Control antibody (BW59) was negative (data not shown).
Surface plasmon resonance analysis
Activation of the CM5 sensor chip, anti-GST IgG, buffers, and binding conditions were per manufacturer's instructions. Anti-GST IgG was immobilized to the activated CM5 sensor chip to capture the FAP-1 peptide GST-PDZd5 (Panomics), or GST as the control. Cyto-CR1 peptide (5 μg/mL) was injected at 5 μL/mL in both channels. The experiments were performed on an upgraded BIAcore 1000 instrument (GE Healthcare, Piscataway, NJ) and the resulting sensograms were analyzed using BIAevaluation version 2.0 software (GE Healthcare). One resonance unit (RU) corresponds to the binding of 1 ng/mm2 of flow cell surface.
Results
CR1 is dispersed in fresh erythrocytes and forms clusters upon cross-linking
For an efficient immune adherence function of human erythroid CR1, multivalent interactions between a single complement-tagged particle or immune complex (IC) and a cluster of several CR1 molecules were thought to be required to assure the formation of stable, high-avidity IC-CR1 interface.9,12 However, the detailed analysis of erythroid CR1 distribution pattern with regard to its native configuration before and during the initial steps of CR1-IC complex formation has not been performed. The expression of CR1 on circulating erythrocytes is genetically determined and varies between 178 and 208 on low CR1 expressors (LL), 478 and 570 on intermediate CR1 expressors (HL), and 708 and 1114 on high CR1 expressors (HH).21 In order to examine the effect of staining methods on distribution of erythroid CR1 in its most innate state, we compared the pattern of CR1 distribution on fresh and fixed erythrocytes from a high CR1 expressor stained with anti-CR1 primary Ab followed by fluorochrome-conjugated secondary Ab. Unlike fresh erythrocytes, which allowed redistribution of CR1 into large clusters after ligation (Figure 1A), on fixed erythrocytes CR1 was scattered randomly in small speckles, with few occasional larger clusters (Figure 1B). After ligation, both the area occupied by CR1 and the intensity of the resulting CR1 clusters increased significantly on fresh erythrocytes in comparison to fixed erythrocytes (Table 1). The most likely explanation for this observation was that CR1 molecules were brought together by ligation, forming larger, fewer CR1 clusters, as erythrocytes do not have the ability to up-regulate plasma membrane proteins. In a separate experiment, the redistribution of CR1 due to ligation on erythrocytes from HH and LL donors was analyzed by counting CR1 molecules in both fresh and fixed erythrocytes using fluorescence microscopy. On erythrocytes from a high CR1 expressor, 96 (± 43) of CR1 spots were counted on fixed erythrocytes and 15 (± 6) on fresh erythrocytes when cells were incubated with primary and secondary Ab. In contrast, on a low CR1 expressor, 21 (± 16) of CR1 spots were visualized on fixed erythrocytes and 8 (± 4) on fresh erythrocytes (n = 20 for both groups). The size of the speckles (fixed erythrocytes) or clusters (fresh erythrocytes) did not depend on the CR1 levels of the donors (data not shown). To exclude the possibility that the observed dispersed distribution of CR1 resulted from a fixation-related artifact, we applied 2 additional staining strategies that avoided fixation. In the first approach (Figure 1C), the identical immunofluorescence staining protocol was applied (ie, primary anti-CR1 Ab followed by fluorescently conjugated secondary Ab) to fresh erythrocytes, and the expression of CR1 was examined immediately after addition of secondary antibody (within 1 minute and without washing steps). As shown in Figure 1C, a similar diffuse CR1 pattern was maintained during the first minutes after secondary antibody addition with CR1 being distributed in speckles with few larger clusters. In a second approach, erythrocytes were incubated with AlexaFluor-594 directly labeled Fab fragments of anti-CR1 mAb 2B11. We have previously shown that this Ab binds a single epitope on CR1 molecule.13 As shown in Figure 1D, the pattern resembles staining of CR1 on fixed erythrocytes (Figure 1B) or on fresh erythrocytes examined immediately after addition of the secondary Ab (Figure 1C). In conclusion, our data show that CR1 is expressed on fresh erythrocytes in a dispersed pattern, whereas redistribution into large clusters is an event promoted by CR1 cross-linking.
Distribution of CR1 on erythrocytes depends on the staining method. (A) Erythrocytes from a HH donor were incubated with anti-CR1 mAb 1F11, followed by AlexaFluor-594 goat anti–mouse Ab. After each step the cells were centrifuged, dispersed, and resuspended in fresh buffer. The majority of CR1 was in large clusters. (B) Fresh erythrocytes from the same HH donor were fixed as described in “Fresh and fixed erythrocytes” and incubated with anti-CR1 mAb 1F11 followed by AlexaFluor-594 goat anti–mouse Ab. CR1 is seen as small speckles throughout the plasma membrane. Bar represents 8 μm. (C) Erythrocytes processed for panel A were imaged within 1 minute after the addition of secondary antibody without any washing. Although larger CR1 clusters can already be observed (white arrows), most of the CR1 is still seen as small speckles. The high background of the image is due to the presence of the secondary antibody in solution during imaging. Bar represents 8 μm. (D) Erythrocytes were incubated with AlexaFluor-594–labeled Fab anti–CR1 mAb 2B11 for 15 minutes at room temperature, washed once, and resuspended in fresh buffer. The majority of CR1 is dispersed with few larger clusters. (E,F) Fresh unfixed (E) or fixed (F) erythrocytes were incubated with C3b-beads AlexaFluor-488 (open histogram) or control beads (IgG + heat-inactivated human serum, filled histogram) for 30 minutes at room temperature. Erythrocytes were then washed and analyzed by flow cytometry. Net mean fluorescence intensity (MFI) was 5.38 for fresh erythrocytes and 3.01 for fixed erythrocytes. (G) On fresh erythrocytes, CR1 partitions in the cytoskeletal fraction. Cytoskeletal (C) and membrane (M) fractions isolated as described in “Immunoblotting and coimmunoprecipitation” were probed for CR1 using rabbit polyclonal IgG. Whole erythrocyte lysate (E, lane 1; 1.5 × 106 erythrocytes/lane) was used as positive control.
Distribution of CR1 on erythrocytes depends on the staining method. (A) Erythrocytes from a HH donor were incubated with anti-CR1 mAb 1F11, followed by AlexaFluor-594 goat anti–mouse Ab. After each step the cells were centrifuged, dispersed, and resuspended in fresh buffer. The majority of CR1 was in large clusters. (B) Fresh erythrocytes from the same HH donor were fixed as described in “Fresh and fixed erythrocytes” and incubated with anti-CR1 mAb 1F11 followed by AlexaFluor-594 goat anti–mouse Ab. CR1 is seen as small speckles throughout the plasma membrane. Bar represents 8 μm. (C) Erythrocytes processed for panel A were imaged within 1 minute after the addition of secondary antibody without any washing. Although larger CR1 clusters can already be observed (white arrows), most of the CR1 is still seen as small speckles. The high background of the image is due to the presence of the secondary antibody in solution during imaging. Bar represents 8 μm. (D) Erythrocytes were incubated with AlexaFluor-594–labeled Fab anti–CR1 mAb 2B11 for 15 minutes at room temperature, washed once, and resuspended in fresh buffer. The majority of CR1 is dispersed with few larger clusters. (E,F) Fresh unfixed (E) or fixed (F) erythrocytes were incubated with C3b-beads AlexaFluor-488 (open histogram) or control beads (IgG + heat-inactivated human serum, filled histogram) for 30 minutes at room temperature. Erythrocytes were then washed and analyzed by flow cytometry. Net mean fluorescence intensity (MFI) was 5.38 for fresh erythrocytes and 3.01 for fixed erythrocytes. (G) On fresh erythrocytes, CR1 partitions in the cytoskeletal fraction. Cytoskeletal (C) and membrane (M) fractions isolated as described in “Immunoblotting and coimmunoprecipitation” were probed for CR1 using rabbit polyclonal IgG. Whole erythrocyte lysate (E, lane 1; 1.5 × 106 erythrocytes/lane) was used as positive control.
Effect of clustering on intensity and area occupied by CR1 on human erythrocytes
| Erythrocyte preparation . | Average area (pixels)/CR1 . | Mean intensity/CR1 . |
|---|---|---|
| Fresh | 26 | 155 |
| Fixed | 6 | 79 |
| Erythrocyte preparation . | Average area (pixels)/CR1 . | Mean intensity/CR1 . |
|---|---|---|
| Fresh | 26 | 155 |
| Fixed | 6 | 79 |
Preclustered CR1 has been believed to be required for binding of complement-opsonized particles or ICs.22 Our new data suggest, however, that the initial CR1-IC association occurs when CR1 is dispersed over the erythrocyte membrane. To determine whether CR1 is able to bind the complement-tagged particles while in a dispersed form, we compared the binding efficiency of in vitro generated, fluorescently labeled, complement-opsonized particles (C3b-beads) by fresh and fixed erythrocytes. We found that fixed cells, which maintain diffused CR1 configuration, efficiently bound and retained C3b-beads (Figure 1F), which suggests that CR1 in a clustered form is not obligatory for an initial association with complement-opsonized cargo. Higher binding efficiency of fresh erythrocytes compared with their fixed counterparts (Figure 1E,F; net mean fluorescence intensity [MFI] 5.38 vs 3.01, respectively) could be the result of 2 factors. First, it could reflect the ability of the former to “mature” into an established cluster of CR1 molecules that retains C3b bead with higher avidity through the washing steps and shear stress of the flow cytometer, clustering which cannot take place once the cell has been fixed. Secondly, as it was shown before for CR1 in neutrophils,23 fixation alters the conformation of CR1, rendering the molecule less antigenic and likely less avid for C3b. We further analyzed the distribution of dispersed and clustered CR1 with respect to the size of the cargo by analyzing the intensity profiles of both CR1 and C3b beads on either fresh (Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article) or fixed erythrocytes (Figure S1D,E). When the intensity of the CR1 clusters was plotted against the intensity of the corresponding cargo (C3b beads), we found that in fresh erythrocytes (Figure S1C) C3b beads were able to cluster CR1 molecules into complexes that correlated significantly with their size (R = 0.9691 and P = .017 as calculated by Spearman rank coefficient), whereas in fixed erythrocytes (Figure S1F) there was no significant correlation between the size of the CR1 cluster and the size of the C3b bead (R = 0.1955, P = .083). The results show that fixation that inhibited CR1 rearrangement into clusters had a minimal effect on binding efficiency of the complement-opsonized beads.
Our data indicate that erythroid CR1 normally undergoes significant rearrangement within the membrane during the immune adherence process. It was therefore important to know what determines the initial dispersed distribution of CR1 at the molecular level. We found that CR1 from fresh erythrocyte lysates fractionated entirely in the insoluble (cytoskeletal) fraction, with no detectable CR1 in the soluble (membrane) fraction (Figure 1G) as assessed by Western blotting. This suggested that dispersed CR1 in “resting” erythrocytes are attached to cytoskeleton. The efficiency of the isolation method used to separate membrane and cytoskeleton fractions was verified by immunoblotting for spectrin (cytoskeletal marker) and CD59 (membrane marker). Spectrin was found exclusively in the cytoskeletal fraction, whereas CD59, which is GPI-anchored, was found only in membrane fraction (Figure S2).
A protein PDZ array identifies PDZ domains 2, 3, and 5 of FAP-1 as human CR1 binding partners
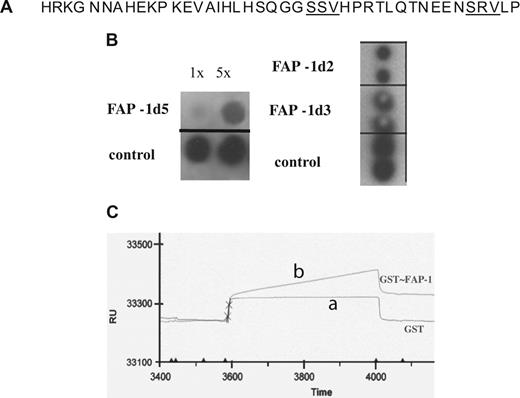
The cytoplasmic tail of CR1 (consisting of 43 amino acids) lacks the identified actin- or actin-spectrin binding motifs.24 This indicates that the observed association between CR1 and the cytoskeleton (Figure 1G) is likely mediated by an intermediate protein. Analysis of the cytoplasmic tail of CR1 revealed 2 canonical PDZ motifs with the sequence of S/T-X-V (Figure 2A; reviewed in Nourry et al25 ), which implies that CR1 has the means to interact with PDZ domain-containing scaffold proteins. To identify the PDZ-containing proteins capable of interacting with CR1, we screened the CR1 cytoplasmic tail peptide (CR1-tail peptide) against 2 commercially available PDZ arrays. Three PDZ domains that bound CR1-tail peptide were identified, all of which were found in 1 protein, FAP-1 (PTPN13, GeneID: 5783). Specifically, of all 5 FAP-1 PDZ domains included in arrays, domains 2, 3 and 5, but not 1 and 4, interacted with CR1-tail peptide (Figure 2B). Because at the time of this experiment no binding partners for FAP-1 PDZ domain 5 were reported, we used surface plasmon resonance (SPR) analysis to confirm the interaction between PDZd5 of FAP-1 and CR1-tail peptide. We found that GST-PDZ domain 5 of FAP-1 specifically bound to CR1-tail peptide (Figure 2C), confirming the specificity of the interaction seen in the PDZ protein array (Figure 2B).
The cytoplasmic domain of CR1 contains 2 PDZ motifs and binds PDZ domains 2, 3, and 5 of FAP-1. (A) Cytoplasmic domain of CR1 with its 2 PDZ motifs underlined. (B) A peptide corresponding to the CR1 cytoplasmic domain (CR1-tail peptide) was used to probe 2 PDZ arrays, which together contained all 5 PDZ domains of FAP-1. CR1-tail peptide (100 ng/mL) bound to FAP-1 PDZd2 and 3 (left blot, duplicate 10 ng/spot) and PDZd5 (right blot, 8 or 40 ng/spot). Bound CR1-tail peptide was detected by rabbit anti–Cyto-CR1 (BW60), followed by HRP-conjugated secondary antibody. (C) Surface plasmon resonance sensorgram confirms binding of CR1-tail peptide to immobilized PDZd5 of FAP-1. GST-PDZd5 was immobilized via anti-GST antibody to CM5 sensor chip and probed with CR1-tail peptide (trace b). On the control channel, GST alone was immobilized to assess the background binding of CR1-tail peptide (trace a). Equal volumes of the same concentration of the CR1 peptide were used for both channels. Sensorgrams are representative of 2 experiments. RU indicates resonance units; time is expressed in seconds.
The cytoplasmic domain of CR1 contains 2 PDZ motifs and binds PDZ domains 2, 3, and 5 of FAP-1. (A) Cytoplasmic domain of CR1 with its 2 PDZ motifs underlined. (B) A peptide corresponding to the CR1 cytoplasmic domain (CR1-tail peptide) was used to probe 2 PDZ arrays, which together contained all 5 PDZ domains of FAP-1. CR1-tail peptide (100 ng/mL) bound to FAP-1 PDZd2 and 3 (left blot, duplicate 10 ng/spot) and PDZd5 (right blot, 8 or 40 ng/spot). Bound CR1-tail peptide was detected by rabbit anti–Cyto-CR1 (BW60), followed by HRP-conjugated secondary antibody. (C) Surface plasmon resonance sensorgram confirms binding of CR1-tail peptide to immobilized PDZd5 of FAP-1. GST-PDZd5 was immobilized via anti-GST antibody to CM5 sensor chip and probed with CR1-tail peptide (trace b). On the control channel, GST alone was immobilized to assess the background binding of CR1-tail peptide (trace a). Equal volumes of the same concentration of the CR1 peptide were used for both channels. Sensorgrams are representative of 2 experiments. RU indicates resonance units; time is expressed in seconds.
FAP-1 protein is expressed in human erythrocytes and colocalizes with CR1
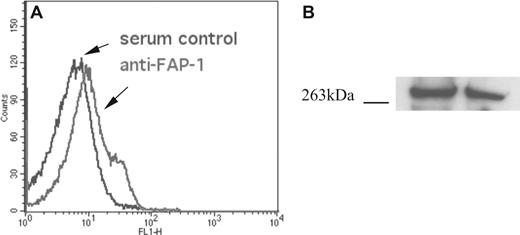
Because FAP-1 contains a FERM domain that was shown in other proteins to interact with actin as well as a KIND domain with similarities to the band 4.1 superfamily,26,27 it is reasonable to speculate that FAP-1 could act as a scaffolding protein for CR1. Despite its well-documented expression in most of the tissues,28 FAP-1 has not yet been identified in erythroid cells.29,30 Using flow cytometry, we now show that human erythrocytes are uniformly positive for FAP-1 protein expression, with a minor subpopulation that demonstrated higher levels of expression (Figure 3A). Immunoblot analysis further confirmed the presence of FAP-1 protein of the expected length in an erythrocyte lysate (Figure 3B). Interestingly, different commercially available anti–FAP-1 antibodies render a single FAP-1 band (C20 from Santa Cruz Biotechnology: 260 kDa, data not shown) or multiple FAP-1 bands (mouse mAb clone 359313 from R&D Systems: 260 kDa and 240 kDa, Figure 4; H-300 from Santa Cruz Biotechnology: 60 kDa, 260 kDa, and 280 kDa, data not shown), when probing erythrocyte lysates. It is possible that of the 8 described isoforms of FAP-1 (http://harvester.fzk.de/harvester/human/IPI00006/IPI00006714.htm), several are present in human erythrocytes.
FAP-1 antigen is detected in all circulating erythrocytes. (A) Fixed and permeabilized erythrocytes were incubated with rabbit anti–FAP-1 antiserum (#B12060, Stratagene) or the same concentration of rabbit nonimmune serum and analyzed by flow cytometry. FAP-1 expression is predominately unimodal, with a small subpopulation expressing more FAP-1. (B) Erythrocyte FAP-1 antigen is the size of full-length FAP-1. Five microliters of fresh erythrocytes were boiled in 90 μL of 1× reducing loading buffer, subjected to SDS-PAGE, and immunoblotted with anti–FAP-1 antiserum17 and HRP-secondary antibody. The detected band had a mobility of 270 kDa, which corresponds to full-length FAP-1. The gel is representative of 4 experiments.
FAP-1 antigen is detected in all circulating erythrocytes. (A) Fixed and permeabilized erythrocytes were incubated with rabbit anti–FAP-1 antiserum (#B12060, Stratagene) or the same concentration of rabbit nonimmune serum and analyzed by flow cytometry. FAP-1 expression is predominately unimodal, with a small subpopulation expressing more FAP-1. (B) Erythrocyte FAP-1 antigen is the size of full-length FAP-1. Five microliters of fresh erythrocytes were boiled in 90 μL of 1× reducing loading buffer, subjected to SDS-PAGE, and immunoblotted with anti–FAP-1 antiserum17 and HRP-secondary antibody. The detected band had a mobility of 270 kDa, which corresponds to full-length FAP-1. The gel is representative of 4 experiments.
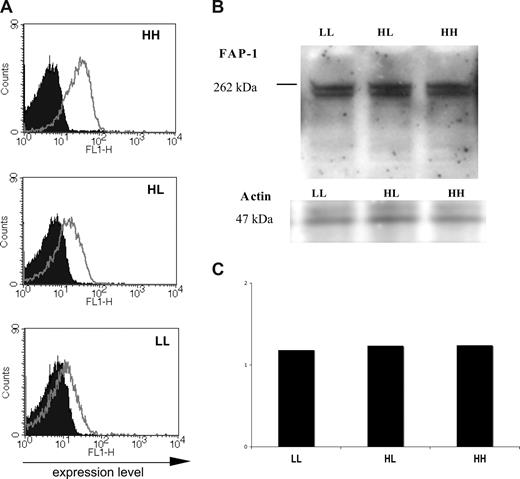
Erythrocyte FAP-1 levels do not parallel those of CR1. (A) Flow cytometric analysis of CR1 expression on erythrocyte from normal donors with low (LL), intermediate (HL), and high (HH) levels of CR1. Finger prick blood from 3 donors (10 μl from each donor) was stained with anti-CR1 mAb 2B11 and incubated with AlexaFluor-488 goat anti–mouse secondary antibody. Cells were then analyzed by FACS within 30 minutes after completion of the staining procedures. The experiment was repeated 4 times with similar results. (B) Western blot analysis of FAP-1 expression in erythrocyte expressing various levels of CR1. Five microliters of freshly purified erythrocyte from the same donors used for Western blot analysis were mixed with 1× reducing- loading buffer and boiled for 3 minutes. Proteins were separated on a 10% Bis-Tris NuPAGE gel and blotted on nitrocellulose membrane. FAP-1 was detected using a mouse mAb. The experiment was repeated 4 times with blood from various HH, HL, and LL donors and different anti–FAP-1 antibodies. (C) Bar graph representing the densitometry results of the levels of FAP-1 in erythrocyte expressing various levels of CR1 after normalization to actin. The loading control was assessed by quantifying the levels of actin (Quantity One; Bio-Rad). The experiment was repeated 3 times with blood from various HH, HL, and LL donors.
Erythrocyte FAP-1 levels do not parallel those of CR1. (A) Flow cytometric analysis of CR1 expression on erythrocyte from normal donors with low (LL), intermediate (HL), and high (HH) levels of CR1. Finger prick blood from 3 donors (10 μl from each donor) was stained with anti-CR1 mAb 2B11 and incubated with AlexaFluor-488 goat anti–mouse secondary antibody. Cells were then analyzed by FACS within 30 minutes after completion of the staining procedures. The experiment was repeated 4 times with similar results. (B) Western blot analysis of FAP-1 expression in erythrocyte expressing various levels of CR1. Five microliters of freshly purified erythrocyte from the same donors used for Western blot analysis were mixed with 1× reducing- loading buffer and boiled for 3 minutes. Proteins were separated on a 10% Bis-Tris NuPAGE gel and blotted on nitrocellulose membrane. FAP-1 was detected using a mouse mAb. The experiment was repeated 4 times with blood from various HH, HL, and LL donors and different anti–FAP-1 antibodies. (C) Bar graph representing the densitometry results of the levels of FAP-1 in erythrocyte expressing various levels of CR1 after normalization to actin. The loading control was assessed by quantifying the levels of actin (Quantity One; Bio-Rad). The experiment was repeated 3 times with blood from various HH, HL, and LL donors.
Levels of CR1 on erythrocytes are both genetically determined and decrease as erythrocytes age, older erythrocytes having approximately 55% to 60% of the CR1 molecules compared with new erythrocytes.31 High CR1 expressors have approximately 3 to 4 times more CR1 than low expressors and approximately twice as many CR1 molecules than intermediate expressors.21 An enticing possibility was that the levels of FAP-1 determined the expression of CR1 on erythrocytes from donors with high, intermediate and low levels of CR1 (Figure 4A,B). We found that FAP-1 levels do not parallel the levels of CR1 expression (Figure 4C).
CR1 colocalizes and coimmunoprecipitates with FAP-1 in human erythrocytes
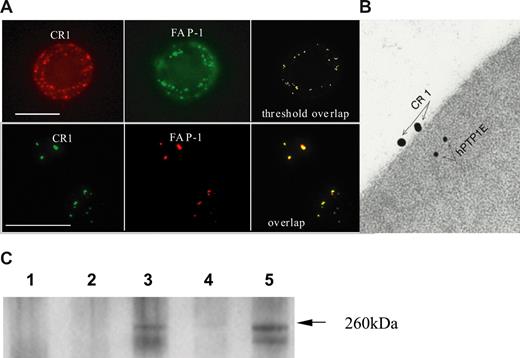
To verify that CR1 and FAP-1 can interact in physiologic conditions in native erythrocytes, we first studied the distribution of FAP-1, with respect to CR1, by immunofluorescence microscopy. We found that when CR1 is in a dispersed configuration (Figure 5A top row), which was achieved by immediate fixation of erythrocytes before staining, FAP-1 was seen predominantly proximate to the plasma membrane, as determined by focusing through the cell. Overlay of the images of FAP-1 and CR1 demonstrated that on 9 analyzed cells, 13.3% of the FAP-1 signals colocalized with CR1 and 20.6% of CR1 colocalized with FAP-1 (Figure 5A top row). When CR1 was allowed to form clusters (by CR1 ligation) before fixation (Figure 5A bottom row), FAP-1 formed large clusters adjoining plasma membrane. An overlay of the images of the 2 cells shows colocalization of 89.9% of the FAP-1 signals with CR1 and 94.5% of CR1 colocalized with FAP-1. Thus, in clustered state the association of CR1 with FAP-1 increases.
CR1 colocalizes and coimmunoprecipitates with FAP-1 in human erythrocytes. (A) Colocalization by fluorescence microscopy. Fixed (top row) or fresh (bottom row) erythrocytes were incubated with anti-CR1 mAb 1F11 followed by AlexaFluor-488 goat anti–mouse Ab. After fixation, quenching, and permeabilization, anti–FAP-1 or control Ab was added, followed by AlexaFluor-594 donkey anti–rabbit Ab. Cells were mounted on a slide and images were recorded using the appropriate filters. Bar represents 5 μm. (B) Colocalization by EM-immunogold method. Fresh erythrocytes were reacted with anti-CR1 mAb and then incubated with 18 nm gold conjugated goat anti–mouse antibody as described in “Methods.” Cells were fixed, embedded, sectioned, and then stained with either control Ab (not shown) or anti–FAP-1 rabbit anti-serum (hPTP1E17 ) followed by 10 nm gold goat anti-rabbit antibody. (C) Fresh (lane 3) or CR1 cross-linked erythrocytes (anti-CR1 mAb YZ1 + goat anti–mouse IgG Ab (lane 5) were lysed and CR1 precipitated with YZ1 coupled to proteins A + G beads (lane 3) or donkey anti–goat coupled to protein A + G beads (lane 5). Controls included: lane 1, erythrocytes + mIgG1 control Ab + protein A + G beads; lane 2, erythrocytes + protein A + G beads; lane 4, erythrocytes + goat anti–mouse coupled to protein A + G beads. The immunoprecipitates were resolved by SDS-PAGE blotted on nitrocellulose membrane and developed with rabbit anti–FAP-1 antiserum.17 FAP-1 band (arrow) was precipitated in erythrocytes both before (lane 3) and after (lane 5) CR1 cross-linking. Control lanes 1, 2, and 4 were negative. The experiment was repeated 3 times, twice with these results.
CR1 colocalizes and coimmunoprecipitates with FAP-1 in human erythrocytes. (A) Colocalization by fluorescence microscopy. Fixed (top row) or fresh (bottom row) erythrocytes were incubated with anti-CR1 mAb 1F11 followed by AlexaFluor-488 goat anti–mouse Ab. After fixation, quenching, and permeabilization, anti–FAP-1 or control Ab was added, followed by AlexaFluor-594 donkey anti–rabbit Ab. Cells were mounted on a slide and images were recorded using the appropriate filters. Bar represents 5 μm. (B) Colocalization by EM-immunogold method. Fresh erythrocytes were reacted with anti-CR1 mAb and then incubated with 18 nm gold conjugated goat anti–mouse antibody as described in “Methods.” Cells were fixed, embedded, sectioned, and then stained with either control Ab (not shown) or anti–FAP-1 rabbit anti-serum (hPTP1E17 ) followed by 10 nm gold goat anti-rabbit antibody. (C) Fresh (lane 3) or CR1 cross-linked erythrocytes (anti-CR1 mAb YZ1 + goat anti–mouse IgG Ab (lane 5) were lysed and CR1 precipitated with YZ1 coupled to proteins A + G beads (lane 3) or donkey anti–goat coupled to protein A + G beads (lane 5). Controls included: lane 1, erythrocytes + mIgG1 control Ab + protein A + G beads; lane 2, erythrocytes + protein A + G beads; lane 4, erythrocytes + goat anti–mouse coupled to protein A + G beads. The immunoprecipitates were resolved by SDS-PAGE blotted on nitrocellulose membrane and developed with rabbit anti–FAP-1 antiserum.17 FAP-1 band (arrow) was precipitated in erythrocytes both before (lane 3) and after (lane 5) CR1 cross-linking. Control lanes 1, 2, and 4 were negative. The experiment was repeated 3 times, twice with these results.
Next, we used electron microscopy to examine the localization of CR1 and FAP-1 in CR1-ligated erythrocytes, the condition that provided maximum colocalization by light microscopy. Because the number of CR1 molecules per erythrocyte is low (between 150-1000, depending on the donor and the age of the cell) there were few positive erythrocyte sections for CR1, but in most of the double positive cells, the signals corresponding to the 2 proteins were adjacent (Figure 5B), further suggesting that CR1 and FAP-1 interact in erythroid cells in vivo. To determine whether CR1 and FAP-1 physically associate in circulating erythrocytes, CR1 was immunoprecipitated from the lysates that were prepared from either fresh erythrocytes (to preserve dispersed CR1 distribution) or after cross-linking of CR1 using primary and secondary antibodies (to induce CR1 clustering). As shown on Figure 5C, FAP-1 antigen of the expected mobility (260-270 kDa range) was detected in the anti-CR1 immunoprecipitates from fresh, as well as from CR1–cross-linked erythrocytes (Figure 5C, lanes 3 and 5, respectively). No signal was detected in the control lanes (Figure 5C, lanes 1, 2 and 4, respectively). These results confirm that the association between FAP-1 and CR1 occurs physiologically in erythrocytes, and identify FAP-1 as the scaffolding molecule that could mediate CR1:cytoskeletal attachment and thus control its lateral mobility.
Discussion
Although erythrocytes are the main IC binding cell in the blood, they express fewer CR1 molecules/cell (∼200-1000) than other CR1-expressing blood cell.21 Initially the efficiency of binding was attributed to the fact that erythrocytes outnumber all other CR1-bearing cells in the blood by at least 400:1. Further studies have shown that even after normalizing the number of CR1 molecules expressed by each cell type, erythrocytes still capture immune complexes more avidly than all the other cells in the blood.22,32 Thus, the native CR1 expression on erythrocytes must be functionally optimized to capture complement-opsonized particles and this phenomenon was attributed to the uniquely clustered expression of CR1 in erythrocytes membrane.12,22 Now we report that CR1 appeared as dispersed speckles when fixed erythrocytes were examined by indirect immunofluorescence (Figure 1B), immediately after CR1 was ligated with secondary antibody (Figure 1C), or after ligation with a non–cross-linking anti-CR1 mAb Fab fragment (Figure 1D). We demonstrated that both fixed erythrocytes with CR1 locked in the dispersed pattern, and fresh erythrocytes, which would have allowed CR1 to rearrange forming large clusters after ligation as in Figure 1A, were able to bind C3b-beads (Figure 1F and E, respectively). Fresh erythrocytes were modestly more efficient in bead-binding than the fixed erythrocytes, which might mean (1) fixation partially denatured CR1, as noted in studies of anti-CR1 binding23 ; (2) clusters favor the retention of bound particles during the perturbation (shear stress) of centrifugation, washing, and analysis by flow cytometry; or a combination of factors 1 and 2. The potential impact of clustering on increasing the avidity and stability of immune adherence binding is discussed below.
That CR1 does cluster after it is ligated is shown in Figure 1A and is well documented in the literature.9,12 There are several potential benefits for CR1 clustering after ligation. First, neighboring CR1 molecules would provide for additional multivalent binding, which would increase the avidity of the interaction. Second, clusters of human CR113 or nonhuman primate GPI-anchored CR1-like molecules6 actually exclude antibody binding to the extracellular domains (long homologous repeats A, B, C, and D) of CR1. In humans there are no data suggesting that CR1 clustering is reversible, but in nonhuman primates dispersion of the preclustered CR1-like molecules has been observed 24 hours after the clearance of IC from circulation.6 It is possible that clustering of CR1 is reversible in humans, too, if the removal of IC in liver and spleen is factor I–dependent, although data supporting an efficient role for factor I in the removal of IC are controversial.33
Factor I is responsible for the sequential degradation of C3b to iC3b and then to C3dg, which has less (iC3b) or no affinity (C3dg) for CR1.34 If clusters exclude antibody, it is possible that they also exclude complement factor I from accessing C3b on the particle. Preventing factor I from degrading C3b would stabilize the binding of complement-opsonized particles to erythrocytes during transportation to macrophages in liver and spleen. The third advantage of having CR1 clustered is keeping opsonic stimuli concentrated in few areas on erythrocyte surface. As an IC-loaded erythrocyte travels through blood, the erythrocyte itself becomes indirectly opsonized by the IgG and the complement fragments on the cargo and a potential target for the phagocytes of the reticuloendothelial system. However, for phagocytosis to be successful, the opsonic stimuli have to be distributed uniformly over the entire surface of target particle at relatively high density to act as a zipper for the phagocyte to surround the target.35-37 Thus, by clustering the cargo-bearing CR1, erythrocytes keep the opsonic stimuli concentrated in few discrete spots on the cell membrane, allowing only a localized, targeted transfer of the CR1 cargo to the macrophages, while sparing the erythrocytes. One liability of CR1 clusters is that, extrapolating again from the antibody binding data,6,13 CR1 clusters would be inefficient in capturing additional complement-opsonized targets.
Finding that full-length FAP-1 is expressed in all erythrocytes (Figure 3A,B) and that CR1 interacts with FAP-1 (Figures 2B,C and 5C) has at least 2 important functional implications: first, it provides a potential means for CR1, which lacks actin- or actin-spectrin–binding domains, to interact with the cytoskeleton,24 which is consistent with CR1 fractionating in the detergent-insoluble cytoskeleton (Figure 1G). It is likely that FAP-1 is anchored to the cytoskeleton by its FERM domain because FERM domains of other proteins were shown to bind actin (reviewed in Bretscher et al16 ). Alternatively, the amino terminus of FAP-1's FERM domain reportedly binds PIP2 and PIP3,38 which would allow FAP-1 to attach reversibly to the plasma membrane. If FAP-1 were anchored by lipid, the fractionation of the CR1-FAP-1 complex with the cytoskeleton that we observed might be due to the large size of the entire FAP-1 scaffolding complex. Our low percentage of CR1-FAP-1 colocalization in erythrocytes before cross-linking would be consistent with this explanation. It was shown that the common form of CR1, which has 4 homologous repeats, binds complement opsonins and complement-opsonized particles that are tagged with multiple opsonins (C3b, C4b, MBL and C1q), insuring that even the interaction of 1CR1 with 1 complement-opsonized particle will be multivalent.39-43 Figure 6 depicts a model of CR1 in the erythrocyte membrane before and after cross-linking with either antibody or complement opsonized particles.
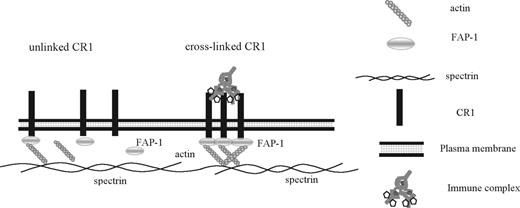
Model of the dynamic CR1 rearrangement induced by cross-linking. In circulating erythrocytes, CR1 is likely attached to the cytoskeleton (Figure 1G), with a subpopulation interacting with FAP-1 (Figure 5A top row). Cross-linking of CR1 by either complement-opsonized beads or anti-CD35 antibodies induces the formation of large CD35 and FAP-1 clusters (Figure 5B,C). Because CD35 and FAP-1 coprecipitate in both fresh and CD35 cross-linked erythrocytes, our model depicts the CD35-FAP-1 complex in both conditions, although in the cross-linked state there are more CR1 molecules interacting with FAP-1 (Figure 5C).
Model of the dynamic CR1 rearrangement induced by cross-linking. In circulating erythrocytes, CR1 is likely attached to the cytoskeleton (Figure 1G), with a subpopulation interacting with FAP-1 (Figure 5A top row). Cross-linking of CR1 by either complement-opsonized beads or anti-CD35 antibodies induces the formation of large CD35 and FAP-1 clusters (Figure 5B,C). Because CD35 and FAP-1 coprecipitate in both fresh and CD35 cross-linked erythrocytes, our model depicts the CD35-FAP-1 complex in both conditions, although in the cross-linked state there are more CR1 molecules interacting with FAP-1 (Figure 5C).
In summary, we have shown for the first time that CR1 has a dispersed appearance on circulating erythrocytes, which may represent 1, 2 or 3 CR1 molecules (as suggested by SPR results) that are attached to PDZ domains 2,3, and 5 of FAP-1. The FERM domain of FAP-l may be linking FAP-1 to the actin cytoskeleton or to lipids of the inner leaflet of the plasma membrane. Finally, this is the first report of FAP-1 as an erythroid protein. Further understanding of the functionality of the CR1-FAP-1 complex in the erythrocyte membrane will shed light on the poorly understood process of immune adherent particle transfer, a process central to host defense and the prevention of autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dan Brown for his help with the electron microscopy studies, the William C. Aird Laboratory for HEK cells, Prof Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden) for rabbit anti-hPTP1E antiserum, and Dr Henry Marsh (Avant Immunotherapeutics, Needham, MA) for anti-CR1 mAb.
This work was supported by National Institutes of Health grants AI42987 (A.N.W.), AI057983 (I.G.), and DK-034854, which supports Core B (Microscopy and Histology Core Facilities at the Beth Israel Deaconess Medical Center) of the Harvard Digestive Diseases Center.
National Institutes of Health
Authorship
Contribution: I.G., A.M.G., G.W., and L.B.K. designed the experiments, performed research, analyzed data, and wrote the paper; and A.N.-W. designed the experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ionita Ghiran, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Center for Life Sciences, R-934, 3 Blackfan Circle, Boston, MA, 02115; e-mail: ighiran@bidmc.harvard.edu.
References
Author notes
*I.G. and A.M.G. are equal first authors.