Abstract
Interleukin-7 (IL-7), a cytokine produced by stromal cells, is required for thymic development and peripheral homeostasis of most major subsets of T cells. We examined whether regulatory T (Treg) cells also required the IL-7 pathway by analyzing IL-7Rα−/− mice. We observed a striking reduction in cells with the Treg surface phenotype (CD4, CD25, GITR (glucocorticoid-induced tumor necrosis factor [TNF]-like receptor), CD45RB, CD62L, CD103) or intracellular markers (cytotoxic T-lymphocyte–associated antigen-4, CTLA-4, and forkhead box transcription factor 3, Foxp3). Foxp3 transcripts were virtually absent in IL-7Rα−/− lymphoid tissues, and no Treg cell suppressive activity could be detected. There are 2 known ligands for IL-7Rα: IL-7 itself and thymic stromal lymphopoietin (TSLP). Surprisingly, mice deficient in IL-7 or the other chain of the TSLP receptor (TSLPR) developed relatively normal numbers of Treg cells. Combined deletion of IL-7 and TSLP receptor greatly reduced Treg cell development in the thymus but was not required for survival of mature peripheral Treg cells. We conclude that Treg cells, like other T cells, require signals from the IL-7 receptor, but unlike other T cells, do not require IL-7 itself because of at least partially overlapping actions of IL-7 and TSLP for development of Treg cells.
Introduction
Regulatory T (Treg) cells have a critical suppressive function for maintenance of self-tolerance and prevention of autoimmunity,1,2 and their deficiency can predispose to gastritis, thyroiditis, diabetes and graft-versus-host disease. Initially, Treg cells were identified as a small percentage, approximately 10% to 15%, of mouse CD4+ T cells that expressed CD25, the α chain of IL-2R.3,4 Treg cells also are reported to express CD45RB,5 CD62L,6 cytotoxic T lymphocyte–associated antigen 4 (CTLA-4),7-9 glucocorticoid-induced tumor necrosis factor (TNF)–like receptor (GITR),10-12 CD103 (αEβ7 integrin)13 and forkhead box transcription factor 3 (Foxp3), an intracellular transcriptional regulator.14-16
In vitro, Treg cells can block proliferative responses of both CD4+ and CD8+ CD25− cells by a mechanism that remains to be clearly defined but appears to be based on cell contact and to be independent of cytokine production.17-21 A very recent paper suggests Tregs suppress target cells by interleukin-2 (IL-2) deprivation and subsequent apoptosis.22 In vivo, Treg cells suppress activation and expansion of self-reactive T cells that have escaped thymic clonal deletion.1,3,23-25 In addition to cell contact, the suppressive cytokines IL-10 and transforming growth factor (TGF)β have been implicated in vivo as mediators of inhibition.26-32
Several studies suggest that CD4+CD25+ T cells mature in the thymus as a distinct T-cell population.5,7,10,15,29,33 High-affinity IL-2 receptors are constitutively expressed on Treg cells, and IL-2 has been implicated in the development, maintenance, and function of these cells.34 It has been reported that IL-2 may be required for peripheral expansion and homeostasis,35-38 and IL-2 appears to be required for Treg cell function in the periphery.17,39-42 Mice deficient in IL-2, IL-2Rα, or IL-2Rβ lack regulatory T cells43,44 and develop severe autoimmune disease.42,45-49
IL-7 is a cytokine that is produced by stromal cells in lymphoid tissues and is required for development and homeostasis of most subsets of T cells.50-52 The IL-7 receptor is composed of IL-7Rα and the common cytokine receptor γ chain, γc53,54 . A related stromal factor, thymic stromal lymphopoietin (TSLP), also shares IL-7Rα but additionally has a distinctive receptor subunit, TSLPR.55,56 Naive and memory CD4+ T cells require IL-7 for homeostatic survival.57,58 In vitro it has been shown that IL-7 can, albeit less well than IL-2 or IL-4, promote the proliferation and suppressor function of CD4+CD25+ cells stimulated with anti-CD3.40 The Tr1 cell, another type of suppressor cell that acts by secreting suppressive cytokines, has been shown to respond to IL-7 in vitro.59 It has recently been reported that Treg cells develop, survive, and function normally in mice that lack IL-7,60 suggesting the Treg cell lineage has requirements quite different from the major lineages of T cells, all of which require IL-7. Moreover, a recent study in human Treg cells noted that IL-7Rα chain is expressed at much lower levels than on other T-cell subsets and suggested that this low expression is a useful marker for distinguishing Treg cells.61 We independently evaluated the role of the IL-7 pathway in the Treg cell lineage, and our results substantiate both the independence of IL-7 and the low expression of IL-7Rα. However, despite this, we find that IL-7R is absolutely required for normal development of the Treg cell lineage. It had previously been reported that Foxp3-positive cells required a γc stimulus in addition to the IL-2 receptor62 ; the present study implicates IL-7Rα as providing both a γc stimulus, and when paired with the TSLPR, a γc-independent stimulus.
Methods
Mice
IL-7Rα−/− (B6.129S7-Il7rtm1Imx/J) and Rag2−/− were originally purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained by homozygous breeding at NCI-Frederick, MD. A different colony of IL-7Rα−/− mice, also originally purchased from The Jackson Laboratory, was maintained at NCI-Bethesda, MD, and provided by C. L. Mackall. TSLPR−/− and TSLPR+/+ mice were generated and maintained at the Laboratory of Molecular Immunology, National Institutes of Health (NIH), Bethesda, MD.63 C57BL/6 mice were purchased from the Animal Production Area, NCI-Frederick Cancer Research and Development Center (Frederick, MD). Rag−/−IL7−/− mice were obtained from R. Murray (EOS Biotechnology, San Francisco, CA). Animal care was provided in accordance with NIH Animal Use and Care guidelines. Experiments were performed following protocols approved by the NIH Committee. In our experiments, we refer to C57BL/6 as the wild-type control for IL-7−/− and IL-7Rα−/− mice, while the littermate TSLPR+/+ mice were the control for TSLPR−/− mice. All untreated mice analyzed were 6 to 8 weeks old and usually sex-matched.
Flow cytometry
For analysis of thymic, splenic, or lymph node cells, single-cell suspensions were prepared immediately after tissue harvesting. Erythrocytes were removed using the ACK lysing buffer (Biosource, Rockville, MD). The following antibodies were purchased from BD PharMingen (San Diego, CA): purified anti-CD16/CD32 (2.4G2); fluorescein isothiocyanate (FITC), peridinin chlorophyll protein (PerCP), allophycocyanin (APC), or phycoerythrin-cyanine 5 (PE-Cy5)-anti–CD4 (RM4-5); FITC-anti–CD3 (∈ chain 145-2C11); PE, FITC, or APC- anti-CD25 (PC61); biotinylated-anti–CD25 (7D4); FITC-anti-CD45RB (16A); FITC-anti–CD103 (integrin αEβ7, M290); FITC-anti–CD62L (L-selectin, LECAM, Ly-22, clone MEL-14); PE-anti–CD152 (cytotoxic T-lymphocyte antigen 4 [CTLA-4, clone UC10-4F10-11)]); PE-anti–phospho-Stat5 (Stat5p, clone Y694); FITC-rat immunoglobulin IgG2aκ; and PE-Ar Ham IgG1κ. FITC-anti–goat IgG was purchased from Sigma-Aldrich (St Louis, MO). Polyclonal anti-glucocorticoid–induced tumor necrosis factor receptor (GITR) was obtained from R&D Systems (Minneapolis, MN). PE-Cy5-anti–CD127 (IL-7Rα, clone A7R34) and APC or PE-anti–Foxp3 (FJK-16s) antibodies were purchased from e-Bioscience (San Diego, CA).
For cell surface staining, cells were washed twice with FACS buffer (phosphate buffered saline [PBS], 0.5% bovine serum albumin [BSA]), treated with Fc-block (anti-CD16/CD32 antibodies) for 15 minutes at 4°C and then incubated with the proper fluorochrome-conjugate antibodies for 30 minutes at 4°C. Cells were then washed twice in FACS buffer and analyzed. For CTLA-4 and Foxp3 intracellular staining, cells were fixed and labeled following the manufacturer's protocols. Intracellular anti-Stat5p staining was performed following the protocol published by Van De Wiele and colleagues.64
Flow cytometric analysis was performed on FACScan using CELLQuest Software (BD Biosciences, San Jose, CA). Postacquisition FACS data were analyzed using FCS Express version 3 software (De Novo Software, Los Angeles, CA).
Isolation and reverse transcription of RNA
Spleens, lymph nodes, and thymi were harvested and immediately homogenized in 600 μL RNeasy lysis buffer (Qiagen, Valencia, CA) with β-mercaptoethanol (Sigma-Aldrich). Total RNA was isolated using the RNeasy Mini Kit (Qiagen). To avoid DNA contamination, in RNA isolated from tissue eluted RNA was incubated with rDNase I for 30 minutes at 37°C (DNA-free kit, Ambion, Austin, TX). An amount of total RNA equal to 500 ng or less was reverse transcribed using SuperScipt III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Briefly, the RNA was incubated at 65°C for 5 minutes in a volume of 20 μL containing 1 μL of 50 μM oligo(dT)20 and 1 μL of 10 mM dNTPs mix, and then quickly chilled on ice. The cDNA synthesis was performed in a total volume of 40 μL containing 20 mM Tris-HCl, 50 mM KCl, 5 mM MgCl2, 10 mM DTT, 40U RNaseOUT, and 100U of SuperScript III RT at 50°C for 50 minutes followed by heat inactivation at 85°C for 5 minutes. The RNA template was removed from the cDNA:RNA hybrid molecules by digestion with 2 U of RNase H at 37°C for 20 minutes. At least 2 μL of this reaction mixture were used for real-time or conventional polymerase chain reaction (PCR) analysis.
Sorted cells were pelleted and resuspended in 1 mL of Trizol (Invitrogen, Carlsbad, CA). Total RNA was isolated using the standard Trizol method. Total RNA equal to 1 μg or less was reverse transcribed using the cDNA archive kit (Applied Biosystems, Foster City, CA). Briefly, RNA was resuspended in a volume of 20 μL containg 2 μL of 10 × RT buffer, 10 × random hexamers, 0.4 uL 10 mM dNTPs, and 1 μL of reverse transcriptase. RNA was incubated at room temperature for 10 minutes and moved to 37°C for 2 hours. 10 ng of cDNA was used for each quantitative real-time PCR.
Foxp3 and IL-7R quantitative real-time PCR
Quantitative real-time PCR.was used to determine the mRNA expression level of Foxp3 in spleens, lymph nodes, and thymus using the following primers for Foxp3 and the housekeeping gene Hprt: Foxp3 F: 5′ CTG CCT ACA GTG CCC CTA G 3′, Foxp3 R: 5′ CAT TTG CCA GCA GTG GGT AG 3′,14 HPRT F: 5′ TCC CAG CGT CGT GAT TAG CGA TGA 3′, HPRT R: 5′ AAT GTG ATG GCC TCC CAT CTC CTT CAT GAC AT 3′.65 Expression was normalized to the level of the housekeeping gene Hprt in each sample. Real-time PCR reactions were performed by an ABI Prism 7900 instrument (Applied Biosystems), and the PCR scheme was the following: 95°C for 15 minutes, then 40 cycles at 95°C for 15 seconds, and 55°C degrees for 30 seconds, followed by the dissociation curve of 95°C for 15 minutes, 55°C for 15 minutes, and 95°C for 15 minutes.
Gene expression assays (Applied Biosystems) for Foxp3 and IL-7 receptor were used to determine the mRNA expression level of Foxp3 and IL-7 receptor in sorted cells. Briefly, 10 ng of cDNA was put in a final volume of 20 μL containing 10 μL of Taqman Univeral PCR mix, 1 μL of primer/probe gene expression assay. The quantitative PCR samples were run on an ABI 7300. The PCR scheme was 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute.
Functional assays
Single-cell suspensions were obtained from mouse spleens. Erythrocytes were removed using the ACK lysing buffer (Biosource). CD3+CD4+CD25+ and CD3+CD4+CD25− cells were sorted using MoFlo cytometer (Cytomation, Fort Collins, CO), yielding a purity of both subsets more than 99%. CD3+CD4+CD25− T cells (5 × 104) were seeded in triplicate in 96-well, round-bottom plate in RPMI-1640 (Mediatech, Herndon, VA) with 10% fetal bovine serum (Hyclone, Logan, UT) containing 2 mM glutamine, 100 IU/mL penicillin (Mediatech), 100 μg/mL streptomycin (Mediatech), 10 mM Hepes (Gibco-BRL, Grand Island, NY), 1 mM sodium pyruvate (Gibco-BRL), and 50 μM β-mercaptoethanol (Sigma-Aldrich), plus 0.5 μg/mL soluble anti-CD3 antibody (∈ chin, 145-2C11; BD PharMingen). T cell–depleted and irradiated spleen cells (2 × 105cell/well) were used as antigen-presenting cells (APCs) and were prepared depleting of CD90+ cells using CD90 (Thy 1.2) microbeads and an LD MACS column (Miltenyi Biotec, Auburn, CA). CD3+CD4+CD25+ T cells were added to the wells at a ratio (CD4+CD25− T cells:CD4+CD25+ T cells) of 10:0, 10:1, 5:1, 2:1, and 1:1. Cells were pulsed with 37 000 becquerels [3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) per well for the last 15 hours of the 72-hour culture period. Cells were then harvested onto filter membranes using a harvester (Inotech Biosystems International, Rockville, MD), and the amount of incorporated [3H]thymidine was measured with a Wallac MicroBeta counter (PerkinElmer Life and Analytical Sciences, Waltham, MA).
In vitro analysis of IL-7Rα expression on splenic CD4+CD25+ cells after IL-7 stimulation
A single-cell suspension was prepared from C57BL/6 spleens. Two million cells were plated in wells of a 24-well plate at a concentration of 2 × 106 cells/mL in RPMI-1640 (Mediatech) with 10% fetal bovine serum (Hyclone) containing 2 mM glutamine, 100 IU/mL penicillin (Mediatech), 100 μg/mL streptomycin (Mediatech), and 55 μM β-mercaptoethanol (Invitrogen, Carlsbad, CA). Cells were treated with IL-7 or IL-2 (PeproTech, Rocky Hill, NJ) at a final concentration of 50 ng/mL and 20 ng/mL, respectively, and cultured overnight at 37°C in a humidified atmosphere with 5% CO2. Fresh cells and overnight untreated cells were used as a reference control.
Cell lines
The IL-7–dependent thymocyte cell line D168 was maintained in RPMI-1640 (Mediatech) supplemented with 10% FBS (Hyclone), 2 mM l-glutamine, 100 IU/mL of penicillin (Mediatech),100 μg/mL of streptomycin (Mediatech), 50 μM β-mercaptoethanol (Invitrogen), and 50 ng/mL murine-recombinant IL-7 (PeproTech). The retroviral package cell line phoenix-Eco69 was maintained in DMEM (Mediatech), supplemented with 10% FBS (Hyclone) and 100 IU/mL of penicillin (Mediatech), and 100 μg/mL of streptomycin (Mediatech).
Retroviral infection
Retroviral constructs pMIGR1-Foxp3 and the empty vector pMIGR1 (a kind gift from Dr S. Sakaguchi14 ) were transfected into the phoenix-Eco package cell line using FuGENE 6 reagent.69 The retrovirus-containing supernatant was collected and loaded onto a Retro-Nectin (Takara, Santa Ana, CA)–coated plate, and then the D1 cells were added and infected overnight. Green fluorescent protein–positive cells were analyzed for IL-7Rα expression 24 hours after infection.
IL-7 response of Treg cells
Splenic CD4+CD25+, CD4+CD25−, and CD4− cells were purified by cell sorting (MoFlo cytometer, Cytomation) or MACS columns (Miltenyi Biotec). Cells (0.5-2 × 106) were resuspended in 500 μL of RPMI-1640 (Mediatech), incubated at 37°C for 5 minutes and then stimulated with IL-7 (PeproTech) at a final concentration of 50 ng/mL for 20 minutes. After stimulation, cells were stained for intracellular Stat5p and then analyzed by flow cytometry.
Adoptive transfer of T cells
IL-7Rα−/− and Rag−/− mice were irradiated with 300 rad of whole body irradiation. Four hours later, the mice were injected in the tail vein with a single-cell suspension of 10 × 106 bone marrow cells obtained from C57BL/6 donor mice and maintained on SMZ antibiotic until the day of sacrifice. Spleens, thymi, and lymph nodes of host mice were harvested after 8 weeks and analyzed by flow cytometry.
C57BL/6 and IL-7−/− mice were irradiated with 300 rad of whole body irradiation. Four hours later, the mice were injected in the tail vein with a single cell suspension of 10 × 106 bone marrow cells obtained from TSLPR−/− or TSLPR+/+ donor mice and maintained on SMZ antibiotic until the day of sacrifice. Spleens and lymph nodes of host mice were harvested after 8 weeks and analyzed by flow cytometry.
IL-7−/−, Rag−/−, IL-7−/−Rag−/−, and C57BL/6 mice were irradiated with 300 rad of whole body irradiation. Four hours later, the mice were injected in the tail vein with a single cell suspension of purified CD4+ cells (15 × 106) obtained from TSLPR−/− or TSLPR+/+ donor mice. Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE). Recipients were maintained on SMZ antibiotic until the day of sacrifice. Spleens and lymph nodes of host mice were harvested after 6 days and analyzed by flow cytometry.
Treatment with anti–IL-7 neutralizing mAb
Eight-week-old TSLPR−/− and TSLPR+/+ mice were intraperitoneally injected 3 times a week for 4 weeks with 1 mg M25, a mouse IgG2b anti–human IL-7 neutralizing monoclonal antibody or M1, anti-FLAG, a mouse IgG2b isotype-matched control (Amgen, Seattle, WA).
Results
Foxp3+ cells are reduced in tissues of IL7Rα−/− mice
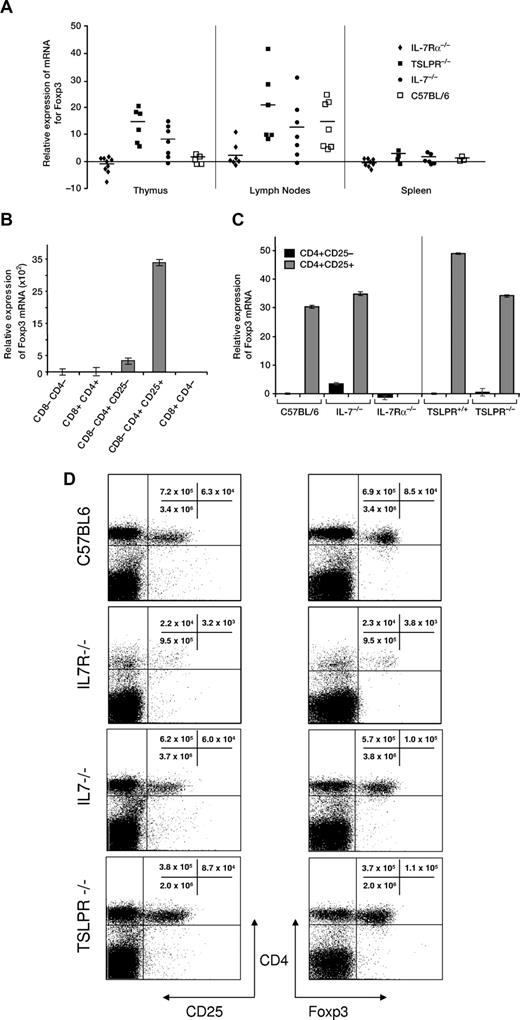
To determine whether Treg cells require the IL-7 system like most subsets of T cells, we first examined the expression of Foxp3, a nuclear protein that is primarily expressed by Treg cells and is essential for their development and function. RNA was extracted from thymus, spleen, lymph nodes, and bone marrow from C57BL/6, IL-7Rα−/−, IL-7−/−, or TSLPR−/− mice. Real-time PCR was used to quantify Foxp3 transcripts in cells pooled from at least 4 mice of each strain. The results shown in Figure 1A indicate that expression was clearly lower in thymus, lymph nodes, and spleen of IL-7Rα−/− mice as compared with TSLPR−/− and IL-7−/− mice. Since the IL-7−/− thymus is deficient in most thymocytes subsets, except Tregs, this could explain the relative enrichment of Foxp3 transcripts compared with C57BL/6. That explanation would not account for the TSLPR−/− result, since there are relatively normal thymocyte numbers in these mice. The other panels of Figure 1A show that in the periphery, there are similar values for C57BL/6, IL-7−/−, and TSLPR−/− mice.
Expression of Foxp3 mRNA in tissues of different strains of mice. (A) Relative expression of Foxp3 mRNA by real-time PCR. RNA was extracted from homogenized suspensions of thymus, spleen, or lymph nodes. Symbols represent the fold increase or decrease expression in individual mice, and the bar indicates the mean. (B) To verify that detection of Foxp3 by real-time PCR was selective for Treg cells, thymocytes from C57BL/6 mice were sorted for Treg cell markers. Expression was highest in the CD4+CD25+ subset that includes Treg cells. (C) Expression of Foxp3 mRNA by real-time PCR in sorted CD4+CD25− or CD4+CD25+ cells from spleens of C57BL/6, IL-7−/−, IL-7Rα−/−, TSLPR+/+, and TSLPR−/− mice. Cells for each strain were pooled from at least 2 mice. (D) Quantification of Treg cells (CD4+CD25+ and CD4+Foxp3+) in spleen of IL-7Rα−/− mice was compared with C57BL/6, IL-7−/−, or TSLPR−/−. Cells were gated on CD3. Representative individual mice from groups of at least 4 mice are shown.
Expression of Foxp3 mRNA in tissues of different strains of mice. (A) Relative expression of Foxp3 mRNA by real-time PCR. RNA was extracted from homogenized suspensions of thymus, spleen, or lymph nodes. Symbols represent the fold increase or decrease expression in individual mice, and the bar indicates the mean. (B) To verify that detection of Foxp3 by real-time PCR was selective for Treg cells, thymocytes from C57BL/6 mice were sorted for Treg cell markers. Expression was highest in the CD4+CD25+ subset that includes Treg cells. (C) Expression of Foxp3 mRNA by real-time PCR in sorted CD4+CD25− or CD4+CD25+ cells from spleens of C57BL/6, IL-7−/−, IL-7Rα−/−, TSLPR+/+, and TSLPR−/− mice. Cells for each strain were pooled from at least 2 mice. (D) Quantification of Treg cells (CD4+CD25+ and CD4+Foxp3+) in spleen of IL-7Rα−/− mice was compared with C57BL/6, IL-7−/−, or TSLPR−/−. Cells were gated on CD3. Representative individual mice from groups of at least 4 mice are shown.
To verify that our real-time PCR reaction for Foxp3 reacted selectively with Treg cells, we compared thymic Treg cells (CD4+CD25+) with other subsets of thymocytes. Thymocytes from C57BL/6 mice were sorted into fractions of double-positive (DP), double-negative (DN), and 3 different single-positive (SP) populations: CD8−CD4+CD25+, CD8−CD4+CD25−, and CD8+CD4−. RNA was amplified and as expected, the expression of Foxp3 in C57BL/6 was abundant in CD4+CD25+ cells and virtually absent in DP, DN, and CD8+CD4− subsets (Figure 1B). Similar results were obtained using sorted splenocytes from IL-7−/−, TSLPR−/−, and TSLPR+/+ mice (Figure 1C), confirming the specific expression of Foxp3 in CD4+CD25+ cells, whereas the few spleen cells obtained from IL-7Rα−/− mice that expressed CD4 and CD25 did not express Foxp3. Similar results were obtained in thymocytes and lymph nodes (data not shown). The number of cells with the phenotypic markers of Tregs (CD4+CD25+ and CD4+Foxp3+) were greatly diminished in spleens of IL-7Rα−/− mice, but present in IL-7−/− or TSLPR−/− mice (Figure 1D). In IL-7Rα−/− mice, the reduction in CD4+Foxp3+ cells (22.7- fold) was similar to the reduction in CD4+Foxp3− cells (30.3-fold), suggesting similar dependency on this receptor for their generation and/or maintenance.
The number of cells with the Treg cell surface markers CD4, CD25, and Foxp3 were determined at different ages in IL-7Rα−/− mice. Throughout life, these cells were greatly reduced in number in both thymus and spleen, compared with WT mice (not shown).
It was surprising from data in Figure 1 that, in contrast to the striking deficiency in IL-7Rα−/− mice, IL-7−/− or TSLPR−/− mice had relatively normal numbers of cells with the Treg cell markers CD4, CD25, and Foxp3. We therefore examined a larger panel of Treg cell markers on CD4+CD25+ cells to determine whether these were also relatively normal in IL-7−/− and TSLPR−/− spleen cells, but deficient in IL-7Rα−/− spleen cells. The frequency and level of expression was relatively normal for CD45RB, CD103, CD62L, GITR and intracellular CTLA-4 (not shown). Thus, the number and phenotype of Treg cells was relatively normal in IL-7−/− or TSLPR−/− mice. However, these markers also confirmed the deficiency in Treg cells in IL-7Rα−/− mice.
It was unexpected to find such a striking difference between IL-7Rα−/− and IL-7−/− mice, since other features of phenotypes in these mice are virtually indistinguishable. To verify that this was not unique to our colony of IL-7Rα−/− mice (NCI-FCRDC), we evaluated a second colony (NCI-Bethesda). Both colonies of IL-7Rα−/− mice showed the same Foxp3 deficiency, the 2 colonies having been founded years apart from breeders purchased from Jackson Laboratories. Thus, development of CD4 cells expressing Foxp3 and CD25, which are essential for function of Treg cells, is dependent on signals from IL-7R. Given this requirement for IL-7R, it was surprising that neither IL-7−/− nor TSLPR−/− mice showed a deficiency in Foxp3, since the only ligands known for IL-7Rα are IL-7 itself and TSLP. One explanation is that, whereas all other subsets of T cells require IL-7 (but not TSLP), Treg cells may use either IL-7 or TSLP interchangeably, and this hypothesis will be supported by findings described later in this report.
IL-7Rα−/− mice lack functional Treg cells
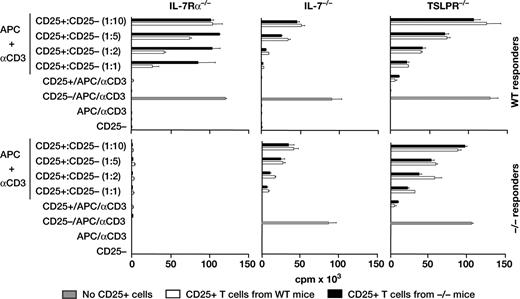
We compared the suppressive function of CD4+CD25+ splenocytes from IL-7Rα−/−, IL-7−/−, TSLPR−/− and wild-type control mice (Figure 2). IL-7Rα−/− cells, despite enrichment for the infrequent CD4+CD25+ cells, showed no significant inhibition of proliferation of wild-type responders. IL-7−/− and TSLPR−/− cells showed suppressive function that was comparable to that of wild-type Treg cells when assayed either on wild-type responders or on responders of their own knockout strain. Thus, Treg cells, defined by the criteria of surface markers and suppressive function, are absent in IL-7Rα−/− mice but present in IL-7−/− and TSLPR−/− mice.
Lack of Treg cell activity from IL-7Rα−/− mice. Suppressor populations were sorted for CD3, CD4, and CD25+ or CD25−. For each individual experiment, spleen cells were pooled from 2 wild-type mice and 10, 6, or 4 spleens from IL-7Rα−/−, IL-7−/−, or TSLPR−/− mice, respectively. The top panel shows CD25+ Treg cells (▭) from various knockout mice mixed with CD25− responders from wild-type mice, whereas the bottom panel shows both CD25+ Treg cells and CD25− responders from the same knockout mice. WT Treg cells ( ) are shown as a control at each mixture ratio.
) are shown as a control at each mixture ratio.
Lack of Treg cell activity from IL-7Rα−/− mice. Suppressor populations were sorted for CD3, CD4, and CD25+ or CD25−. For each individual experiment, spleen cells were pooled from 2 wild-type mice and 10, 6, or 4 spleens from IL-7Rα−/−, IL-7−/−, or TSLPR−/− mice, respectively. The top panel shows CD25+ Treg cells (▭) from various knockout mice mixed with CD25− responders from wild-type mice, whereas the bottom panel shows both CD25+ Treg cells and CD25− responders from the same knockout mice. WT Treg cells ( ) are shown as a control at each mixture ratio.
) are shown as a control at each mixture ratio.
IL-7Rα expression and signaling in Treg cells
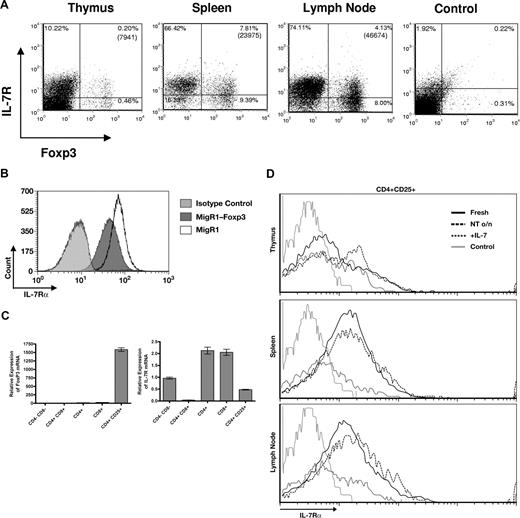
Having shown that Foxp3+ cells required IL-7Rα for development, we examined the expression of IL-7Rα on Foxp3+ cells from C57BL/6 mice. Thymus, spleen, and lymph node cells were stained for surface expression of IL-7Rα versus intracellular content of Foxp3 protein. IL-7Rα was only detectable by flow cytometry on a minority of the Foxp3+ cells from thymus and spleen and lymph node (Figure 3A). This expression on a subset of murine Treg cells therefore differs from human peripheral blood Treg cells, which lack IL-7Rα.61
Expression of IL-7Rα on Treg cells. (A) Surface IL-7Rα versus intracellular Foxp3 were evaluated in cells from thymus, spleen, and lymph nodes of normal C57BL/6 mice. IL-7Rα was variably expressed on Foxp3-positive cells. (B) Effect of overnight culture on expression of IL-7Rα. The level of IL-7Rα increased on some cells from thymus spleen and lymph node of normal C57BL/6 mice. On Treg cells (CD4+CD25+) from thymus, the IL-7Rα level increased, but it did not rise on Treg cells from spleen or lymph node. Culture with IL-7 down-regulated IL-7Rα expression on Treg cells as well as other T cells. (C) Foxp3 down-regulates IL-7Rα expression. The IL-7–dependent T-cell line D1 was infected with the retrovirus MigR1 expressing Foxp3, or with an empty MigR1 vector. After 24 hours, cells were analyzed for expression of IL-7Rα. The result is representative of 3 separate experiments. (D) Subsets of thymocytes were analyzed for expression of Foxp3 versus IL-7R. Cells were sorted according to expression of CD4, CD8, and CD25, then assayed by real-time PCR.
Expression of IL-7Rα on Treg cells. (A) Surface IL-7Rα versus intracellular Foxp3 were evaluated in cells from thymus, spleen, and lymph nodes of normal C57BL/6 mice. IL-7Rα was variably expressed on Foxp3-positive cells. (B) Effect of overnight culture on expression of IL-7Rα. The level of IL-7Rα increased on some cells from thymus spleen and lymph node of normal C57BL/6 mice. On Treg cells (CD4+CD25+) from thymus, the IL-7Rα level increased, but it did not rise on Treg cells from spleen or lymph node. Culture with IL-7 down-regulated IL-7Rα expression on Treg cells as well as other T cells. (C) Foxp3 down-regulates IL-7Rα expression. The IL-7–dependent T-cell line D1 was infected with the retrovirus MigR1 expressing Foxp3, or with an empty MigR1 vector. After 24 hours, cells were analyzed for expression of IL-7Rα. The result is representative of 3 separate experiments. (D) Subsets of thymocytes were analyzed for expression of Foxp3 versus IL-7R. Cells were sorted according to expression of CD4, CD8, and CD25, then assayed by real-time PCR.
We then considered that the low expression on freshly isolated Foxp3+ could reflect a recent encounter with IL-2 or IL-7 in vivo, which down-regulates IL-7Rα on other T-cell subsets.70-72 Cells were placed in culture overnight, which indeed raised expression of IL-7Rα on other T-cell subsets in thymus, spleen, and lymph node. However, only thymic Treg cells up-regulated IL-7Rα, whereas splenic or lymph node Treg cells did not up-regulate IL-7Rα (Figure 3B). Culture with IL-7 down-regulated IL-7Rα expression on Treg cells from thymus, spleen, or lymph node. Taken together, these results suggest that the low level of IL-7Rα on the majority of freshly isolated Treg cells from spleen or lymph node does not necessarily result from recent encounters with IL-7 or IL-2, although thymic Treg cells may have had such a recent encounter.
Because Treg cells expressed lower levels of IL-7Rα than other T-cell subsets, we examined whether Foxp3 could influence the expression of IL-7Rα. Transduction of Foxp3 into an IL-7–dependent murine thymocyte line induced a significant decline in IL-7Rα expression (Figure 3C). Thus, the low levels of IL-7Rα on mature Treg cells may alternatively reflect down-regulation by Foxp3, rather than a recent encounter with IL-7 or IL-2. In thymocytes, if Foxp3 inhibited IL7-Rα expression, different subsets might reflect this relationship. Thymocytes were sorted into different subpopulations and indeed, CD4+ cells bearing CD25 expressed much higher levels of Foxp3, and correspondingly lower levels of IL-7Rα, than their CD25− counterparts (Figure 3D). This relationship is consistent with Foxp3 inhibiting IL-7Rα expression. The lack of IL-7Rα in CD4+CD8+ cells is well known72 and, from these results, is unrelated to Foxp3 expression.
Based on the level of receptor expression, the response of Treg cells to IL-7 was expected to occur in only a minority of cells. However, a vigorous phosphorylation of Stat5 was observed in the majority of Treg cells as shown in Figure 4 and was comparable to other CD4 cells. This indicates that despite the low expression of IL-7 receptors on the majority of mature murine Treg cells, there were sufficient numbers of receptors to signal a response. This raises the possibility that a requirement for signals from IL-7 receptor could occur both in thymic development and peripheral survival of Treg cells, as in other T-cell subsets; however, as we will show, other data support a developmental but not a survival role.
IL-7 stimulation of Treg cells induces phosphorylation of Stat5. Spleen cells freshly isolated from C57BL/6 mice were sorted into different populations, stimulated for 20 minutes with IL-7, then stained for intracellular phospho-Stat5. Most Treg cells responded to IL-7 despite low expression of IL-7Rα on the majority of cells.
IL-7 stimulation of Treg cells induces phosphorylation of Stat5. Spleen cells freshly isolated from C57BL/6 mice were sorted into different populations, stimulated for 20 minutes with IL-7, then stained for intracellular phospho-Stat5. Most Treg cells responded to IL-7 despite low expression of IL-7Rα on the majority of cells.
Anti–IL-7 antibody blocks the development of Treg cells in TSLPR−/− mice
Since Treg cells were deficient in IL-7Rα−/− mice but not in either IL-7−/− or TSLPR−/− mice, it suggested that the 2 ligands of IL-7Rα might have a redundant activity. To test this, we injected anti–IL-7 monoclonal antibody (M25) into either TSLPR−/− or TSLPR+/+ littermates, as a control. The depletion of splenic Treg cells by anti–IL-7 was more complete in TSLPR−/− mice than in TSLPR+/+ littermates (Figure 5), supporting a redundant function for IL-7 and TSLP in inducing the development of Treg cells through an IL-7 receptor signal. It should be noted that there was also a depleting effect of anti–IL-7 in TSLPR+/+ mice, suggesting that of the 2 ligands, Treg cell progenitors may somewhat prefer IL-7 over TSLP.
Depletion of Treg cells in TSLPR−/− mice by injection of anti–IL-7 Ab. Anti–IL-7 (M25) or control antibody (M1) were injected 3 times per week for 4 weeks into either TSLPR−/− or TSLPR+/+ littermates. The number of Treg cells in thymus and spleen was determined by staining for intracellular Foxp3. Values shown are for 3 individual mice per group, and the bar indicates the mean in each group.
Depletion of Treg cells in TSLPR−/− mice by injection of anti–IL-7 Ab. Anti–IL-7 (M25) or control antibody (M1) were injected 3 times per week for 4 weeks into either TSLPR−/− or TSLPR+/+ littermates. The number of Treg cells in thymus and spleen was determined by staining for intracellular Foxp3. Values shown are for 3 individual mice per group, and the bar indicates the mean in each group.
IL-7 or TSLP are required for thymic development, but not peripheral survival, of Treg cells
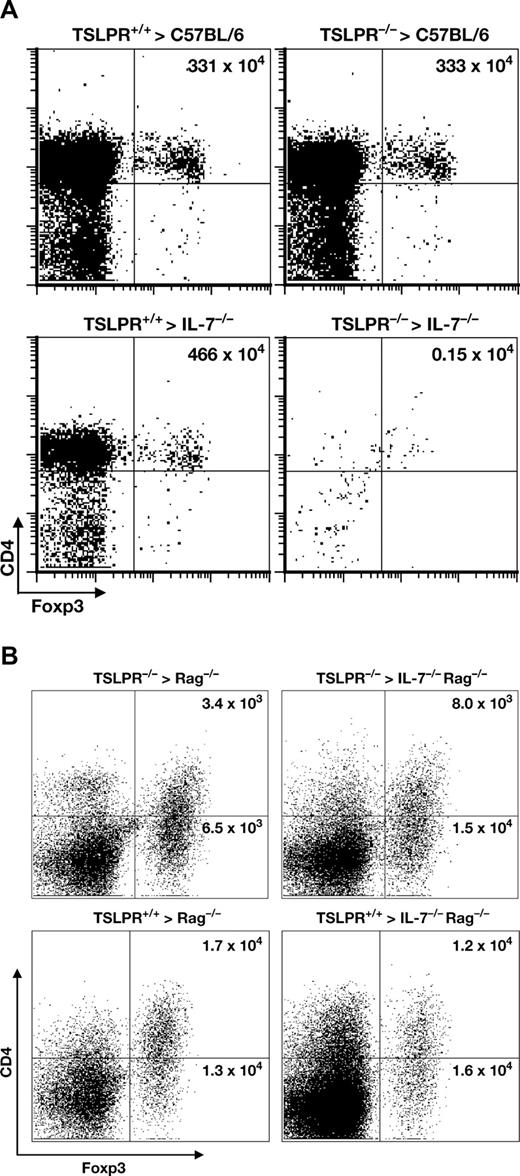
The results using antibody injections showed a block of thymic development of Treg cells, and it remained possible that peripheral survival of these cells also depended on an IL-7 receptor signal, analogous to other subsets of T cells. We therefore performed chimerism and adoptive transfer experiments that would distinguish whether the IL-7 receptor signal was required for both development and survival. Bone marrow chimeras verified the redundant requirement for IL-7 or TSLP in thymic development of Treg cells (Figure 6A). Thus, normal numbers of thymic Treg cells developed in mice lacking either IL-7 or TSLPR, but not both.
Treg cells require an IL-7Rα signal for thymic development but not for peripheral survival. (A) Treg cells require IL-7 or TSLP for thymic development. TSLPR−/− or TSLPR+/+ bone marrow was used to reconstitute irradiated IL-7−/− or C57BL/6 recipients. Eleven weeks later, the thymus was analyzed for CD4+Foxp3+ cells. Representative data from 3 separate experiments is shown, and the total number of Treg cells per thymus is indicated in the upper right quadrant. (B) Mature Treg cells do not require IL-7 or TSLP for survival. Purified CD4+ spleen cells from TSLPR−/− or TSLPR+/+ mice were labeled with CFSE and transferred into irradiated recipients that expressed IL-7 or lacked it, as indicated. Six days later, spleen cells were analyzed for CD4 and Foxp3 expression. TSLPR−/− Treg cells survived equally well in hosts with or without IL-7.
Treg cells require an IL-7Rα signal for thymic development but not for peripheral survival. (A) Treg cells require IL-7 or TSLP for thymic development. TSLPR−/− or TSLPR+/+ bone marrow was used to reconstitute irradiated IL-7−/− or C57BL/6 recipients. Eleven weeks later, the thymus was analyzed for CD4+Foxp3+ cells. Representative data from 3 separate experiments is shown, and the total number of Treg cells per thymus is indicated in the upper right quadrant. (B) Mature Treg cells do not require IL-7 or TSLP for survival. Purified CD4+ spleen cells from TSLPR−/− or TSLPR+/+ mice were labeled with CFSE and transferred into irradiated recipients that expressed IL-7 or lacked it, as indicated. Six days later, spleen cells were analyzed for CD4 and Foxp3 expression. TSLPR−/− Treg cells survived equally well in hosts with or without IL-7.
To determine whether an IL-7 receptor signal was required for peripheral survival of Treg cells, spleen cells were labeled with CFSE, and then transferred into recipient mice. Analysis of spleen cells 6 days later showed no apparent decrease in survival if Treg cells were deprived of both IL-7 and TSLPR (Figure 6B). We conclude that Treg cells require an IL-7 receptor signal for development, but unlike other T cell subsets, do not require this signal for survival.
Discussion
Treg cells have attracted much attention in recent years due to their potency in suppressing immune responses, suggesting applications of enhancing their activity to treat autoimmune diseases and inhibiting them to promoting immune responses. It was previously reported that Treg cells are independent of IL-7,60 and human Treg cells express low levels of IL-7Rα,61 distinguishing them from most other major subsets of T cells that require IL-7 during thymic development and in the periphery. Here we show that in IL-7Rα−/− mice, Treg cells do not develop in the thymus and are absent in peripheral lymphoid organs. It was previously reported that Treg cell development requires a γc signal in addition to that from the IL-2 receptor.62 The present study implicates the IL-7Rα chain as providing both a γc stimulus, and when paired with the TSLPR, a γc-independent stimulus. The identification of a key role for TSLP in Treg development indicates another important role for TSLP beyond its importance in mediating inflammatory processes such as allergic inflammation in the lung and atopic skin diseases.73-77
We verified the report60 that, unlike other subsets of T cells, Treg cells developed relatively normally in IL-7−/− mice, suggesting that the IL-7 receptor is activated by more than one ligand during Treg cell development and implicating TSLP. Of the 2 ligands, Treg cell progenitors may somewhat favor IL-7 over TSLP, since anti–IL-7 treatment reduces Treg cells in mice that have TSLPR (Figure 5), although as we have emphasized, this effect is stronger in mice lacking TSLPR. A recent study78 using cultures of human thymocytes reported that TSLP can indirectly induce Treg cell development; TSLP (but not IL-7) promoted dendritic cell development, which in turn supported Treg cell development. However, it is now clear that both mouse and human TSLP can act directly on CD4+ T cells as well as on DCs.63,79 Our study did not find that TSLPR was obligatory for Treg cell development in the mouse thymus.
Our interpretation that TSLP has a redundant function (with IL-7) is actually based on studies with TSLPR-, rather than TSLP-deficient cells. It remains a formal possibility that TSLP is not the only ligand for TSLPR, although none have yet been identified. Thus, an alternative ligand for TSLPR paired with IL-7Rα would also explain our results.
The IL-2 pathway was shown to be required for thymic development of Treg cells in some studies,48,49,80,81 but not others.35 Other reports show a requirement for IL-2 in persistence of Treg cells in peripheral organs37 or in peripheral development from nonsuppressive T cells.35 Thus, mice deficient in IL-2 itself, IL-2Rα, or IL-2Rβ all show a deficiency in Treg cells, excessive immune responses, and colitis. From our study, IL-7Rα deficiency leads to a Treg cell defect but, unlike other Treg cell deficiency models, there has been no colitis reported; this suggests that IL-7Rα−/− mice also lack, in addition to Treg cells, the effector T cells that are capable of excessive immune responses.
A recent study82 investigated whether the required regions of IL-2Rβ in Treg cell development could be satisfied by regions of IL-7Rα. They concluded that, although the intracellular domain of IL-7Rα was capable of delivering signals for Treg cell development, apparently only IL-2, and not IL-7, was present in the microenvironment in which Treg cells developed Foxp3 expression. Taken together with our findings, this suggests that in thymic development, the IL-7Rα requirement could occur early, at the pro–T-cell stage, as for other T-cell lineages. Later in thymic development, IL-2 may be required for induction of Foxp3 and the differentiation to Treg cells.
In other types of lymphocytes, the IL-7 receptor delivers signals for survival, proliferation, and differentiation. In pro-T cells, the most important response to IL-7 receptor signals appears to be survival, since IL-7Rα deficiency can be largely reversed by overexpressing the survival protein Bcl-283,84 or deleting the death protein Bax.85 In CD8 T-cell development, IL-7 delivers a differentiation signal,86 and in γδ T-cell development it induces rearrangement of the TCRγ locus.87 In peripheral T cells, IL-7 receptor signals also induce proliferation88,89 mediated by cell cycle regulators as well as survival based on protection from the death protein Bim.90 The requirement for IL-7Rα in Treg cell development could be an effect on survival, proliferation, or differentiation.
Mature peripheral Treg cells did not require IL-7 or TSLP for survival, and only a minority of freshly isolated Foxp3+ cells expresses enough IL-7Rα to fall above control levels detected by flow cytometry (Figure 3A). Despite the low receptor expression, all Treg cells were capable of responding to IL-7 by phosphorylating Stat5 (Figure 4); this indicates that a very low level of receptor does not preclude responses to IL-7, and also suggests that IL-7 could have important actions on mature Treg cells in vivo, but not be absolutely required. This effect on Treg cells is consistent with direct actions of TSLP on both mouse63 and human79 CD4+ T cells. We initially thought that the low receptor expression might have been explained by a recent encounter with IL-2 or IL-7, which down-regulate IL-7Rα70,71 ; but other subsets of CD4 or CD8 cells, once removed from their IL-7–containing environment, spontaneously up-regulate IL-7Rα. However, peripheral Treg cells, after overnight culture in the absence of IL-7, did not up-regulate IL-7Rα, unlike other subsets of T cells. The low expression of IL-7Rα on Treg cells could be partly due to an effect of Foxp3, which we show down-regulated IL-7Rα expression (Figure 3B).
It should be noted that treatment of TSLPR+/+ mice with anti–IL-7 antibody for a month showed modest splenic depletion of Tregs (approximately a third in Figure 5), whereas transfer of Tregs into IL-7−/− mice showed no depletion in 6 days (Figure 6B). The control antibody injections did not deplete Tregs (Figure 5), thus a completely nonspecific antibody effect is unlikely, although immune complexes of IL-7/αIL-7 might be a factor. One explanation is that a month of anti–IL-7 treatment partially inhibits thymic Treg generation in the adult mouse thymus (Figure 5), which in turn inhibits seeding of peripheral Tregs. Although IL-7−/− mice had substantial numbers of Treg cells in our experiments (Figure 1D), the study of de Latour60 reported an overall reduction in Tregs in IL-7−/− mice, although the reduction was less severe than other T-cell subsets.
Although a direct effect of IL-7 receptor on Treg cells is the simplest explanation of our findings, an indirect effect cannot be ruled out as yet; for example, it may induce production of other cytokines that could directly act on Treg cells. Other cytokines have been reported to promote Treg cells, including IL-2, TGFβ, IL-10, IL-4, and IL-13 91 ), although only IL-2 has been implicated in the naturally occurring Treg cells that are addressed in our studies. Since IL-2 is required for Treg cells, it is possible that the IL-7Rα requirement is for production of IL-2. However, this seems unlikely given the difference in IL-7Rα– versus IL-7–deficient mice: the former lack Treg cells but have a general T-cell deficiency identical to the latter, presumably including an identical degree of IL-2 deficiency.
If the requirement for IL-7Rα stimulation is through direct action on a thymic progenitor, our findings suggest a distinction between the progenitor for Treg cells and that of other αβT cells. The latter depend exclusively on the ligand IL-7, whereas we observe that Treg cells depend on IL-7Rα but not exclusively on IL-7. Pro-T2 and -T3 cells depend on IL-7 for survival.92 Thus, deletion of IL-7 (or IL-7Rα) results in loss of pro-T2 and -T3 cells93,94 and the majority of αβT cells that descend from them. Our results show that Treg cell development is relatively normal in the absence of IL-7 itself, verifying the findings of Peffault de Latour60 ) and suggesting that unlike the majority of αβT cells, Treg cells may not develop from typical IL-7–dependent pro-T2 and -T3 cells. This adds to the previously noted distinctive characteristics of thymic Treg cells.48,80,81
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank C. Willis (Amgen) for providing anti–IL-7 antibody; M. Guimond and C. L. Mackall for providing a verifying source of IL-7Rα−/− mice; R. Wyles and S. Stull for animal technical assistance; K. Noer, R. Matthai, S. Bauchiero, and M. Anderson for flow cytometry assistance; and J. J. Oppenheim for comments on the manuscript.
This work was supported by the Intramural Research Program of the NIH, NCI, and with federal funds from the NCI, NIH, under contract N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
National Institutes of Health
Authorship
Contribution: R.M. designed and performed research, analyzed data, and wrote the paper; J.A.H., R.S., X.C., W.L., V.L.H., and J.W.B. performed research; A.A.H. and W.J.L. designed research, and S.K.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: R.S. and W.J.L. are listed as coinventors on applications for and/or issued patents related to TSLP. The remaining authors declare no competing financial interests.
Correspondence: Scott K. Durum, NCI-FCRDC, Building 560, Room 31-71, Frederick, MD 21702-1201; e-mail: durums@ncifcrf.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal