Abstract
The role of FoxP3+CD4+ regulatory T (Treg) cells in HIV-1 disease in vivo is poorly understood due to the lack of a robust model. We report here that CD4+FoxP3+ T cells are developed in all lymphoid organs in humanized Rag2−/−γC−/− (DKO-hu HSC) mice and they display both Treg phenotype and Treg function. These FoxP3+ Treg cells are preferentially infected and depleted by a pathogenic HIV-1 isolate in HIV-infected DKO-hu HSC mice; and depletion of Treg cells is correlated with induction of their apoptosis in vivo. When CD4+CD25+/hi Treg cells are depleted with the IL-2–toxin fusion protein (denileukin diftitox), HIV-1 infection is significantly impaired. This is demonstrated by reduced levels of productively infected cells in lymphoid organs and lower plasma viremia. Therefore, FoxP3+ Treg cells are productively infected and play an important role in acute HIV-1 infection in vivo. The DKO-hu HSC mouse will be a valuable model to study human Treg functions and their role in HIV-1 pathogenesis in vivo.
Introduction
Regulatory CD4+CD25+ T (Treg) cells have been implicated in a number of pathologic processes including cancers,1,2 infectious diseases,3-6 as well as autoimmune diseases.7-9 Treg cells are induced (or recruited and expanded) during the host's immune responses to modulate overreactive immunity. As a result, Treg cells play a critical role in immune responses and in immunopathogenesis of infectious diseases. For example, Leishmania infection leads to induction of Treg cells that help to maintain the balance of the hosts immune response against the pathogen and the pathogen's persistence in the host.10,11 Depletion of Treg cells leads to clearance of Leishmania in the host. However, Treg-depleted mice have become susceptible to reinfection by Leishmania, indicating that persistence is beneficial to maintain effective host immunity against Leishmania reinfection.10 Treg cells also play an important role in chronic viral infections. They can be induced to subdue the antiviral immune response, which allows for persistent viral infection. This is demonstrated during hepatitis C virus (HCV) infection in humans and chimps,12 and during Friend leukemia virus infection in mice.4
To understand the origins of a Treg cell, the molecular mechanism of Treg lineage development has been investigated, but it is still not completely clear. Recent genetic studies in both mice and humans have identified Scurfin or FoxP3, a fork-head transcription factor, as a determinant of Treg development.5,13-17 This finding has been confirmed in several different studies. First, scurfy (sf) mice carrying mutations in the FoxP3 gene lack functional Treg cells and exhibit lymphoproliferative diseases and autoimmune phenotypes. These findings have been confirmed by targeted inactivation of FoxP3 in mice.16,17 Second, human immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) patients, due to mutations in the human FoxP3 gene, also develop multiple organ autoimmune symptoms consistent with a lack of functional Treg cells. In newborn mice, generation of functional Treg cells in the thymus correlates with induction of FoxP3 through mechanisms not clearly elucidated.18,19 Finally, ectopic expression of FoxP3 in naive CD4+CD25− T cells converts them to look and function like Treg cells.5,15 Similarly, CD4+CD25+ T cells with suppressive activity (Tregs) can also be generated from activation of naive CD4+CD25− T cells in the presence of TGFβ, which correlates with expression of FoxP3.20,21 Therefore, FoxP3 has been firmly established as a molecular marker of Treg cells.
The role of Treg cells in HIV-1 infection and pathogenesis is poorly understood due to a lack of robust models for studying human immune functions in vivo. Unique to HIV-1 diseases, CD4+ Treg cells are potential target cells for HIV-1 infection. However, there is no report of direct infection of Treg cells by HIV-1 in human patients. In SIV-infected rhesus monkeys, recent evidence has documented that 13% of the FoxP3+ T cells are productively infected by SIV in the gut-associated lymphoid organs of acutely infected animals.22 This is consistent with recent findings that Treg cells (CD4+CD25+) are direct target cells that support higher levels of infection by HIV-1 or FIV in vitro.5,23 Both elevated entry and LTR expression contribute to enhanced FIV infection of Treg cells in vitro.5,23 Although FoxP3 has been reported to inhibit NFAT or NF-κB activity in transfected 293 cells or T cells,24-26 FoxP3 has also been reported to enhance HIV gene expression via an NF-κB–dependent pathway.25
Since the beginning of the AIDS epidemic, it has been documented that chronic immune activation is a hallmark and reliable predictor of AIDS progression.27 It has been proposed that Treg cells may be impaired or depleted by HIV-1, which contributes to HIV disease progression, leading to immune hyperactivation. There are conflicting reports, however, in human patients about whether HIV-1 infection depletes or increases Treg cells during AIDS progression. Some early reports support the idea that HIV infection leads to decreased Treg number or activity in the peripheral blood.5,28-31 However, elevated FoxP3 expression in lymphoid organs has also been documented in HIV-infected patients.22,32-34 In a recent comprehensive study involving analysis of Treg cells from SIV-infected rhesus monkeys (RMs) during acute, early chronic, and late chronic stages of infection, a better picture of Treg dynamics in SIV pathogenesis is reported.35 Interestingly, a transient increase in the frequency of Treg cells is detected after acute infection. During SIV disease progression, however, the number of Treg cells decreased and their function was impaired, which was inversely correlated with both plasma viremia and immune activation. Thus, Treg cells may play an important role in SIV infection/pathogenesis in RMs. These reports highlight the importance of studying the interaction between HIV-1 and Treg cells in relevant robust in vivo models.
A number of human-mouse chimeric models have been developed, but with only limited success. This is due to selective engraftment of xenoreactive human T cells in hu-PBL-SCID mice,36,37 or lack of significant human immune responses in the SCID-hu Thy/Liv mouse.38,39 However, the SCID-hu Thy/Liv model may be improved with cotransplantation of hematopoietic progenitor cells.40 A new mouse model with a functional human immune system has recently been reported. The Rag2-γC double knockout (DKO) mouse lacks T lymphocytes, B lymphocytes, and natural killer (NK) cells, and supports efficient human hematopoietic stem cell (HSC) engraftment and reconstitution of a functional human immune system in central and peripheral lymphoid organs.41 Remarkably, long-term human lymphoid (T, B, NK) and myeloid lineages (monocytes and dendritic cells) are stably detected in peripheral lymphoid tissues such as spleen, lymph nodes (LNs), and peripheral blood (PB). Human T cells developed in the DKO-hu mouse are tolerant to both human and mouse self-antigens, indicating efficient negative selection by both murine and human antigen presenting cells (APCs). Importantly, de novo human B- and T-cell responses are elicited in the DKO-hu HSC mouse by standard immunization (human TT-specific IgG induction) or infection with the human tumor virus Epstein-Barr virus (EBV; expansion of EBV-specific CD8 T cells). These EBV-reactive T cells respond to EBV antigens in a human MHC-restricted fashion. This suggests that human T cells are positively selected by human MHC as well as by murine MHC in humanized DKO-hu mice.42
We and others have shown that HIV-1 infection is efficiently established and persistently detectable in the PB of DKO-hu HSC mice with X4, R5, or X4/R5 dual-tropic isolates. In addition, human CD4+ T cells are depleted during HIV infection in a dose-dependent manner.43-45 We show here that FoxP3+ Treg cells are present and functional in all lymphoid organs in DKO-hu mice. The FoxP3+ Treg cells support high levels of HIV-1 infection and are preferentially depleted by pathogenic HIV-1 isolates in HIV-infected DKO-hu HSC mice. Finally, depletion of Treg cells in DKO-hu mice leads to reduced HIV infection and replication, which underscores the importance of Treg cells in HIV-1 infection.
Methods
Construction of DKO-hu HSC mice
Approval for animal work was obtained from the University of North Carolina Institutional Animal Care and Use Committee (IACUC). We constructed DKO-hu HSC mice as previously reported.45 Briefly, human CD34+ cells were isolated from 17- to 20-week-old fetal liver tissues. Tissue was first perfused with Liver Perfusion Medium and then with Liver Digest Medium (Invitrogen, Frederick, MD). The cell suspension released from the liver was filtered through a 70-μm cell strainer (BD Falcon, Lincoln Park, NJ) and was centrifuged at 150g for 5 minutes to collect mononuclear cells. Cell viability, measured using Guava via-count (Hayward, CA), generally exceeded 90%. After selection with the CD34 magnetic-activated cell sorting (MACS) kit, the purity of CD34+ HSCs was greater than 95%. CD34+ HSCs (0.5 × 106) were injected into the liver of each 1- to 3-day-old DKO mouse, which had been previously irradiated at 400 rad (sublethal). More than 95% of the DKO-hu mice were stably reconstituted with human leukocytes in the blood (10%-50% at 12-14 weeks). DKO-hu mice in each cohort (DKO-hu mice reconstituted from the same human donor fetal liver tissue) had similar levels of engraftment. All mice were housed at the University of North Carolina at Chapel Hill.
Analysis of human leukocytes in PB or tissues by multicolor FACS
Stable human hematolymphopoiesis was monitored in the blood (tail vein or retro-orbital bleeding) at 8, 10, and 12 weeks after transplantation. At termination, all lymphoid organs including thymus, bone marrow, spleen, cervical/axillary/inguinal lymph nodes (pLNs), and mesenteric lymph nodes (mLNs) were harvested.18,46 Human leukocytes (CD45+) were analyzed for CD3, CD4, CD8, CD45RO, CD25, CD45RA, HLA-DR, CD127, FoxP3, CCR5 (2D7), and CXCR4 (12G5) by CyAn FACS machine (Dako North America, Carpinteria, CA). FITC-conjugated anti–human CCR5, HLA-DR, and CD45RA; PE-conjugated anti–human CD25, CXCR4, active caspase-3, and CD127; and PE/Cy7 anti–human CD3 were purchased from BD Pharmingen (San Diego, CA). PE/Cy5-conjugated anti–human CD4, APC/Cy7-conjugated anti–human CD45, Pacific blue–conjugated anti–mouse CD45, and Alexa Fluor 647–conjugated anti–human Foxp3 Abs were purchased from BioLegend (San Diego, CA). PE/Texas red–conjugated anti–human CD8 antibody was purchased from Caltag (Invitrogen, Carlsbad, CA). Live/dead fixable violet dead cell dye (VLD) was purchased from Molecular Probes (Eugene, OR). FITC-conjugated anti–HIV p24 (clone FH190-1-1) was purchased from Beckman Coulter (Fullerton, CA).
Immunohistochemistry staining
Paraffin-embedded sections of lymphoid organs (mesenteric lymph node and spleen) from DKO-hu mice were stained18,46 with the anti–human FoxP3 antibody (provided by Dr Alison H. Banham, University of Oxford, Oxford, United Kingdom), washed in PBS, then incubated with goat antimouse biotin and the avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA) and substrate DAB (Pierce, Rockford, IL). Slides were observed using a Nikon Microphot FXA microscope (Nikon, Garden City, NY) with a 20× nonoil objective and 10× ocular lens. Images were captured using a QImaging Micropublisher 3.3 CCD digital camera and QCapture software version 3.0 (QImaging, Surrey, BC).
Human Treg suppression assay
To isolate human Treg cells, spleens (Spl's) and mesenteric lymph nodes (mLNs) from 3 to 5 DKO-hu HSC mice of the same donor-derived cohort were harvested and pooled. Cells were stained with antimouse CD45, antihuman CD45, antihuman CD4, and antihuman CD25 antibodies and live/dead fixable violet dead cell dye (VLD; Molecular Probes), VLD-mCD45-huCD45+huCD4+huCD25+ Treg cells were sorted on a MoFlo sorter (Dako North America, Carpinteria, CA). Human CD4+CD25− cells from normal human peripheral blood were purified on the autoMACS system using human Treg isolation kit (Miltenyi Biotec, Auburn, CA). The suppression assay of CD4+CD25− T-cell proliferation by Treg cells was performed as described.18 Briefly, purified human CD4+CD25− responder cells were labeled with CFSE for 3 minutes at 37°C, quenched by adding 50% of FBS, washed with medium 3 times, then cocultured with sorted human Treg cells (Treg/responders at 1:2) from humanized DKO-hu mice for 4 days with anti-CD3/CD28 stimulations. Cells were harvested and analyzed by flow cytometry.
A human Treg suppression assay was also performed using monocyte-derived dendritic cells (mDCs) in the presence of superantigen, staphylococcal enterotoxin B (SEB), to stimulate naive CD4+CD25− T cells.5,47 Briefly, human CD14+ monocytes were purified using the MACS system (Miltenyi Biotec) and cultured in the presence of IL-4 (100 ng/mL) and granulocyte-macrophage colony-stimulating factor (GM-CSF, 50 ng/mL) (both from R&D Systems, Minneapolis, MN) for 4 to 6 days. Purified human CD4+CD25− responder cells were labeled with CFSE and cocultured with dendritic cells (DCs) and purified human Treg cells from DKO-hu HSC mice in a round-bottom well of a 96-well plate, in the presence of SEB (10 ng/mL-0.1 pg/mL). Human CD4+CD25− T cells from DKO-hu mice were also used as controls. The ratio of effector T cells to responder cells was 1:1. Four to 6 days later, cells were harvested and analyzed by flow cytometry.
HIV-1 infection in DKO-hu HSC mice
At 12 to 14 weeks after CD34 HSC transfer, DKO-hu HSC mice with stable human leukocyte reconstitution (> 10% human CD45+ leukocytes in their blood) were intravenously infected with HIV-1 R3A (CCR5/CXCR4 dual tropic, highly pathogenic)48-50 stocks (1 ng p24/mouse in 50-100 μL). DKO-hu HSC mice infected with mock stocks were included as control groups. At termination time (1-2 weeks after infection), spleen and mesenteric lymph nodes were harvested and cell numbers were counted by Guava Easycytes. Cells were stained with surface markers with different antibodies. Next, the cells were permeabilized with BD cytofix/cytoperm buffer, followed by intracellular staining with anti-HIV p24-FITC, antihuman FoxP3-Alexa Fluor 647, and/or anti–active caspase-3 PE antibodies. Control antibodies including mouse IgG1-FITC (for anti-p24), mouse IgG1–Alexa Fluor 647 (for anti-FoxP3), and rabbit IgG-PE (for anti–active caspase-3) were used. Cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry.
HIV-1 replication and pathogenesis assays
HIV-1 replication (genome copy number per milliliter in the plasma) was measured by the Roche Amplicor Monitor v.1.5 quantitative reversetranscription–polymerase chain reaction (qRT-PCR) assay (Roche Diagnostics, Indianapolis, IN) or by p24-FACS detection of productively infected human T cells.45
HIV-1–induced T-cell death
Lymphoid organs were harvested at 1 to 2 weeks after infection and human cells were analyzed for HIV infection and T-cell depletion. Active caspase-3 levels (% Casp3+) were measured in CD3+CD8−FoxP3+ human T cells of mock- or HIV-infected spleen and mLN samples and as we have reported previously.50,51
Treg cell depletion by denileukin diftitox (DAB389IL-2, denileukin diftitox) in DKO-hu HSC mice
Denileukin diftitox (150 μg/mL, in citrate buffer) was provided by Ligand Pharmaceuticals (San Diego, CA). For in vivo treatment, 12-week-old DKO-hu HSC mice were injected intraperitoneally with 0.75 μg denileukin diftitox diluted in 100 μL PBS. PBS-injected mice were used as control. Treg cells in the blood and lymphoid organs were monitored at 1, 3, 6, and 12 days after injection. For infection cohorts, denileukin diftitox– or PBS-treated mice were infected with HIV-R3A at 2 days after injection. HIV replication and T-cell depletion were measured at 7 or 10 days after infection.
Statistical analysis
The significance of all comparisons was calculated using a Student 2-tailed t test assuming unequal variance between mock- and HIV-1–infected groups.
Results
Development of functional Treg cells in DKO-hu HSC mice
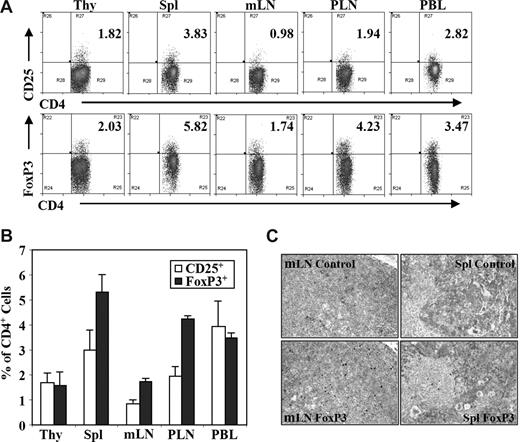
We analyzed human CD4+FoxP3+ regulatory T (Treg) cells from DKO-hu HSC mice by multiple-color flow cytometry (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Treg cells were detected in the blood and other lymphoid organs at a frequency similar to human or mouse lymphoid organs, either as CD4+CD25+ or as CD4+FoxP3+ T cells (Figure 1A-C). Similar to findings from human peripheral blood mononuclear cells (PBMCs), approximately 60% of human CD3+CD4+CD25+ T cells were FoxP3+, and approximately 40% to 50% of CD3+CD4+FoxP3+ T cells expressed CD25 (Figure S1). Only a small fraction of FoxP3+CD4+ Treg cells from spleen or LN expressed IL-7Rα (< 20%), CD45RA (10%-25%), or HLA-DR (< 5%), whereas most FoxP3−CD4+ T cells express IL-7Rα and CD45RA (Figure S2).
Human regulatory T cells (Treg cells) in DKO-hu HSC mice. Lymphoid organs from each DKO-hu HSC mouse were individually analyzed for development of human Treg cell by flow cytometry analysis at 12 to 40 weeks after transplantation. Experiments were repeated using 5 independent cohorts of DKO-hu HSC mice (generated using 5 different human donor fetal liver tissues). (A) Human CD4 T cells (mCD45−hCD45+CD3+CD4+) in different lymphoid organs of one representative DKO-hu HSC mouse were stained with CD25 or FoxP3 antibodies (see Figure S1 for FoxP3 vs CD25 plots). Numbers on plots are percentages of double-positive cells from the mCD45−huCD45+ CD3+CD4+ gated cells. (B) Bar graphs represent percentages of CD3+CD4+CD25+ or CD3+CD4+FoxP3+ Treg cells of total CD3+CD4+ T cells in different lymphoid organs. Data are summarized from DKO-hu mice derived from 5 different human fetal liver donor tissues (n > 30 DKO-hu mice). Error bars display standard deviations. (C) Human FoxP3+ Treg cells in lymphoid tissues of DKO-hu HSC mice were detected by immunostaining. Immunohistochemistry (IHC) staining was performed with anti–human FoxP3 antibody on paraffin sections of mesenteric lymph node (mLN) and spleen from DKO-hu HSC mice. Control nonspecific hybridoma supernatant was used as negative control.
Human regulatory T cells (Treg cells) in DKO-hu HSC mice. Lymphoid organs from each DKO-hu HSC mouse were individually analyzed for development of human Treg cell by flow cytometry analysis at 12 to 40 weeks after transplantation. Experiments were repeated using 5 independent cohorts of DKO-hu HSC mice (generated using 5 different human donor fetal liver tissues). (A) Human CD4 T cells (mCD45−hCD45+CD3+CD4+) in different lymphoid organs of one representative DKO-hu HSC mouse were stained with CD25 or FoxP3 antibodies (see Figure S1 for FoxP3 vs CD25 plots). Numbers on plots are percentages of double-positive cells from the mCD45−huCD45+ CD3+CD4+ gated cells. (B) Bar graphs represent percentages of CD3+CD4+CD25+ or CD3+CD4+FoxP3+ Treg cells of total CD3+CD4+ T cells in different lymphoid organs. Data are summarized from DKO-hu mice derived from 5 different human fetal liver donor tissues (n > 30 DKO-hu mice). Error bars display standard deviations. (C) Human FoxP3+ Treg cells in lymphoid tissues of DKO-hu HSC mice were detected by immunostaining. Immunohistochemistry (IHC) staining was performed with anti–human FoxP3 antibody on paraffin sections of mesenteric lymph node (mLN) and spleen from DKO-hu HSC mice. Control nonspecific hybridoma supernatant was used as negative control.
Functionally, these CD4+CD25+ Treg cells are anergic in vitro as demonstrated by decreased IL-2 production and cell proliferation (data not shown). We also examined the suppressive activity of the Treg cells in suppression assays. We showed that CD4+CD25+/hi cells (pooled from the spleen and mLN) were able to suppress proliferation of CD4+CD25− T cells either directly with responder T cells activated with anti-CD3/CD28 (Figure 2A-C) or when cocultured with primary DCs pulsed with the superantigen SEB (Figure 2D-F). Therefore, Treg cells with normal phenotypes developed in all lymphoid organs of the DKO-hu HSC mouse, and these Treg cells are functional with suppressor activity.
Human CD3+CD4+CD25+ T cells from DKO-hu HSC mice are functional Treg cells. Human Treg cells (huCD45+CD3+CD4+CD25+) from DKO-hu HSC mice were isolated by FACS (> 95% purity), and cocultured with naive CD4+CD25− T cells from human PBMCs labeled with CFSE. Cells were activated either with antihuman CD3/CD28 mAb (A-C) or with mDCs pulsed with SEB (10 pg/mL; D-F). At 4 to 5 days after activation, cells were analyzed for CFSE levels by flow cytometry. Numbers indicate percentage of CFSElow (proliferated) responder cells. (A) CFSE-labeled responder cells without CD3/CD28 stimulation. (B) CFSE-labeled responder cells with CD3/CD28 stimulation.(C) Treg cells plus CFSE-labeled responder cells (at a 1:2 Treg/responder ratio) with CD3/CD28 stimulation. (D) CFSE-labeled responder cells with SEB-pulsed mDCs. (E) CFSE-labeled responder cells and SEB-pulsed mDCs plus CD4+CD25− naive T cells isolated from DKO-hu mice. (F) CFSE-labeled responder cells and SEB-pulsed mDCs plus CD4+CD25+ Treg cells. Both CD4+CD25− control and CD4+CD25+ Treg cells were used at 1:1 ratio relative to responder cells. Data shown are representative of 2 independent experiments.
Human CD3+CD4+CD25+ T cells from DKO-hu HSC mice are functional Treg cells. Human Treg cells (huCD45+CD3+CD4+CD25+) from DKO-hu HSC mice were isolated by FACS (> 95% purity), and cocultured with naive CD4+CD25− T cells from human PBMCs labeled with CFSE. Cells were activated either with antihuman CD3/CD28 mAb (A-C) or with mDCs pulsed with SEB (10 pg/mL; D-F). At 4 to 5 days after activation, cells were analyzed for CFSE levels by flow cytometry. Numbers indicate percentage of CFSElow (proliferated) responder cells. (A) CFSE-labeled responder cells without CD3/CD28 stimulation. (B) CFSE-labeled responder cells with CD3/CD28 stimulation.(C) Treg cells plus CFSE-labeled responder cells (at a 1:2 Treg/responder ratio) with CD3/CD28 stimulation. (D) CFSE-labeled responder cells with SEB-pulsed mDCs. (E) CFSE-labeled responder cells and SEB-pulsed mDCs plus CD4+CD25− naive T cells isolated from DKO-hu mice. (F) CFSE-labeled responder cells and SEB-pulsed mDCs plus CD4+CD25+ Treg cells. Both CD4+CD25− control and CD4+CD25+ Treg cells were used at 1:1 ratio relative to responder cells. Data shown are representative of 2 independent experiments.
Productive infection of FoxP3+ Treg cells by HIV-1 in DKO-hu HSC mice
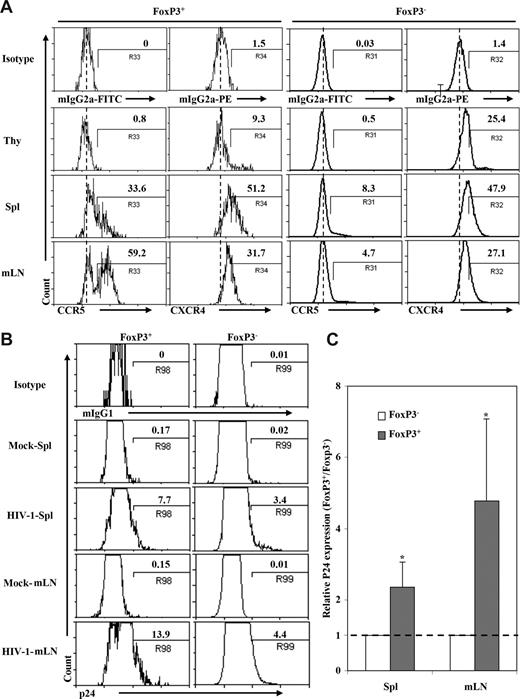
DKO-hu HSC mice are susceptible to infection with CCR5- or CXCR4-tropic HIV-1 in the thymus, spleen, and lymph nodes.45 Interestingly, a higher fraction of Treg cells in the spleen and LN (30% and 60%, respectively) from DKO-hu mice expressed CCR5 compared with FoxP3−CD4 T cells (4%-8%): however, they expressed similar levels of CXCR4 (Figure 3A). To analyze productive infection of CD4+FoxP3+ Treg cells by HIV-1, human cells from DKO-hu HSC mice infected with the dual-tropic and pathogenic HIV-R3A48,50 isolate were analyzed by p24 intracellular staining. Due to CD4 down-regulation in CD4+ T cells productively infected with HIV-1,45 we analyzed p24 and FoxP3 expression in CD45+CD3+CD8− T cells. Productive HIV infection was detected in both FoxP3+ and FoxP3− T cells. Strikingly, a higher percentage of FoxP3+ Treg cells were infected compared with FoxP3− T cells from the spleen or mLN (2- to 5-fold increase, P < .05, Figure 3B,C). Thus, 13% to 15% of CD3+CD8−FoxP3+ Treg cells were productively infected, whereas only 3% to 5% of CD3+CD8−FoxP3− T cells were infected by HIV-R3A (P < .05). In addition, the CCR5-tropic HIV-JRCSF also infected FoxP3+ T cells more efficiently than FoxP3−CD4 T cells (25% vs 7%; data not shown). Therefore, HIV-1 is able to efficiently infect and replicate in FoxP3+ Treg cells in vivo.
HIV preferentially infected Treg cells in DKO-hu HSC mice in vivo. (A) Expression of HIV-1 coreceptors on human CD4+ T cells from different lymphoid organs in DKO-hu HSC mice. Cells shown were gated on human CD45+CD3+CD4+CD8− cells. CD4+FoxP3+ or CD4+FoxP3− cells were further analyzed for expression of CCR5 and CXCR4. Numbers indicate percentage of positive cells, and the dashed line indicates isotype control signals (the median fluorescence intensity of control staining). (B) Infection of FoxP3+ and FoxP3− CD4 T cells in DKO-hu HSC mice by HIV-R3A (X4/R5 dual tropic). Mock supernatant was used as negative control. DKO-hu HSC mice were killed at 1 week after infection, and levels of intracellular p24 on FoxP3+CD4+ or FoxP3−CD4+ (CD45+CD3+CD8−) T cells from spleen and mLN were analyzed. Numbers indicate percentage of p24+ cells. (C) Summary of relative p24 expression in FoxP3+ or FoxP3− CD4 T cells from spleen and mLN. Data shown are representative of 2 independent experiments with at least 3 mice in each group. *P < .05. Error bars display standard deviations.
HIV preferentially infected Treg cells in DKO-hu HSC mice in vivo. (A) Expression of HIV-1 coreceptors on human CD4+ T cells from different lymphoid organs in DKO-hu HSC mice. Cells shown were gated on human CD45+CD3+CD4+CD8− cells. CD4+FoxP3+ or CD4+FoxP3− cells were further analyzed for expression of CCR5 and CXCR4. Numbers indicate percentage of positive cells, and the dashed line indicates isotype control signals (the median fluorescence intensity of control staining). (B) Infection of FoxP3+ and FoxP3− CD4 T cells in DKO-hu HSC mice by HIV-R3A (X4/R5 dual tropic). Mock supernatant was used as negative control. DKO-hu HSC mice were killed at 1 week after infection, and levels of intracellular p24 on FoxP3+CD4+ or FoxP3−CD4+ (CD45+CD3+CD8−) T cells from spleen and mLN were analyzed. Numbers indicate percentage of p24+ cells. (C) Summary of relative p24 expression in FoxP3+ or FoxP3− CD4 T cells from spleen and mLN. Data shown are representative of 2 independent experiments with at least 3 mice in each group. *P < .05. Error bars display standard deviations.
HIV-1 infection preferentially depletes FoxP3+ Treg cells in DKO-hu HSC mice
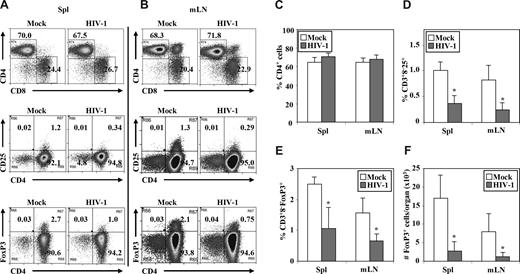
We also measured relative depletion of human Treg cells by HIV-1 infection (Figure 4A,B). The highly pathogenic HIV-R3A virus replicated to high levels at 1 week after infection, but only a slight depletion of total CD4 T cells in lymphoid organs was observed (Figure 4). In contrast, HIV-R3A infection significantly depleted Treg cells in the LN and spleen, determined either by relative percentage or by total cell number/organ when CD3+CD8−CD25+ T cells (Figure 4D) or CD3+CD8−FoxP3+ Treg cells (Figure 4E,F) were measured. At 2 weeks after infection, HIV-R3A infection led to almost complete depletion of human CD4+ T cells, including Treg cells (Zhang et al45 and data not shown).
HIV-R3A infection preferentially depleted Treg cells in DKO-hu HSC mice. Cells from spleen and mLN were harvested from each HIV- or mock-infected mouse at 1 week after infection. Mouse CD45− and human CD45+ VLD−CD3+ cells were gated to analyze CD4/CD8, CD4/CD25, and CD4/FoxP3 from spleen (Spl; A) or mLN (B). The data shown are representative of 3 independent experiments with at least 3 DKO-hu mice in each group. Summarized data of percentage of CD4+ cells (C), percentage of CD3+CD8−CD25+ cells (D), percentage of CD3+CD8− FoxP3+ cells (E), and number of FoxP3+ cells per organ (F) in spleen and mLN of HIV-1– versus mock-infected mice are shown. Error bars indicate standard errors. *P < .05.
HIV-R3A infection preferentially depleted Treg cells in DKO-hu HSC mice. Cells from spleen and mLN were harvested from each HIV- or mock-infected mouse at 1 week after infection. Mouse CD45− and human CD45+ VLD−CD3+ cells were gated to analyze CD4/CD8, CD4/CD25, and CD4/FoxP3 from spleen (Spl; A) or mLN (B). The data shown are representative of 3 independent experiments with at least 3 DKO-hu mice in each group. Summarized data of percentage of CD4+ cells (C), percentage of CD3+CD8−CD25+ cells (D), percentage of CD3+CD8− FoxP3+ cells (E), and number of FoxP3+ cells per organ (F) in spleen and mLN of HIV-1– versus mock-infected mice are shown. Error bars indicate standard errors. *P < .05.
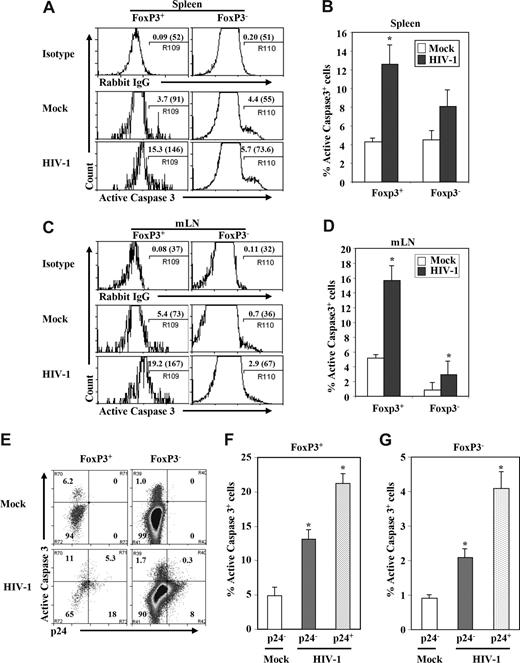
We next measured levels of active caspase-3+ cells to test whether HIV infection induced apoptosis in FoxP3+ Treg cells from DKO-hu mice. We observed that a high percentage of Treg (FoxP3+CD3+CD8−) cells was caspase-3+ compared with FoxP3−CD3+CD8− T cells isolated from spleen or LN (Figure 5). In the spleen of mock-infected DKO-hu mice, lower levels of caspase-3+ cells were detected in both FoxP3+CD3+CD8− and FoxP3−CD3+CD8− T cells. However, HIV-1 infection preferentially induced apoptosis in FoxP3+ T cells compared with FoxP3− T cells (Figure 5A,C). In the mLN, preferential induction of apoptosis of FoxP3+ T cells was also observed; however, FoxP3+ T cells from mock-infected mice have higher levels of basal caspase-3+ expression than FoxP3− T cells (Figure 5B,D). We then measured relative apoptosis induction in p24+ and p24− CD3+CD8− T cells. We observed that both HIV-infected and uninfected bystander FoxP3+CD3+CD8− T cells were apoptotic, by caspase-3+ staining, although a higher percentage of the p24+FoxP3+ T cells expressed active caspase-3 (Figure 5E,F). When caspase-3 levels were analyzed in FoxP3−CD3+CD8− T cells, a lower but significant level of both p24+ and p24− T cells were apoptotic (Figure 5E,G). Thus, the highly pathogenic HIV-R3A virus preferentially infects and depletes FoxP3+CD3+CD8− T cells in vivo, and correlates with induction of apoptosis.
Depletion of Treg cells by HIV-1 was associated with induction of apoptosis. Cells from spleen (A) and mLN (B) in HIV-1– or mock-infected mice were harvested; human FoxP3+ Treg cells and FoxP3− T cells (CD45+CD3+CD8−) were analyzed for the expression of activated caspase-3 in the spleen and mLN. Numbers indicate percentage active caspase-3+ cells. Numbers in parentheses indicate relative caspase-3 signals of total CD3+CD8−FoxP3+ or CD3+CD8−FoxP3− T cells. Summarized data of percentage of active caspase-3 of FoxP3+ Treg cells or FoxP3− T cells in spleen (C) and mLN (D) are shown. (E-G) Induction of apoptosis in HIV-infected (p24+) and bystander (p24−) cells of FoxP3+ or FoxP3− (CD3+CD8−) T cells. (E) FACS plots with mLN CD45+CD3+CD8− T cells from mock- and HIV-infected DKO-hu mice. Numbers indicate percentage of cells in each subpopulation. Relative induction of apoptosis in p24+ and p24− FoxP3+ Treg cells (F) and FoxP3− T cells (G) is summarized. Data shown are representative of 3 independent experiments with at least 3 mice in each group. Error bars indicate standard errors. *P < .05.
Depletion of Treg cells by HIV-1 was associated with induction of apoptosis. Cells from spleen (A) and mLN (B) in HIV-1– or mock-infected mice were harvested; human FoxP3+ Treg cells and FoxP3− T cells (CD45+CD3+CD8−) were analyzed for the expression of activated caspase-3 in the spleen and mLN. Numbers indicate percentage active caspase-3+ cells. Numbers in parentheses indicate relative caspase-3 signals of total CD3+CD8−FoxP3+ or CD3+CD8−FoxP3− T cells. Summarized data of percentage of active caspase-3 of FoxP3+ Treg cells or FoxP3− T cells in spleen (C) and mLN (D) are shown. (E-G) Induction of apoptosis in HIV-infected (p24+) and bystander (p24−) cells of FoxP3+ or FoxP3− (CD3+CD8−) T cells. (E) FACS plots with mLN CD45+CD3+CD8− T cells from mock- and HIV-infected DKO-hu mice. Numbers indicate percentage of cells in each subpopulation. Relative induction of apoptosis in p24+ and p24− FoxP3+ Treg cells (F) and FoxP3− T cells (G) is summarized. Data shown are representative of 3 independent experiments with at least 3 mice in each group. Error bars indicate standard errors. *P < .05.
Depletion of CD4+CD25hi Treg cells in DKO-hu HSC mice reduced HIV-1 replication in vivo
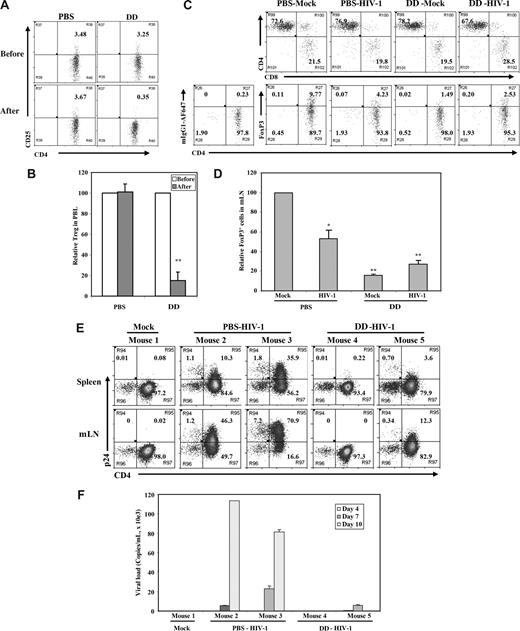
To define the role of Treg cells in acute HIV-1 infection, we used the IL-2–toxin fusion protein (denileukin diftitox) that has been used clinically in leukemia patients to remove CD4+CD25hi Treg cells.52,53 Denileukin diftitox treatment of DKO-hu mice efficiently depleted human CD4+CD25hi Treg cells, with no significant effect on other human leukocytes, including CD4+CD25− or CD8 T cells (Figure 6A,B; Figure S3; Table 1). The reduced level of human CD4+CD25+/hi Treg cells in lymphoid organs of DKO-hu mice was maintained for 10 to 12 days and correlated with elevated levels of HLA-DR on both CD4 and CD8 T cells (Figure S3). Once we confirmed the depletion of Treg cells, we infected PBS- or denileukin diftitox–treated DKO-hu mice with HIV-R3A. At 1 week after infection, HIV-1 infection led to preferential depletion of FoxP3+ Treg cells in the PBS-treated mice, compared with mock-treated mice (Figure 6C,D; Table 1), as observed in Figure 4. In this cohort, the level of CD4+FoxP3+ Treg cells was higher than average (Figure 1C; Table 1). In denileukin diftitox–treated mice, low levels of Treg cells were maintained (Figure 6C,D; Figure S3). To follow the kinetics of HIV-1 infection after denileukin diftitox treatment, DKO-hu mice were infected with HIV-1, and peripheral blood samples were collected at 3, 7, and 10 days after infection. When HIV-1 replication levels were measured, a lower level of HIV infection was detected in denileukin diftitox–treated DKO-hu mice as measured by p24 intracellular staining of human leukocytes from lymphoid organs (Figure 6E) and by RT-PCR of plasma viremia (Figure 6F). Therefore, reduced levels of Treg cells led to lower levels of HIV-1 during the acute stage of infection.
Denileukin diftitox (DAB389IL-2) treatment depleted Treg cells and reduced HIV-1 infection in DKO-hu HSC mice. (A) Human CD4+CD25+/hi Treg cells in peripheral blood of DKO-hu HSC mice were analyzed by FACS 24 hours after PBS or denileukin diftitox injection. Shown are CD45+CD3+CD4+ T cells. (B) Relative depletion of CD4+CD25+ T cells in the blood was shown, using preinjection levels as 100. Error bars are standard deviations (n = 4 DKO-hu mice for PBS or denileukin diftitox). (C) Forty-eight hours after PBS or denileukin diftitox injection, DKO-hu HSC mice were infected with HIV-R3A virus. At 7 days after infection, mLN from HIV-1– and mock-infected mice were analyzed for CD4/CD8 or CD4/FoxP3 expression by FACS. Shown are pregated CD45+CD3+ human T cells for CD4/CD8 and CD45+CD3+CD8− human T cells for CD4/FoxP3. Numbers indicate percentage of each cell population, and mIgG1-AF647 was used as isotype control for the FoxP3 staining. (D) Relative percentage of FoxP3+CD3+CD8− Treg cells is summarized with data from 2 to 4 mice. (E) DKO-hu mice were similarly treated as in panel C and infected with mock or HIV-R3A. At 10 days after infection, CD45+CD3+CD8− T cells from spleen or mLN were analyzed for expression of CD4 and p24 by FACS. (F) HIV-1 viremia in plasma samples (copies/mL) from all infected DKO-hu mice were measured at 4, 7, and 10 days after infection. Data shown are representative of 3 independent experiments with 2 to 3 mice in each group. Error bars indicate standard deviations.
Denileukin diftitox (DAB389IL-2) treatment depleted Treg cells and reduced HIV-1 infection in DKO-hu HSC mice. (A) Human CD4+CD25+/hi Treg cells in peripheral blood of DKO-hu HSC mice were analyzed by FACS 24 hours after PBS or denileukin diftitox injection. Shown are CD45+CD3+CD4+ T cells. (B) Relative depletion of CD4+CD25+ T cells in the blood was shown, using preinjection levels as 100. Error bars are standard deviations (n = 4 DKO-hu mice for PBS or denileukin diftitox). (C) Forty-eight hours after PBS or denileukin diftitox injection, DKO-hu HSC mice were infected with HIV-R3A virus. At 7 days after infection, mLN from HIV-1– and mock-infected mice were analyzed for CD4/CD8 or CD4/FoxP3 expression by FACS. Shown are pregated CD45+CD3+ human T cells for CD4/CD8 and CD45+CD3+CD8− human T cells for CD4/FoxP3. Numbers indicate percentage of each cell population, and mIgG1-AF647 was used as isotype control for the FoxP3 staining. (D) Relative percentage of FoxP3+CD3+CD8− Treg cells is summarized with data from 2 to 4 mice. (E) DKO-hu mice were similarly treated as in panel C and infected with mock or HIV-R3A. At 10 days after infection, CD45+CD3+CD8− T cells from spleen or mLN were analyzed for expression of CD4 and p24 by FACS. (F) HIV-1 viremia in plasma samples (copies/mL) from all infected DKO-hu mice were measured at 4, 7, and 10 days after infection. Data shown are representative of 3 independent experiments with 2 to 3 mice in each group. Error bars indicate standard deviations.
Denileukin diftitox (DAB389IL-2) treatment reduced HIV-1 infection in DKO-hu HSC mice
| Donor . | Mice no. . | Treatment . | Organ . | Percentage cell population . | Absolute cell number per organ . | Plasma viral load, copies/mL . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD45+* . | CD3+† . | CD4+‡ . | CD8+‡ . | Foxp3+§ . | p24+§ . | CD45+ . | CD3+ . | CD4+CD8− . | CD8+CD4− . | Foxp3+ . | Day 4‖ . | Day 7 . | Day 10 . | ||||
| 1 | M56 | Mock | Spleen | 11.7 | 27.5 | 66 | 29.4 | 2.22 | 0.09 | 7.5E+06 | 2.1E+06 | 1.4E+06 | 1.5E+06 | 3.2E+04 | ND | 0 | 0 |
| 1 | M58 | PBS, HIV-1 | Spleen | 14.2 | 17.7 | 66.5 | 27.5 | 0.59 | 11.4 | 2.5E+05 | 4.4E+04 | 2.9E+04 | 3.2E+04 | 1.9E+02 | ND | 5.5E+03 | 1.1E+05 |
| 1 | M59 | PBS, HIV-1 | Spleen | 15.6 | 15 | 55 | 36.7 | 1.24 | 37.7 | 8.1E+06 | 1.2E+06 | 6.7E+05 | 7.7E+05 | 9.5E+03 | ND | 2.3E+04 | 8.1E+04 |
| 1 | M54 | DD, HIV-1 | Spleen | 26.1 | 18.9 | 51 | 43.3 | 2.64 | 0.23 | 1.9E+07 | 3.6E+06 | 1.9E+06 | 2.1E+06 | 5.4E+04 | ND | ND | ND |
| 1 | M57 | DD, HIV-1 | Spleen | 21.2 | 19.3 | 40 | 39.2 | 1.62 | 4.3 | 2.4E+07 | 4.7E+06 | 1.9E+06 | 2.9E+06 | 4.7E+04 | ND | 5.8E+03 | ND |
| 1 | M56 | Mock | mLN | 86.2 | 58.7 | 68.4 | 26.7 | 3 | 0.02 | 2.1E+06 | 1.2E+06 | 8.3E+05 | 8.8E+05 | 2.7E+04 | — | — | — |
| 1 | M58 | PBS, HIV-1 | mLN | 83.8 | 52.5 | 67.1 | 29.8 | 0.72 | 47.5 | 8.4E+05 | 4.4E+05 | 2.9E+05 | 3.1E+05 | 2.2E+03 | — | — | — |
| 1 | M59 | PBS, HIV-1 | mLN | 85.2 | 23.8 | 32.5 | 62 | 0.85 | 78.1 | 1.4E+06 | 3.2E+05 | 1.1E+05 | 1.2E+05 | 1.0E+03 | — | — | — |
| 1 | M54 | DD, HIV-1 | mLN | 95.4 | 72 | 57.1 | 40.9 | 1.71 | 0 | 3.6E+06 | 2.6E+06 | 1.5E+06 | 1.5E+06 | 2.6E+04 | — | — | — |
| 1 | M57 | DD, HIV-1 | mLN | 93.3 | 67.7 | 57.4 | 38.6 | 0.3 | 12.64 | 3.6E+06 | 2.5E+06 | 1.4E+06 | 1.5E+06 | 4.5E+03 | — | — | — |
| 2 | 582 | Mock | Spleen | 2.4 | 54.1 | 77.9 | 18.5 | 3.37 | 0.35 | 2.3E+06 | 1.2E+06 | 9.7E+05 | 1.0E+06 | 3.4E+04 | ND | ND | ND |
| 2 | 580 | Mock | Spleen | 12.0 | 58.6 | 68.2 | 27.1 | 11.07 | 0.18 | 4.9E+06 | 2.9E+06 | 2.0E+06 | 2.1E+06 | 2.3E+05 | ND | ND | ND |
| 2 | 576 | PBS, HIV-1 | Spleen | 1.4 | 17.9 | 4.1 | 84.2 | 0.21 | 23.1 | 1.2E+06 | 2.2E+05 | 9.0E+03 | 3.5E+04 | 7.3E+01 | 6.1E+04 | 1.9E+05 | 2.2E+05 |
| 2 | 583 | PBS, HIV-1 | Spleen | 1.4 | 45.1 | 5.2 | 84.8 | 0.32 | 22.4 | 2.0E+06 | 9.1E+05 | 4.7E+04 | 1.4E+05 | 4.4E+02 | ND | 5.7E+04 | 9.3E+04 |
| 2 | 577 | DD, HIV-1 | Spleen | 5.1 | 9.8 | 34.6 | 53.1 | 0.52 | 47.5 | 4.8E+06 | 4.7E+05 | 1.6E+05 | 2.2E+05 | 1.1E+03 | ND | 8.2E+04 | 1.7E+05 |
| 2 | 579 | DD, HIV-1 | Spleen | 5.4 | 29 | 70.2 | 22.5 | 0.42 | 14.77 | 3.5E+06 | 1.0E+06 | 7.2E+05 | 8.0E+05 | 3.3E+03 | 3.1E+03 | 2.3E+04 | 6.6E+04 |
| 2 | 582 | Mock | mLN | 72.5 | 72.3 | 62.5 | 33.5 | 1.22 | 0.04 | 6.7E+05 | 4.8E+05 | 3.0E+05 | 3.2E+05 | 3.9E+03 | — | — | — |
| 2 | 580 | Mock | mLN | 91.5 | 81.9 | 67.5 | 29.3 | 4.82 | 0.02 | 3.2E+06 | 2.6E+06 | 1.8E+06 | 1.9E+06 | 9.0E+04 | — | — | — |
| 2 | 576 | PBS, HIV-1 | mLN | 77.1 | 50.7 | 0.37 | 94.5 | 1 | 8.7 | 4.8E+05 | 2.5E+05 | 9.1E+02 | 1.4E+04 | 1.4E+02 | — | — | — |
| 2 | 583 | PBS, HIV-1 | mLN | 56.5 | 98.2 | 14.8 | 78.5 | 2.6 | 70.9 | 1.8E+05 | 1.8E+05 | 2.6E+04 | 3.8E+04 | 9.8E+02 | — | — | — |
| 2 | 577 | DD, HIV-1 | mLN | 75.4 | 37.7 | 16.6 | 78.2 | 0.91 | 71.1 | 5.0E+05 | 1.9E+05 | 3.1E+04 | 4.1E+04 | 3.7E+02 | — | — | — |
| 2 | 579 | DD, HIV-1 | mLN | 87.6 | 71.1 | 60.6 | 35 | 0.22 | 57 | 1.3E+06 | 9.0E+05 | 5.5E+05 | 5.9E+05 | 1.3E+03 | — | — | — |
| 3 | 454 | PBS, mock | mLN | 14.6 | 60.7 | 83.3 | 10.8 | 8.43 | 0.2 | 7.6E+03 | 4.6E+03 | 3.9E+03 | 5.0E+02 | 3.9E+02 | — | ND | — |
| 3 | 463 | PBS, mock | mLN | 67.9 | 63.2 | 72.6 | 21.5 | 9.77 | 0.23 | 1.3E+05 | 8.2E+04 | 5.9E+04 | 1.8E+04 | 8.0E+03 | — | ND | — |
| 3 | 461 | DD, mock | mLN | 93.5 | 64.3 | 78.2 | 19.5 | 1.49 | 0.05 | 1.1E+06 | 6.8E+05 | 5.4E+05 | 1.3E+05 | 1.0E+04 | — | ND | — |
| 3 | 462 | DD, mock | mLN | 79.0 | 49.1 | 75.9 | 21.6 | 1.15 | 0.33 | 3.8E+05 | 1.9E+05 | 1.4E+05 | 4.0E+04 | 2.1E+03 | — | ND | — |
| 3 | 457 | PBS, HIV-1 | mLN | 27.1 | 51.9 | 67.1 | 29.8 | 5.33 | 1.35 | 7.3E+03 | 3.8E+03 | 2.5E+03 | 1.1E+03 | 2.0E+02 | — | 9.3E+05 | — |
| 3 | 460 | PBS, HIV-1 | mLN | 83.2 | 65.8 | 76.9 | 19.8 | 4.23 | 2.07 | 2.9E+05 | 1.9E+05 | 1.5E+05 | 3.7E+04 | 8.0E+03 | — | 1.1E+05 | — |
| 3 | 452 | DD, HIV-1 | mLN | 81.4 | 54.4 | 67.6 | 28.5 | 6.72 | 0.19 | 2.0E+06 | 1.1E+06 | 7.2E+05 | 3.0E+05 | 7.2E+04 | — | 7.6E+03 | — |
| 3 | 459 | DD, HIV-1 | mLN | 91.5 | 51.4 | 81.6 | 14.8 | 2.27 | 0.34 | 1.0E+06 | 5.3E+05 | 4.3E+05 | 7.9E+04 | 1.2E+04 | — | ND | — |
| Donor . | Mice no. . | Treatment . | Organ . | Percentage cell population . | Absolute cell number per organ . | Plasma viral load, copies/mL . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD45+* . | CD3+† . | CD4+‡ . | CD8+‡ . | Foxp3+§ . | p24+§ . | CD45+ . | CD3+ . | CD4+CD8− . | CD8+CD4− . | Foxp3+ . | Day 4‖ . | Day 7 . | Day 10 . | ||||
| 1 | M56 | Mock | Spleen | 11.7 | 27.5 | 66 | 29.4 | 2.22 | 0.09 | 7.5E+06 | 2.1E+06 | 1.4E+06 | 1.5E+06 | 3.2E+04 | ND | 0 | 0 |
| 1 | M58 | PBS, HIV-1 | Spleen | 14.2 | 17.7 | 66.5 | 27.5 | 0.59 | 11.4 | 2.5E+05 | 4.4E+04 | 2.9E+04 | 3.2E+04 | 1.9E+02 | ND | 5.5E+03 | 1.1E+05 |
| 1 | M59 | PBS, HIV-1 | Spleen | 15.6 | 15 | 55 | 36.7 | 1.24 | 37.7 | 8.1E+06 | 1.2E+06 | 6.7E+05 | 7.7E+05 | 9.5E+03 | ND | 2.3E+04 | 8.1E+04 |
| 1 | M54 | DD, HIV-1 | Spleen | 26.1 | 18.9 | 51 | 43.3 | 2.64 | 0.23 | 1.9E+07 | 3.6E+06 | 1.9E+06 | 2.1E+06 | 5.4E+04 | ND | ND | ND |
| 1 | M57 | DD, HIV-1 | Spleen | 21.2 | 19.3 | 40 | 39.2 | 1.62 | 4.3 | 2.4E+07 | 4.7E+06 | 1.9E+06 | 2.9E+06 | 4.7E+04 | ND | 5.8E+03 | ND |
| 1 | M56 | Mock | mLN | 86.2 | 58.7 | 68.4 | 26.7 | 3 | 0.02 | 2.1E+06 | 1.2E+06 | 8.3E+05 | 8.8E+05 | 2.7E+04 | — | — | — |
| 1 | M58 | PBS, HIV-1 | mLN | 83.8 | 52.5 | 67.1 | 29.8 | 0.72 | 47.5 | 8.4E+05 | 4.4E+05 | 2.9E+05 | 3.1E+05 | 2.2E+03 | — | — | — |
| 1 | M59 | PBS, HIV-1 | mLN | 85.2 | 23.8 | 32.5 | 62 | 0.85 | 78.1 | 1.4E+06 | 3.2E+05 | 1.1E+05 | 1.2E+05 | 1.0E+03 | — | — | — |
| 1 | M54 | DD, HIV-1 | mLN | 95.4 | 72 | 57.1 | 40.9 | 1.71 | 0 | 3.6E+06 | 2.6E+06 | 1.5E+06 | 1.5E+06 | 2.6E+04 | — | — | — |
| 1 | M57 | DD, HIV-1 | mLN | 93.3 | 67.7 | 57.4 | 38.6 | 0.3 | 12.64 | 3.6E+06 | 2.5E+06 | 1.4E+06 | 1.5E+06 | 4.5E+03 | — | — | — |
| 2 | 582 | Mock | Spleen | 2.4 | 54.1 | 77.9 | 18.5 | 3.37 | 0.35 | 2.3E+06 | 1.2E+06 | 9.7E+05 | 1.0E+06 | 3.4E+04 | ND | ND | ND |
| 2 | 580 | Mock | Spleen | 12.0 | 58.6 | 68.2 | 27.1 | 11.07 | 0.18 | 4.9E+06 | 2.9E+06 | 2.0E+06 | 2.1E+06 | 2.3E+05 | ND | ND | ND |
| 2 | 576 | PBS, HIV-1 | Spleen | 1.4 | 17.9 | 4.1 | 84.2 | 0.21 | 23.1 | 1.2E+06 | 2.2E+05 | 9.0E+03 | 3.5E+04 | 7.3E+01 | 6.1E+04 | 1.9E+05 | 2.2E+05 |
| 2 | 583 | PBS, HIV-1 | Spleen | 1.4 | 45.1 | 5.2 | 84.8 | 0.32 | 22.4 | 2.0E+06 | 9.1E+05 | 4.7E+04 | 1.4E+05 | 4.4E+02 | ND | 5.7E+04 | 9.3E+04 |
| 2 | 577 | DD, HIV-1 | Spleen | 5.1 | 9.8 | 34.6 | 53.1 | 0.52 | 47.5 | 4.8E+06 | 4.7E+05 | 1.6E+05 | 2.2E+05 | 1.1E+03 | ND | 8.2E+04 | 1.7E+05 |
| 2 | 579 | DD, HIV-1 | Spleen | 5.4 | 29 | 70.2 | 22.5 | 0.42 | 14.77 | 3.5E+06 | 1.0E+06 | 7.2E+05 | 8.0E+05 | 3.3E+03 | 3.1E+03 | 2.3E+04 | 6.6E+04 |
| 2 | 582 | Mock | mLN | 72.5 | 72.3 | 62.5 | 33.5 | 1.22 | 0.04 | 6.7E+05 | 4.8E+05 | 3.0E+05 | 3.2E+05 | 3.9E+03 | — | — | — |
| 2 | 580 | Mock | mLN | 91.5 | 81.9 | 67.5 | 29.3 | 4.82 | 0.02 | 3.2E+06 | 2.6E+06 | 1.8E+06 | 1.9E+06 | 9.0E+04 | — | — | — |
| 2 | 576 | PBS, HIV-1 | mLN | 77.1 | 50.7 | 0.37 | 94.5 | 1 | 8.7 | 4.8E+05 | 2.5E+05 | 9.1E+02 | 1.4E+04 | 1.4E+02 | — | — | — |
| 2 | 583 | PBS, HIV-1 | mLN | 56.5 | 98.2 | 14.8 | 78.5 | 2.6 | 70.9 | 1.8E+05 | 1.8E+05 | 2.6E+04 | 3.8E+04 | 9.8E+02 | — | — | — |
| 2 | 577 | DD, HIV-1 | mLN | 75.4 | 37.7 | 16.6 | 78.2 | 0.91 | 71.1 | 5.0E+05 | 1.9E+05 | 3.1E+04 | 4.1E+04 | 3.7E+02 | — | — | — |
| 2 | 579 | DD, HIV-1 | mLN | 87.6 | 71.1 | 60.6 | 35 | 0.22 | 57 | 1.3E+06 | 9.0E+05 | 5.5E+05 | 5.9E+05 | 1.3E+03 | — | — | — |
| 3 | 454 | PBS, mock | mLN | 14.6 | 60.7 | 83.3 | 10.8 | 8.43 | 0.2 | 7.6E+03 | 4.6E+03 | 3.9E+03 | 5.0E+02 | 3.9E+02 | — | ND | — |
| 3 | 463 | PBS, mock | mLN | 67.9 | 63.2 | 72.6 | 21.5 | 9.77 | 0.23 | 1.3E+05 | 8.2E+04 | 5.9E+04 | 1.8E+04 | 8.0E+03 | — | ND | — |
| 3 | 461 | DD, mock | mLN | 93.5 | 64.3 | 78.2 | 19.5 | 1.49 | 0.05 | 1.1E+06 | 6.8E+05 | 5.4E+05 | 1.3E+05 | 1.0E+04 | — | ND | — |
| 3 | 462 | DD, mock | mLN | 79.0 | 49.1 | 75.9 | 21.6 | 1.15 | 0.33 | 3.8E+05 | 1.9E+05 | 1.4E+05 | 4.0E+04 | 2.1E+03 | — | ND | — |
| 3 | 457 | PBS, HIV-1 | mLN | 27.1 | 51.9 | 67.1 | 29.8 | 5.33 | 1.35 | 7.3E+03 | 3.8E+03 | 2.5E+03 | 1.1E+03 | 2.0E+02 | — | 9.3E+05 | — |
| 3 | 460 | PBS, HIV-1 | mLN | 83.2 | 65.8 | 76.9 | 19.8 | 4.23 | 2.07 | 2.9E+05 | 1.9E+05 | 1.5E+05 | 3.7E+04 | 8.0E+03 | — | 1.1E+05 | — |
| 3 | 452 | DD, HIV-1 | mLN | 81.4 | 54.4 | 67.6 | 28.5 | 6.72 | 0.19 | 2.0E+06 | 1.1E+06 | 7.2E+05 | 3.0E+05 | 7.2E+04 | — | 7.6E+03 | — |
| 3 | 459 | DD, HIV-1 | mLN | 91.5 | 51.4 | 81.6 | 14.8 | 2.27 | 0.34 | 1.0E+06 | 5.3E+05 | 4.3E+05 | 7.9E+04 | 1.2E+04 | — | ND | — |
DD indicates denileukin diftitox; ND, not detectable; and —, not done.
Percentage of human CD45+ of total cells.
Percentage of CD3+ from human CD45+ cells.
Percentage of CD4+ or CD8+ from CD45+ CD3+ cells.
Percentage of Foxp3+ or p24+ from CD45+CD3+CD8− cells.
Days after HIV-1 infection.
Discussion
The role of FoxP3+ Treg cells in HIV-1 disease is not clearly defined due to several reasons. First, basic immunobiology of Treg cells in humans is poorly investigated due to lack of relevant models. Second, Treg cells from the blood, the primary source of Treg cells in most studies, may not reflect the dynamics of Treg cells in lymphoid tissues. Third, Treg cells may be affected differently at acute stages of infection compared with the late chronic or AIDS stages of HIV-1 infection. Finally, individual differences in age and genetics within and between study populations may lead to discrepancy in results. To overcome these obstacles, a robust animal model that addresses these limitations is direly needed to study the modulation and role of Treg cells in HIV-1 infection. The DKO-hu HSC model is ideal for this purpose. With a stable, quiescent, and functional human immune system, HIV-1 establishes a persistent infection that leads to depletion of human CD4 T cells. We report here that suppressive human Treg cells developed in normal proportions in all lymphoid organs in DKO-hu mice, and that the FoxP3+ Treg cells support efficient HIV-1 infection in vivo. In addition, Treg cells were preferentially depleted by a pathogenic HIV-1 isolate in infected DKO-hu HSC mice, and this correlated with preferential induction of apoptosis in vivo. Finally, depletion of Treg cells in DKO-hu mice significantly decreased HIV-1 infection and replication in vivo.
A recent report has documented that more than 10% of the FoxP3+ T cells are productively infected (SIV RNA+ by in situ hybridization [ISH]) in the mucosal lymphoid organs of acutely infected rhesus macaques.22 In the DKO-hu HSC model, our data show that HIV-1 infects and replicates in CD4+FoxP3+ Treg cells more efficiently than in CD4+FoxP3− T cells. This is consistent with recent findings that Treg cells (CD4+CD25+) are direct target cells that support higher levels of infection by HIV-1 or FIV in vitro.5,23 Although FoxP3 has been reported to inhibit NFκB activity in transfected 293 cells or T cells,24 we and others have not observed such inhibition in human T cells.25,26 In fact, we have discovered that FoxP3 enhances HIV gene expression via an NFκB-dependent pathway.25 Therefore, FoxP3+ Treg cells are important target cells for HIV infection and replication, particularly in lymphoid tissues during acute infection when most human CD4 T cells are quiescent. As a consequence, accumulation of CD4+FoxP3+ Treg cells in lymphoid organs during HIV-1 acute infection may contribute to increased amounts of HIV target cells, as well as to suppressed anti-HIV immune responses in lymphoid tissues.
Chronic immune activation is a reliable predictor of AIDS progression.27 Impairing or depleting Treg cells by HIV-1 can conceivably contribute to immune hyperactivation. Some early reports support the idea that HIV infection leads to reduced Treg number and activity in peripheral blood.5,28-31 There are conflicting reports, however, in human patients regarding whether HIV-1 infection decreases or increases Treg cells during AIDS progression. Elevated FoxP3 expression in lymphoid organs has been documented in HIV-infected patients and in SIV-infected rhesus macaques.22,32-34 In a recent comprehensive study involving analysis of Treg cells from SIV-infected rhesus macaques during acute, early chronic, and late chronic stages of infection, a more defined role of Treg dynamics in SIV pathogenesis is reported.35 After acute infection, a transient increase in the frequency of Treg cells is detected. During SIV disease progression, however, a decrease in Treg numbers and suppressive activity is detected, which is inversely correlated with both plasma viremia and immune activation. Thus, Treg cells may play distinct roles during different stages of SIV or HIV infection/pathogenesis. It is possible that Treg cells accumulate in HIV-infected lymphoid organs during acute infection, similar to other viral infections, to coordinate a concerted immune response. However, HIV-1 can target these CD4+ Treg cells for productive infection, and the Treg cells can suppress anti-HIV immunity, which may contribute to establishment of persistent infection. During chronic- or late-stage disease progression, HIV infection may impair and/or deplete Treg cells, resulting in immune hyperactivation.54,55 Our findings using the pathogenic HIV-R3A isolate from a rapid progressor suggest that highly pathogenic HIV isolates, which are present during transmission or evolved during HIV disease progression, may rapidly infect and deplete Treg cells to cause immune hyperactivation and disease progression.49 This model system allows for the study of HIV isolates with different pathogenic phenotypes and tropisms. CCR5-tropic HIV-1 isolates, such as JR-CSF and YU2, establish a chronic infection with slow depletion of CD4+ T cells in DKO-hu mice.43-45 Therefore, we will be able to test the role of Treg cells at different stages of HIV infection by modulating the level of Treg cells in the DKO-hu model with different HIV-1 isolates.
Our data from HIV-R3A infection also indicate that HIV infection preferentially depletes FoxP3+CD4+ Treg cells, and this correlates with an induction of apoptosis in vivo. This is in contrast to findings from a recent report demonstrating that Treg cells incubated with HIV Env proteins in vitro appeared to rescue CD4+ Treg cells from apoptosis via CD4-dependent mechanisms.33 One possible explanation for this discrepancy is that HIV infection in lymphoid organs in vivo may have distinct effects on Treg cell survival compared with Treg cells cultured with HIV-1 Env or virions in vitro. In addition, the X4/R5 dual-tropic HIV-R3A Env may be more pathogenic than the R5 or X4 tropic Env used in the previous report.
One of the most critical questions in HIV infection and pathogenesis is the role of Treg cells during HIV-1 infection–induced immune hyperactivation. Our Treg depletion study with denileukin diftitox prior to HIV infection suggests that Treg cells may promote an increase in viral load and infected cells during acute infection by HIV-1. This may be partly because they are highly permissive to and support HIV infection5,23 (and this report). In addition, reduced Treg levels may also lead to enhanced anti-HIV immunity, which contributes to lower HIV infection and viral load. Our preliminary results indicate that elevated levels of T-cell activation correlate with reduced HIV replication in the denileukin diftitox–treated DKO-hu mice (Q.J. and L.S., unpublished results, November 2007). We will measure quantitatively the specific anti-HIV T-cell responses in these denileukin diftitox–treated and HIV-1–infected DKO-hu mice. It will also be interesting to deplete Treg cells from DKO-hu mice that have been chronically infected with CCR5-tropic HIV isolates, which maintain high viremia for more than 2 weeks43,45 (Q.J. and L.S., unpublished results, June 2007). Although denileukin diftitox appears to specifically deplete CD4+CD25hi Treg cells in vitro and in vivo,53 denileukin diftitox treatment does not completely deplete Treg cells and may have other nonspecific activity. With the DKO-hu HSC model, we will also be able to genetically test the role of FoxP3 in human Treg development and in HIV infection and pathogenesis. Therefore, the DKO-hu HSC model will be a valuable tool in further understanding how Treg cells respond to HIV infection and how HIV-1 interacts with the Treg cell population in HIV-1 pathogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr A. Banham (University of Oxford) for providing the anti-FoxP3 antibody and Dr G. Sempowski (Duke University, Durham, NC) for providing the denileukin diftitox. We thank the University of North Carolina Division of Laboratory Animal Medicine for animal care and Dr L. Arnold for help with the flow cytometry assay. We thank members of the Su laboratory for critical reading and/or discussion of the paper, and for their input and assistance.
The project was supported by grants from the National Institutes of Health (Bethesda, MD; AI41356, AI048407), a pilot grant from Southeast Regional Center of Excellence for Emerging Infections and Biodefense (Chapel Hill, NC) Developmental Projects to L.S., AI065303 to D.U., T32 AI007273-24 to M.L.W., and by amfAR (New York, NY; 106693-39-RFMT) to L.Z.
National Institutes of Health
Authorship
Contribution: Q.J. and L.Z. planned, designed, and performed the experiments and wrote the paper; R.W. and D.U. con-tributed to Treg suppression assays; D.B. performed the HIV replication assays; J.J., M.L.W., G.I.K., and S.B. performed the humanized mouse construction and assays; and L.S. conceived the research project, planned and designed the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lishan Su, Lineberger Comprehensive Cancer Center, Department of Microbiology and Immunology, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: lsu@med.unc.edu.
References
Author notes
*Q.J. and L.Z. contributed equally to this work.