Abstract
The persistence of human T-cell leukemia/lymphoma virus-I (HTLV-I)–infected cells is dependent upon clonal expansion and up-regulation of telomerase (hTERT). We have previously found that in interleukin (IL)–2–independent transformed HTLV-I cells, Tax strongly activates the hTERT promoter through nuclear factor-κB (NF-κB)–mediated Sp1 and c-Myc activation. In IL-2–dependent cells and adult T-cell leukemia/lymphoma (ATLL) patient samples, however, Tax expression is very low to undetectable, yet these cells retain strong telomerase activity. This suggests the existence of compensatory mechanisms in IL-2–dependent cells and ATLL patients. In this study, we demonstrate that telomerase activity is significantly decreased upon IL-2 withdrawal in immortalized HTLV-I cell lines. Inhibition of PI3K or AKT signaling pathways reduced telomerase activity in HTLV-I cells. We found that IL-2/IL-2R signaling was associated with a PI3K-dependent/AKT-independent transcriptional up-regulation of the endogenous hTERT promoter. We found that activation of the PI3K pathway mediated cytoplasmic retention of the Wilms tumor (WTI) protein, which strongly suppressed the hTERT promoter. The importance of this regulatory pathway for telomerase expression is underscored by findings that the PI3K pathway is commonly found activated in cancer cells.
Introduction
Interleukin-2 (IL-2) is required for the differentiation and long-term proliferation of T cells. IL-2 induces activation of Janus kinases and signal transducers and activators of transcription (JAK/STATs), leading to the induction of Shc/Ras/Raf/mitogen-activated protein kinase (MAPK) and PI3K/AKT pathways.1 These cell signaling pathways promote cellular proliferation, survival, and differentiation2 and are frequently deregulated in hematologic malignancies, including B-cell acute and chronic lymphoblastic leukemias, T-cell childhood and adult acute lymphoblastic leukemias, and various myeloid/lymphoid leukemias.
Human T-cell leukemia/lymphoma virus-I (HTLV-I) is the causative agent of adult T-cell leukemia/lymphoma (ATLL).3 In the early phases of infection, HTLV-I–infected cells are dependent upon the presence of IL-2, possibly contributing to the early clonal expansion of infected T cells through an IL-2/IL-2R autocrine/paracrine loop. Disease progression, however, occurs in the absence of IL-2 secretion or expression, and when HTLV-I–infected cells become transformed they no longer require IL-2. Although the steps leading to IL-2 independence remain to be elucidated, HTLV-I–transformed cells constitutively express the IL-2R and acquire constitutive activation of PI3K and JAK/STAT pathways required for the growth of HTLV-I–infected cells.4-7
To sustain long-term proliferation, leukemic cells must acquire several oncogenic changes, 2 of which are bypassing apoptosis and replicative senescence. In ATLL cells, apoptosis is inhibited by increased expression of the antiapoptotic protein, Bcl-xL,8 and alternate mechanisms. The avoidance of replicative senescence in ATLL cells is associated with an increased expression of telomerase,9 a cellular reverse transcriptase that prolongs the life span of cells by extending the ends of chromosomes, or telomeres. During successive replication cycles, telomerase (hTERT) lays down repetitive TTAGGG repeats provided by the template RNA (hTR).10 Whereas hTR is constitutively expressed in all the cells, the catalytic subunit of telomerase, hTERT is transiently expressed, and its expression is the rate-limiting step for telomerase activity.11,12
We have shown that hTERT mRNA is overexpressed in HTLV-I–infected cells and in ATLL patients.13 In these cells, Tax stimulation of NF-κB induced activation of c-Myc and Sp1, thereby up-regulating hTERT promoter expression. We also demonstrated that IL-2–dependent and –independent HTLV-I cell lines and ATLL cells possess strong levels of telomerase activity, independent of their transformation status.14 More importantly, inhibition of telomerase activity by treatment with interferon and azidothymidine (AZT) results in cellular senescence of HTLV-I–infected cells, and disease remission in ATLL patients carrying a functional p53.9 In the present study, we demonstrate that after IL-2 withdrawal telomerase activity is rapidly and substantially reduced in IL-2–dependent HTLV-I–infected cells. Using inhibitors of downstream IL-2R targets, we have identified a novel PI3K-dependent/AKT-independent pathway that potently regulates transcription from the hTERT promoter.
Methods
Cell culture
The HTLV-I–transformed cell lines MT4, MT2, and C8166, and the HTLV-I–immortalized cell lines LAF and 1185, were cultivated in RPMI 1640 with 10% fetal bovine serum and, when indicated in the text, with IL-2 (50 U/mL). Human 293T cells were grown in DMEM. HTLV-I cell lines were treated with either 20 μM AKT Inhibitor II (Calbiochem, San Diego, CA), 10 μM LY294002 (Sigma-Aldrich, St Louis, MO), or 50 μM AG490 (Biomol, Plymouth Meeting, PA), as indicated in the figure legends. Treatment with either dimethyl sulfoxide (DMSO) or 10 μM LY303511 (Sigma-Aldrich) served as negative control.
Telomeric repeat amplification protocol assays
Telomeric repeat amplification protocol (TRAP) assays were performed using the TRAPeze telomerase detection kit according to the manufacturer's instructions (Chemicon International, Temecula, CA). Quantification of the total product generated (TPG) was performed using the following equation: [(X/CX)]/[(R/CR)]*100, where X is the TRAP product generated in the experimental sample, R is the TRAP product generated in the control sample, CX is the internal control band for the experimental sample, and CR is the internal control band for the control sample. Telomeric products were separated on 8% Tris boric acid EDTA gels and stained with SYBR green for visualization. Standardization of the assay in our laboratory has been previously reported.9
RNA extraction, RT-PCR, and real-time PCR
Total RNA was extracted from cells using Trizol (Invitrogen, Carlsbad, CA), and DNA contamination was removed from RNA samples as previously described.14 Reverse-transcriptase–polymerase chain reaction (RT-PCR) was performed in nonsaturating conditions using the OneStep RT-PCR kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. RT-PCR was performed with the following sets of primers: hTERT, F: 5′-CTG GGT GGC ACG GCT TTT GTT C-3′ and R: 5′-CCC CGG GAG CTT CCG ACT-3′; GAPDH, F: 5′-GAA GGT GAA GGT CGG AGT C-3′ and R: 5′-GAA GAT GGT GAT GGG ATT TC-3′. RT-PCR was performed without the reverse-transcriptase step to verify absence of DNA. cDNA for real-time PCR was generated using the Transcriptor first strand cDNA synthesis kit (Roche, Indianapolis, IN). The cDNA was diluted (1:2) and real-time PCR was performed using the RT2 SYBR Green/ROX qPCR Master Mix (SuperArray, Bethesda, MD), with the following hTERT primers, F: 5′-GCG GAA GAC AGT GGT GAA CT-3′ and R: 5′-AGC TGG AGT AGT CGC TCT GC-3′
Western blots
Cells were lysed in radioimmunoprecipitation assay (RIPA) and subject to Western blotting using the following sets of antibodies: monoclonal anti-Tax (National Institutes of Health [NIH] AIDS Reagent Repository, Germantown, MD), c-Myc (A-14; Santa Cruz Biotechnology, Santa Cruz, CA), WT1 (C-19; Santa Cruz Biotechnology), α-actin (C-11; Santa Cruz Biotechnology), AKT (9272; Cell Signaling), and phospho-AKT (Ser473) (9271; Cell Signaling). For Western blots after IL-2 withdrawal, the cell pellets were lysed in hypotonic buffer (10 mM HEPES, pH 7.9, 1 mM MgCl2, 0.5 mM NaCl, 0.5% NP-40), then centrifuged. The supernatants were collected for cytoplasmic proteins and the cell pellets were lysed in hypertonic buffer (5 mM HEPES, pH 7.9, 5 mM MgCl2, 0.1 mM EDTA, 0.4 M NaCl, 1 mM DTT). After centrifugation, the supernatant was collected for nuclear proteins. Western blots were then performed using NF-κB p65 (A; Santa Cruz Biotechnology), c-Myc (A-14; Santa Cruz Biotechnology), and IκB-α (H-4; Santa Cruz Biotechnology). All secondary antibodies were from Santa Cruz Biotechnology.
Cell-cycle analysis
Cells were cultured with and without IL-2 or treated with LY29 (10 μM) and/or AKT II (20 μM) as indicated in the text. Cells were fixed with 80% EtOH for 30 minutes on ice, followed by treatment with RNase for 15 minutes at 37°C, stained with 50 μg/mL propidium iodine for 15 minutes at room temperature, and analyzed by flow cytometry.
Annexin V/PI staining
HTLV-I–infected cells were treated for 48 hours with either LY29 (10 μM), AG490 (50 μM), AKT II (20 μM), or DMSO control. After treatment, cells were collected and stained with annexin V/propidium iodine using the Vybrant Apoptosis Assay Kit no. 2 (Molecular Probes, Eugene, OR), according to the manufacturer's instructions.
Immunofluorescence
HTLV-I–infected cells were treated with or without LY29 (10 μM) or AKT II (20 μM) for 48 hours and/or cultivated with or without IL-2 overnight. After treatment, cells were fixed with 4% paraformaldehyde and permeabilized using 0.5% Triton X-100. Cells were then incubated in blocking buffer (10% BSA and 0.1% Tween 20), followed by incubation with WT1 antibody (C-19; Santa Cruz Biotechnology). Secondary antibody was Alexa Fluor 488 goat α-rabbit IgG (Molecular Probes). Cells were stained with DAPI for nuclear visualization, and images were captured using a Nikon EFD3 microscope (Boyce Scientific, Gray Summit, MO) and Nikon camera (100× Eplan [160/0.17] objective; Nikon, Melville, NY).
Biotin pull-down assay
The hTERT promoter was isolated from the hTERT-Luc-3915 plasmid (a generous gift from I. Horikawa) and labeled with C14-biotin (Invitrogen). Labeled DNA was bound to streptavidin-agarose beads and incubated with protein extracts from cells cultured with or without IL-2 for 24 hours. The pull-down was subsequently washed in binding buffer (10 mM Tris [pH 7.05], 50 mM NaCl, 50 mM NaF, 0.2 mM Na3VO4, 30 mM Na2P2O7, 5 μM ZnCl2, 0.5% Triton X-100) and bound proteins were analyzed by Western blot.
Luciferase
Luciferase assays were performed with the Luciferase Reporter Assay Kit (Promega, Madison, WI). 293T cells were cotransfected with the hTERT-Luc-3915 vector, along with either wild-type WT1 (SS) or mutant WT1 (FF) expression vectors (generous gifts from T. Hunter from the Salk Institute, La Jolla, CA). All transfections were performed using Polyfect (Qiagen). The binding site for WT1 in the hTERT-Luc-3915 promoter (GCGCGGGCG) was mutated to (GCGAAGGCG) using the QuickChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA), according to the manufacturer's instructions. Mutated nucleotides (CG→AA) are in bold/italics. DNA was sequenced to verify the correct mutations. HTLV-I–infected cell lines were transfected using Nucleofector Kit V (Amaxa Biosystems, Gaithersburg, MD), according to manufacturer's instructions. Twelve hours after transfection, MT4 and MT2 cells were treated with either LY29 (10 μM) or DMSO control for 8 hours. For IL-2 withdrawal, 1185 cells were cultivated in media with or without IL-2 for 24 hours.
Results
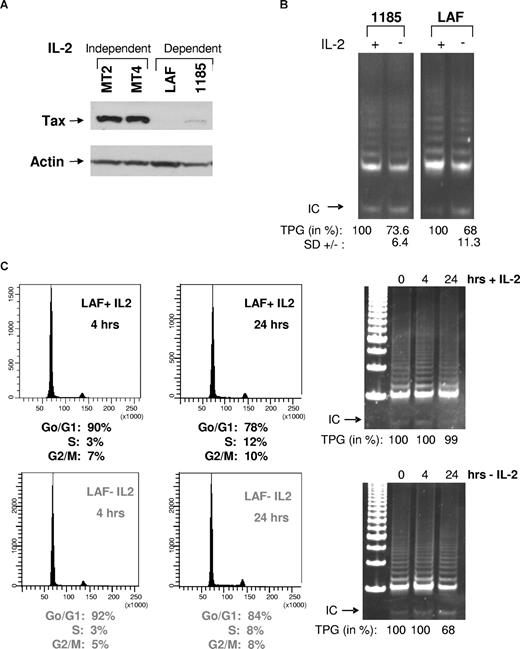
In agreement with previous studies,15 immunoblot analysis revealed higher levels of Tax in HTLV-I–transformed cells lines (MT2 and MT4) compared with HTLV-I–immortalized cell lines (LAF and 1185; Figure 1A). These data were further confirmed in 2 additional IL-2–dependent cell lines, 2.94 and SP (data not shown). Previous studies, however, demonstrate that HTLV-I–immortalized cell lines have levels of telomerase activity similar to those present in transformed cell lines,14 suggesting that in HTLV-I–immortalized cell lines, activation of telomerase occurs, in part, through a Tax-independent pathway. Because HTLV-I–immortalized cells are strictly IL-2–dependent for long-term growth but not for short-term proliferation, we hypothesize that IL-2/IL-2R may transduce a signal that compensates for the low levels of Tax expression and stimulates telomerase activity in preleukemic stages. Consistent with this hypothesis, when IL-2 was removed from HTLV-I cell lines (LAF and 1185), telomerase activity was decreased (Figure 1B). These data were further confirmed in 2 additional HTLV-I IL-2–dependent cell lines, SP and 2.94 (data not shown). IL-2 withdrawal in 1185 cells was less sensitive compared with other HTLV-I–immortalized cell lines, possibly due to higher levels of Tax expression (Figure 1A).
Removal of IL-2 from HTLV-I–immortalized cells decreases telomerase activity. (A) Total cell extracts from IL-2–independent (MT2 and MT4) and IL-2–dependent (LAF and 1185) cell lines were immunoblotted with anti-Tax. Actin served as a loading control. (B) LAF and 1185 cells were cultured in the presence (+) or absence (−) of IL-2 for 24 hours, followed by TRAP analysis for telomerase activity. TPGs are calculated as the percentage of activity compared with cells grown with IL-2 (100%), as previously reported.9 Results are representative of at least 2 independent experiments, and standard deviation (SD) is indicated. (C) LAF cells were analyzed at either 4 hours or 24 hours after growth in either IL-2–containing or IL-2–deprived media. For each time point, cells were collected and analyzed by FACS for cell-cycle and TRAP for telomerase activity. TPGs were calculated as the percentage of activity compared with cells grown with IL-2 (100%).
Removal of IL-2 from HTLV-I–immortalized cells decreases telomerase activity. (A) Total cell extracts from IL-2–independent (MT2 and MT4) and IL-2–dependent (LAF and 1185) cell lines were immunoblotted with anti-Tax. Actin served as a loading control. (B) LAF and 1185 cells were cultured in the presence (+) or absence (−) of IL-2 for 24 hours, followed by TRAP analysis for telomerase activity. TPGs are calculated as the percentage of activity compared with cells grown with IL-2 (100%), as previously reported.9 Results are representative of at least 2 independent experiments, and standard deviation (SD) is indicated. (C) LAF cells were analyzed at either 4 hours or 24 hours after growth in either IL-2–containing or IL-2–deprived media. For each time point, cells were collected and analyzed by FACS for cell-cycle and TRAP for telomerase activity. TPGs were calculated as the percentage of activity compared with cells grown with IL-2 (100%).
To determine whether IL-2 removal affected cell cycle progression, LAF and 1185 cells were grown in the presence or absence of IL-2 for 4 and 24 hours. Fluorescence-activated cell sorting (FACS) analysis revealed marginal differences in cell-cycle distribution between cells cultivated with or without IL-2 (Figure 1C). Similar results were seen with 1185 cells (data not shown). Although IL-2 withdrawal for 24 hours had no significant effect on cell-cycle progression, our data show a significant decrease in telomerase activity (Figure 1C). This is in agreement with published studies,16,17 showing that hTERT enzymatic activity is constitutive and not significantly affected throughout the cell cycle and is independent of IL-2. In contrast to constitutive hTERT activity, accessibility of the telomeric ends by hTERT is restricted to the S phase.18
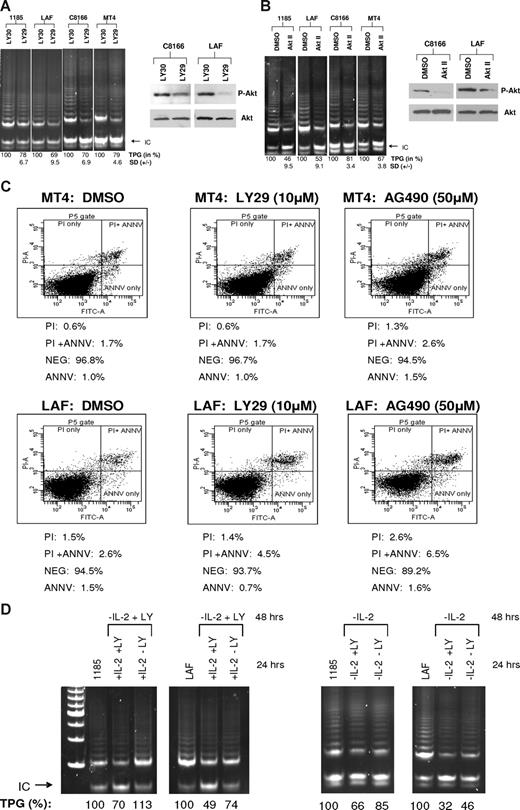
PI3K is a downstream target of IL-2R signaling and is constitutively activated in HTLV-I–infected cells.19 To determine whether PI3K has a role in telomerase activity, 2 HTLV-I IL-2–dependent cell lines and 2 HTLV-I IL-2–independent cell lines, with constitutive PI3K activation, were assayed for telomerase activity after LY294002 (LY29) treatment, a pharmacologic inhibitor of PI3K.20 Our results demonstrate a decrease in telomerase activity in all the cell lines tested, confirming that PI3K activation is essential for sustaining strong telomerase activity in HTLV-I–infected cell lines (Figure 2A). To verify the efficacy of LY29 treatment, cell extracts were assayed for AKT phosphorylation, a downstream target of PI3K. Western blot analysis showed decreased AKT phosphorylation at Ser473 (60% and 37% decrease for C8166 and LAF, respectively), whereas levels of total AKT remained unchanged.
The PI3K and AKT pathways are associated with increased telomerase activity in HTLV-I–immortalized and –transformed cells. (A,B) IL-2–independent (MT4 and C8166) and –dependent (LAF and 1185) cells were treated with LY29 (LY294002, 10 μM) or control, LY30 (LY303511, 10 μM), for 48 hours, followed by TRAP analysis for telomerase activity. (B) Cells were treated with AKT Inhibitor II (20 μM) or DMSO control (solvent control). TPGs are calculated as the percentage activity compared with cells grown without inhibitor (100%). Results are representative of at least 2 independent experiments, and SD is indicated. To verify inhibitor efficacy, total cell extracts were immunoblotted with anti-phosphorylated AKT (P-AKT). Anti-AKT served as a loading control. The percentage of decreased expression of P-AKT, as stated in the text, was calculated by spot densitometry with cells grown in either LY30 or DMSO considered to be 100%. (C) HTLV-I cell lines, MT4 and LAF, were treated for 48 hours with either LY29 (10 μM), AG490 (50 μM), or control DMSO. Cells were subsequently stained for annexin V and propidium iodine (PI), and analyzed for apoptosis by FACS analysis. The percentage of dead cells for each treatment is indicated. (D) 1185 and LAF cells were cultivated without IL-2 and in the presence of LY29 (10 μM) for 48 hours. After the 48-hour incubation period, the cultures were split and IL-2 was added in the absence or presence of LY29 (10 μM) for an additional 24 hours. TRAP analysis was performed and TPGs were calculated as the percentage of activity compared with cells grown without treatment (100%). As a control, cells were grown for 48 hours without IL-2, then treated an additional 24 hours without IL-2, with or without LY29 (10 μM). Cells grown continuously in IL-2 served as a positive control.
The PI3K and AKT pathways are associated with increased telomerase activity in HTLV-I–immortalized and –transformed cells. (A,B) IL-2–independent (MT4 and C8166) and –dependent (LAF and 1185) cells were treated with LY29 (LY294002, 10 μM) or control, LY30 (LY303511, 10 μM), for 48 hours, followed by TRAP analysis for telomerase activity. (B) Cells were treated with AKT Inhibitor II (20 μM) or DMSO control (solvent control). TPGs are calculated as the percentage activity compared with cells grown without inhibitor (100%). Results are representative of at least 2 independent experiments, and SD is indicated. To verify inhibitor efficacy, total cell extracts were immunoblotted with anti-phosphorylated AKT (P-AKT). Anti-AKT served as a loading control. The percentage of decreased expression of P-AKT, as stated in the text, was calculated by spot densitometry with cells grown in either LY30 or DMSO considered to be 100%. (C) HTLV-I cell lines, MT4 and LAF, were treated for 48 hours with either LY29 (10 μM), AG490 (50 μM), or control DMSO. Cells were subsequently stained for annexin V and propidium iodine (PI), and analyzed for apoptosis by FACS analysis. The percentage of dead cells for each treatment is indicated. (D) 1185 and LAF cells were cultivated without IL-2 and in the presence of LY29 (10 μM) for 48 hours. After the 48-hour incubation period, the cultures were split and IL-2 was added in the absence or presence of LY29 (10 μM) for an additional 24 hours. TRAP analysis was performed and TPGs were calculated as the percentage of activity compared with cells grown without treatment (100%). As a control, cells were grown for 48 hours without IL-2, then treated an additional 24 hours without IL-2, with or without LY29 (10 μM). Cells grown continuously in IL-2 served as a positive control.
The Tax protein of HTLV-I has been shown to stimulate AKT activity,21 a target of PI3K signaling. Inhibition of AKT after treatment with the inhibitor, AKT Inhibitor II (AKT II), resulted in decreased telomerase activity in both immortalized and HTLV-I–transformed cell lines (Figure 2B). This is in agreement with published reports, in which AKT increased telomerase activity by phosphorylating hTERT, thereby increasing hTERT activity at the posttranscriptional level.22 The levels of phosphorylated AKT decreased by 45% and 76% for C8166 and LAF, respectively, verifying that treatment of HTLV-I cells with AKT II inhibited AKT activity.
Previous studies have reported growth inhibition and induction of apoptosis after treatment with LY29 and AG490, a Jak inhibitor.23,24 To demonstrate that inhibition of PI3K signaling and not an increase in cellular death caused the decrease in telomerase activity, we treated MT4 and LAF cells with LY29 or AG490 for 48 hours and analyzed cells for apoptosis by annexin V/propidium iodine (PI) staining. AG490 was used as control, because our studies showed no effect on telomerase activity, after 48 hours of AG490 treatment (data not shown). FACS analysis demonstrated marginal increases of 0.1% and 0.8% in cell death for MT4 and LAF cells, respectively, after treatment with LY29 (Figure 2C). Treatment with AG490 caused a slightly greater increase in MT4 and LAF cell death (0.8% and 4.5%, respectively). These results are in agreement with a previously published report in which treatment with AG490 did not inhibit proliferation in HTLV-I cell lines.25 Because AG490 had no effect on telomerase activity, these data demonstrate that inhibition of telomerase activity by LY29 is specifically due to inhibition of the PI3K pathway and is independent of cell death.
To confirm the role of the IL-2/IL-2R/PI3K pathway in stimulation of telomerase activity we cultured 1185 and LAF cells in media without IL-2 and with LY29 for 48 hours. These cells were then divided into cultures with IL-2 in the presence or absence of LY29 for an additional 24 hours. IL-2 pulse increased hTERT activity only when LY29 was absent (Figure 2D). Unlike 1185 cells, LAF cells pulsed with IL-2 were not completely rescued for telomerase activity in 24 hours. This could be due to the fact that inhibition by LY29 was more pronounced in LAF cells, which may require a longer period of recovery. These data suggest that most of the IL-2R signaling involved in hTERT promoter regulation occurs through the IL-2/IL-2R/PI3K pathway. As a control, cells were grown in the absence of IL-2 for 48 hours followed by treatment with or without LY29. As expected, telomerase activity decreased after IL-2 removal, which was further decreased with the addition of LY29 inhibitor (Figure 2D).
The PI3K pathway transcriptionally regulates hTERT expression in HTLV-I–infected cells
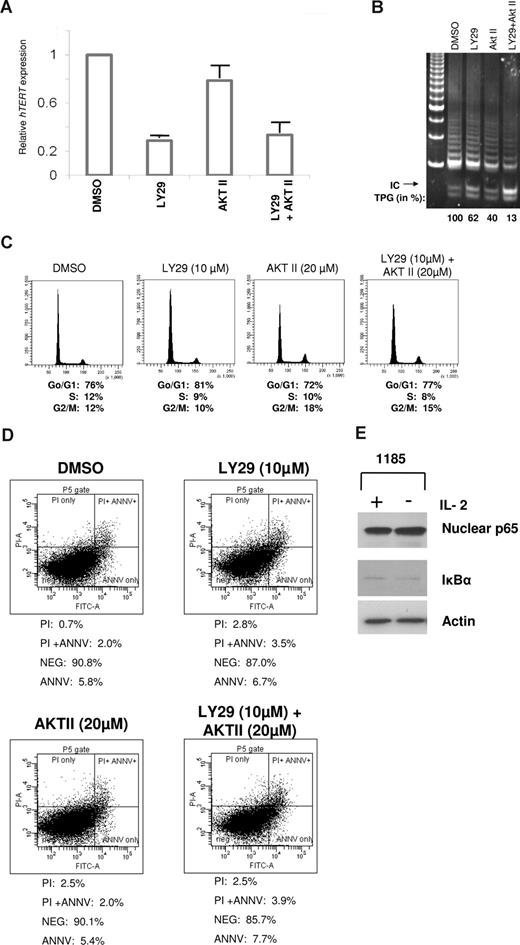
To determine whether PI3K and AKT signaling acted at the transcriptional and/or posttranscriptional levels in HTLV-I–infected cells, we treated 1185 cells with PI3K and/or AKT inhibitors for 4 hours. Quantitative real-time PCR revealed a rapid and considerable loss of hTERT expression after treatment with LY29 compared with AKT II (Figure 3A). A combination of LY29 and AKT II led to a decrease in hTERT expression comparable to treatment with LY29 alone, suggesting that PI3K is the primary regulator of hTERT expression at the transcriptional level. PI3K and not AKT inhibition led to significant transcriptional repression of hTERT (approximately 80%). Of note, the stronger effect seen at the level of transcription compared with the TRAP activity is indicative of the long half-life of the hTERT protein complex (24 hours).26 In fact, inhibition of PI3K for an extended period of time further decreased telomerase activity by more than 50% (data not shown), a level previously shown to have biologic effects on HTLV-I–infected cells.9 Our results suggest that PI3K regulates telomerase activity through an AKT-independent pathway (transcriptional). To confirm this hypothesis, 1185 cells were treated with a combination of PI3K and AKT inhibitors. Results indicated that treatment with both inhibitors reduced telomerase activity to a much larger extent than treatment with either drug alone (Figure 3B), thereby demonstrating that PI3K and AKT have additive, independent effects on telomerase activity. These effects were independent of cell-cycle distribution (Figure 3C) or cell death (Figure 3D), as demonstrated by FACS analysis of PI staining and annexin V/PI staining, respectively.
Telomerase activity is regulated at the level of transcription by a PI3K-dependent pathway in HTLV-I cell lines. (A) 1185 cells were treated with LY29 (10 μM), AKT II (20 μM), LY29 (10 μM)/AKT II (20 μM), or DMSO (solvent control) for 4 hours, followed by analysis of hTERT RNA expression by quantitative real-time PCR. GAPDH expression was used as a control. The expression level in 1185 cells treated with DMSO was defined as 1.0. Results are representative of 3 independent experiments and standard deviations are indicated by error bars. (B-D) 1185 cells were treated with LY29 (10 μM), AKT II (20 μM), LY29/AKT II (10 μM/20 μM), or DMSO (solvent control) for 48 hours, followed by TRAP analysis for telomerase activity (B), FACS analysis for cell cycle (C), or FACS analysis for apoptosis (D). TPGs were calculated as the percent-age of activity compared with DMSO control (100%). (E) 1185 cells were grown with or without IL-2 for 4 hours followed by Western blot analysis. Nuclear extracts were probed with anti–NF-κB RelA/p65, and cytoplasmic extracts with anti–IκB-α.
Telomerase activity is regulated at the level of transcription by a PI3K-dependent pathway in HTLV-I cell lines. (A) 1185 cells were treated with LY29 (10 μM), AKT II (20 μM), LY29 (10 μM)/AKT II (20 μM), or DMSO (solvent control) for 4 hours, followed by analysis of hTERT RNA expression by quantitative real-time PCR. GAPDH expression was used as a control. The expression level in 1185 cells treated with DMSO was defined as 1.0. Results are representative of 3 independent experiments and standard deviations are indicated by error bars. (B-D) 1185 cells were treated with LY29 (10 μM), AKT II (20 μM), LY29/AKT II (10 μM/20 μM), or DMSO (solvent control) for 48 hours, followed by TRAP analysis for telomerase activity (B), FACS analysis for cell cycle (C), or FACS analysis for apoptosis (D). TPGs were calculated as the percent-age of activity compared with DMSO control (100%). (E) 1185 cells were grown with or without IL-2 for 4 hours followed by Western blot analysis. Nuclear extracts were probed with anti–NF-κB RelA/p65, and cytoplasmic extracts with anti–IκB-α.
A downstream target of PI3K/AKT signaling is NF-κB. NF-κB increases hTERT activity transcriptionally and posttranscriptionally: the former, by nuclear translocation of hTERT through p65-mediated interactions,27 and the latter, by increases in c-Myc and Sp1 expression.28 Because Tax can directly stimulate NF-κB through activation of PI3K, AKT, and IKK, we did not expect to see a difference in NF-κB–mediated control of hTERT upon IL-2 withdrawal. As expected, 1185 cells showed no significant differences in nuclear RelA/p65 or cytoplasmic IκB-α expression after removal of IL-2 from the culture media (Figure 3E).
We next assayed the effect of IL-2 on hTERT promoter expression in HTLV-I–infected cells. We found a strong decrease in hTERT expression only 4 hours after IL-2 removal in both 1185 and LAF cells, and hTERT expression was completely suppressed after 24 hours of IL-2 removal (Figure 4A). To demonstrate that IL-2 removal was responsible for the transcriptional control of hTERT expression, LAF cells were cultivated in the absence of IL-2 and then pulsed with IL-2 for 4 or 24 hours. Results indicated that hTERT expression levels were increased in less than 4 hours and restored to the original levels after overnight culturing with IL-2 media (Figure 4B). This confirms a potent and rapid effect of IL-2R signaling on hTERT mRNA expression. Because our data showed a sudden loss of hTERT RNA levels in only 4 hours, we next investigated the stability of the RNA transcript by measuring the half life of the hTERT mRNA in1185 cells cultured in the presence or absence of IL-2. Our results indicated no difference in hTERT half-life (around 2 hours) in 1185 cells cultivated in the presence or absence of IL-2 (Figure 4C). These results are consistent with the previously reported half-life of hTERT mRNA (approximately 2 hours)29 and suggest that the effect of IL-2 removal occurs mainly at the transcriptional level in the absence of hTERT RNA destabilization.
IL-2R/IL-2 signaling regulates hTERT promoter expression. (A) IL-2 was withdrawn from 1185 and LAF cells for 4 or 24 (overnight [O/N]) hours, followed by hTERT RNA expression by RT-PCR. (B) LAF cells were cultured in the absence of IL-2 for 4 hours, and subsequently pulsed with IL-2 for 4 and 24 hours before hTERT RT-PCR analysis. Cells grown continuously in IL-2 media served as a control (first lane). (C) 1185 cells were treated with actinomycin D (5 μg/mL) in the presence or absence of IL-2 for 0, 1, 2, and 4 hours. RNA was extracted and analyzed for hTERT expression by RT-PCR. (A-C) GAPDH was used as an amplification control.
IL-2R/IL-2 signaling regulates hTERT promoter expression. (A) IL-2 was withdrawn from 1185 and LAF cells for 4 or 24 (overnight [O/N]) hours, followed by hTERT RNA expression by RT-PCR. (B) LAF cells were cultured in the absence of IL-2 for 4 hours, and subsequently pulsed with IL-2 for 4 and 24 hours before hTERT RT-PCR analysis. Cells grown continuously in IL-2 media served as a control (first lane). (C) 1185 cells were treated with actinomycin D (5 μg/mL) in the presence or absence of IL-2 for 0, 1, 2, and 4 hours. RNA was extracted and analyzed for hTERT expression by RT-PCR. (A-C) GAPDH was used as an amplification control.
The activation of PI3K signaling pathways in HTLV-I–infected cells results in cytoplasmic retention of WT1, a negative regulator of hTERT expression
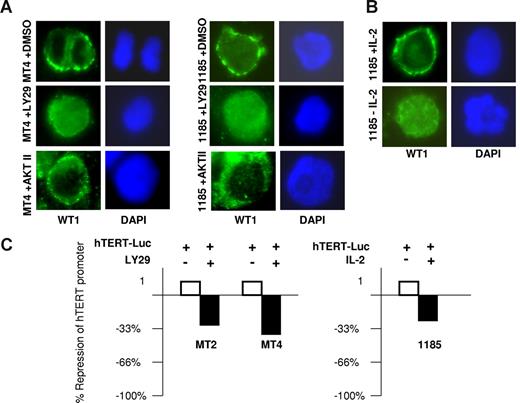
Protein phosphatase 2A (PP2A) lies directly downstream of PI3K and targets AKT and protein kinases A and C (PKA and PKC). PKC acts as a posttranscriptional regulator of telomerase, by phosphorylating hTERT and allowing for assembly of the holoenzyme complex.30 In contrast, PKA increases phosphorylation of the Wilms tumor suppressor (WT1) protein, a potent transcriptional repressor that inhibits hTERT expression by direct binding to the hTERT promoter.31 PKA-mediated phosphorylation of WT1 sequesters WT1 in the cytoplasm.32,33 Consistent with this model and the constitutive activation of PI3K found in HTLV-I–infected cells, we observed a prominent cytoplasmic distribution of WT1 in MT4 and 1185 cells (Figure 5A). However, inhibition of PI3K, by treatment with LY29 in HTLV-I–infected cell lines caused a redistribution of WT1 to the nucleus, an effect that was not seen after AKT inhibition. Importantly, WT1 had a prominent cytoplasmic staining in 1185 cells cultivated with IL-2, which was lost upon IL-2 removal (Figure 5B). These results demonstrate that IL-2R signaling via PI3K leads to cytoplasmic retention of WT1, effectively preventing WT1 from reaching and repressing the hTERT promoter.
Cellular localization of WT1 dictates repression of hTERT expression. (A) MT4 and 1185 cells were treated with LY29 (10 μM), AKT II (20 μM), or DMSO (solvent control) for 48 hours followed by immunostaining with anti-WT1 (green). Nuclear staining was visualized by DAPI (blue). (B) 1185 cells were cultured overnight with or without IL-2 and stained with anti-WT1, as described in panel A. (C) The HTLV-I–transformed cells, MT2 and MT4, or the HTLV-I–immortalized cells, 1185, were electroporated with the hTERT-Luc-Promoter using the Amaxa transfection kit (Amaxa Biosystems). Twelve hours after transfection, MT2 and MT4 cells were treated with either LY29 (10 μM) or DMSO (solvent control) for 8 hours. 1185 cells were electroporated and cultured in media with and without IL-2 for 24 hours. Cell extracts were normalized and assayed for luciferase activity.
Cellular localization of WT1 dictates repression of hTERT expression. (A) MT4 and 1185 cells were treated with LY29 (10 μM), AKT II (20 μM), or DMSO (solvent control) for 48 hours followed by immunostaining with anti-WT1 (green). Nuclear staining was visualized by DAPI (blue). (B) 1185 cells were cultured overnight with or without IL-2 and stained with anti-WT1, as described in panel A. (C) The HTLV-I–transformed cells, MT2 and MT4, or the HTLV-I–immortalized cells, 1185, were electroporated with the hTERT-Luc-Promoter using the Amaxa transfection kit (Amaxa Biosystems). Twelve hours after transfection, MT2 and MT4 cells were treated with either LY29 (10 μM) or DMSO (solvent control) for 8 hours. 1185 cells were electroporated and cultured in media with and without IL-2 for 24 hours. Cell extracts were normalized and assayed for luciferase activity.
To confirm the role of IL-2/IL-2R signaling through PI3K on hTERT expression in HTLV-I–infected cells, we electroporated MT2 and MT4 cells with the hTERT luciferase vector. Consistent with the results described in Figures 2 and 3, inhibition of PI3K by LY29 reduced hTERT luciferase activity by 32% and 34% for MT2 and MT4 cells, respectively (Figure 5C). In the HTLV-I–immortalized cell line 1185, we found a 28% reduction in hTERT luciferase promoter transactivation when cells were grown for 24 hours in media without IL-2 compared with cells grown in the presence of IL-2. The differences in hTERT transactivation in the 1185 luciferase assays (28% reduction) compared with the endogenous levels of hTERT expression (approximately 80% reduction) seen in Figure 3A are probably due to the presence of multiple copies of the hTERT luciferase plasmid after Amaxa transfection (Amaxa Biosystems). In such conditions the limiting amounts of WT1 in HTLV-I–infected cells, in relation to the number of plasmid copies, would explain that the suppression of the hTERT luciferase promoter upon transfection is not as pronounced compared with the endogenous hTERT promoter.
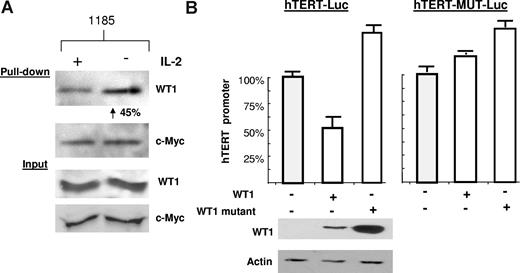
To further characterize the role of IL-2R signaling on WT1 activity, we performed a biotin pull-down assay using the hTERT promoter as bait. We found that cells cultured in the absence of IL-2 had a significant increase (45%) in WT1 binding to the hTERT promoter, thereby explaining the repression of hTERT expression (Figure 6A). Increased binding of WT1 was due to an increase in DNA binding affinity, rather than overall levels, as shown by equal input of WT1 and c-Myc. In fact, it has been shown that PKA-mediated phosphorylation of WT1 impairs both its nuclear localization and its DNA binding activity.32 As an internal control for this assay, the levels of c-Myc bound to the hTERT promoter did not change in response to IL-2 withdrawal. To confirm that WT1 is a transcriptional repressor of the hTERT promoter, we transfected wild-type WT1 into 293T cells along with an hTERT luciferase construct. hTERT promoter activity decreased by approximately 50% in the presence of wild-type WT1 (Figure 6B). In contrast, mutant WT1 (WT1-KK), which cannot be phosphorylated by PKA, was unable to repress the hTERT promoter. To further demonstrate that the WT1 effect was mediated by direct binding, we mutated the WT1 binding site (GCGCGGGCG)31 within the hTERT luciferase vector. This hTERT promoter luciferase construct (hTERT-MUT-Luc) could no longer be repressed upon coexpression of WT1 (Figure 6B). Our data demonstrate that upon activation of the PI3K pathway, WT1 is sequestered in the cytoplasm of HTLV-I–infected cells, thus contributing to the overall elevated telomerase activity seen in HTLV-I–infected cells.
IL-2–dependent WT1 binding to the hTERT promoter. (A) The hTERT-3915 promoter was biotin labeled and bound to streptavidin-agarose beads, followed by incubation with 1185 cellular extracts from cells cultivated overnight in the presence or absence of IL-2. Bound proteins were visualized using anti-WT1 or anti–c-Myc. The percentage of increased binding of WT1 to the hTERT promoter was calculated by spot densitometry as indicated, with 1185 cells grown in the presence of IL-2 considered to be 100%. (B) 293T cells were transfected with wild-type WT1 (SS) or mutant WT1 (FF), along with the hTERT-Luc promoter, or wild-type WT1 (SS) or mutant WT1 (FF), along with the hTERT-Mut-Luc promoter (defective for WT1 binding). Extracts were assayed 48 hours after transfection for luciferase activity. Cell extracts were probed with anti-WT1 and antiactin to verify expression. Average luciferase values were as follows: hTERT-Luc promoter (150 518), + WT1 (SS) (79 578), + WT1 (FF) (241 727) (SD measured from at least 2 independent experiments) and for the hTERT-Mut Luc promoter (1 166 792), + WT1 (SS) (1 468 345), + WT1 (FF) (1 736 347) (SD measured from multiple readings from the same experiment).
IL-2–dependent WT1 binding to the hTERT promoter. (A) The hTERT-3915 promoter was biotin labeled and bound to streptavidin-agarose beads, followed by incubation with 1185 cellular extracts from cells cultivated overnight in the presence or absence of IL-2. Bound proteins were visualized using anti-WT1 or anti–c-Myc. The percentage of increased binding of WT1 to the hTERT promoter was calculated by spot densitometry as indicated, with 1185 cells grown in the presence of IL-2 considered to be 100%. (B) 293T cells were transfected with wild-type WT1 (SS) or mutant WT1 (FF), along with the hTERT-Luc promoter, or wild-type WT1 (SS) or mutant WT1 (FF), along with the hTERT-Mut-Luc promoter (defective for WT1 binding). Extracts were assayed 48 hours after transfection for luciferase activity. Cell extracts were probed with anti-WT1 and antiactin to verify expression. Average luciferase values were as follows: hTERT-Luc promoter (150 518), + WT1 (SS) (79 578), + WT1 (FF) (241 727) (SD measured from at least 2 independent experiments) and for the hTERT-Mut Luc promoter (1 166 792), + WT1 (SS) (1 468 345), + WT1 (FF) (1 736 347) (SD measured from multiple readings from the same experiment).
Discussion
Tax plays an important role in the early steps of transformation by HTLV-I through deregulation of host cell signaling pathways, including those involved with cell cycle, apoptosis, and proliferation. We have previously shown Tax stimulates high telomerase activity in HTLV-I–infected cells.13 However, IL-2–dependent cell lines express very little Tax, yet retain the high levels of telomerase activity seen in transformed cell lines. Here, we demonstrate that HTLV-I cell lines rely upon IL-2R signaling pathways to sustain high telomerase activity in the absence of high Tax expression.
To gain better insights into IL-2– and Tax-mediated regulation of telomerase activity, we looked at the level of hTERT expression. In the absence of exogenous IL-2, Tax can activate PI3K and AKT signaling pathways. In accordance, inhibition of downstream IL-2R targets, PI3K and AKT, leads to a significant loss of telomerase activity in both immortalized and transformed cell lines. Importantly, such levels of repression were previously shown to be sufficient for telomere attrition and induction of senescence in HTLV-I–infected cells.9 AKT inhibition had marginal effects on hTERT promoter expression, consistent with its role as a posttranscriptional regulator of telomerase activity.22 In contrast, we found that PI3K inhibition substantially decreased hTERT mRNA expression, suggesting that a PI3K-dependent/AKT-independent pathway controls hTERT transcription. PI3K stimulates PKA, which phosphorylates WT1, leading to its cytoplasmic retention.32,33 By sequestering WT1 in the cytoplasm, HTLV-I–infected cells remove a negative regulator of hTERT expression. We have previously demonstrated that c-Myc and Sp1 have increased binding to the hTERT promoter upon transfection of Tax. We now report a new mechanism by which HTLV-I increases hTERT activity by relieving WT1-mediated hTERT promoter repression in both Tax-expressing and Tax-nonexpressing cells. The biologic significance of our data is underscored by the fact that similar levels of telomerase inhibition by AZT (as those reported here) have been shown to induce p53-dependent senescence and death of HTLV-I cell lines and, in combination with interferon, ATL patients.34 Therefore, IL-2 may be essential in the early stages of transformation to ensure high telomerase activity and extend the life span of infected cells, therefore increasing the risk of cumulative genetic defects and transformation.
WT1 is expressed in Wilms tumors, colon cancer, breast cancer, and leukemia.35 In hematopoietic cells, WT1 behaves like a tumor suppressor, promoting differentiation by decreasing cellular proliferation, contributing to growth arrest, and reducing colony formation.36,37 However, it has been suggested that WT1 acts as an oncogene in leukemic cells as it is highly expressed in the peripheral blood of many leukemias.35 Our study supports a role for WT1 as a tumor suppressor gene in HTLV-I–infected cell lines, by decreasing the level of telomerase expression. Decreased telomerase activity in cancer cells has been linked to cellular senescence and death. WT1 expression is elevated in leukemias beyond the levels found in normal hematopoietic cells. The use of RNA interference and antisense oligonucleotides against endogenous WT1 in acute myeloid leukemia (AML) and chronic myelogenous leukemia (CML) cell lines, including leukemia patient samples, leads to decreased growth and apoptosis.38,39 HTLV-I infection may allow for WT1 expression to enhance the proto-oncogenic effects required for tumor proliferation, while at the same time inhibiting the transcriptional suppressive functions of WT1 by sequestering the protein in the cytoplasm to allow for elevated telomerase activity.
Tax is barely detectable in ATLL leukemic cells,40,41 yet these cells maintain high levels of telomerase expression and activity.13,14 We propose that ATLL cells rely upon an IL-2R signaling pathway similar to those in HTLV-I–immortalized cell lines to maintain high telomerase activity in the absence of Tax expression. This is supported by the fact that ATLL cells have constitutive activation of the high-affinity IL-2R.42 It remains to be clarified whether PI3K is responsible for retaining WT1 in the cytoplasm, and whether additional IL-2R signaling pathways are activated and/or repressed to enhance telomerase expression in ATLL patients.
All oncogenic viruses have been shown to increase telomerase activity by various transcriptional and posttranscriptional methods.43 We now demonstrate a novel way by which HTLV-I mediates telomerase activity and suggests an important role for the PI3K pathway in transcriptional regulation of hTERT. This underscores the importance of the PI3K regulatory pathway for telomerase expression in cancer cells, especially given the fact that the PI3K pathway is commonly found activated in tumor cells.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by grants CA106258 and CA115398 from the National Cancer Institute to C.N.
National Institutes of Health
Authorship
Contribution: M.B. performed all experiments and wrote the paper; and C.N. was responsible for design of the project and interpretation of the results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christophe Nicot, University of Kansas Medical Center, Department of Microbiology, Immunology, and Molecular Genetics, 3025 Wahl Hall West, 3901 Rainbow Blvd, Kansas City, KS 66160; e-mail: cnicot@kumc.edu.

![Figure 4. IL-2R/IL-2 signaling regulates hTERT promoter expression. (A) IL-2 was withdrawn from 1185 and LAF cells for 4 or 24 (overnight [O/N]) hours, followed by hTERT RNA expression by RT-PCR. (B) LAF cells were cultured in the absence of IL-2 for 4 hours, and subsequently pulsed with IL-2 for 4 and 24 hours before hTERT RT-PCR analysis. Cells grown continuously in IL-2 media served as a control (first lane). (C) 1185 cells were treated with actinomycin D (5 μg/mL) in the presence or absence of IL-2 for 0, 1, 2, and 4 hours. RNA was extracted and analyzed for hTERT expression by RT-PCR. (A-C) GAPDH was used as an amplification control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/7/10.1182_blood-2008-01-134692/6/m_zh80160822250004.jpeg?Expires=1765029527&Signature=IDxW6yxyvbp9UPFvWr~Ed-DlxqpvcwCjUJWBzHppDkAYCMkhtieKa7oyQqOBktlC51B4lyPqyXEgjQKjtdzxqOJ0STIwUyP6Fq8X6t5431atPzRX0Fi41g06guNBywwyv-CEGV9ev-VmdcVmNU46ygDfrMTIYK67cgyjCfiuQQWGuH1habLsM~bYl4rfHiLWoPZFENmzkyJKLOnCu-VRBZ5-pOw~NnNTxX6cRdyC-C05CT5peF-ySz6WmISx~pKlrZuldysXreUrej1Ku7DQr8SUVW20nHqk7fKiD1ijUy6NulxioQBtG-xa6hyNBGLjLil4u7UI7aM2LPoVPygJzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal