Abstract
B cells are antibody (Ab)–secreting cells as well as potent antigen (Ag)–presenting cells that prime T-cell activation, which evokes great interest in their use for vaccine development. Here, we targeted ovalbumin (OVA) to B cells via CD19 and found that a single low dose of anti–CD19-OVA conjugates, but not isotype mAb-OVA, stimulated augmented CD4 and CD8 T-cell proliferation and expansion. Administration of TLR9 agonist CpG could significantly enhance long-term T-cell survival. Similar results were obtained when the tumor-associated Ag MUC1 was delivered to B cells. MUC1 transgenic (Tg) mice were previously found to lack effective T-cell help and produce low-titer of anti-MUC1 Abs after vaccination. Targeting MUC1 to B cells elicited high titer of anti-MUC1 Abs with different isotypes, predominantly IgG2a and IgG2b, in MUC1 Tg mice. The isotype switching of anti-MUC1 Ab was CD4 dependent. In addition, IFN-γ–producing CD8 T cells and in vivo cytolytic activity were significantly increased in these mice. The mice also showed significant resistance to MUC1+ lymphoma cell challenge both in the prophylactic and therapeutic settings. We conclude that Ags targeting to B cells stimulate CD4 and CD8 T-cell responses as well as Th-dependent humoral immune responses.
Introduction
The enormous advantage of cancer immunotherapy as a targeted tumor therapy rests on the specificity of antibodies (Abs) and antigen (Ag)–specific T cells, which presumably allow the immune system to distinguish cancerous cells from normal cells. Among most cancer immunotherapies, dendritic cell (DC)–based tumor vaccines have achieved considerable success in preclinical animal studies.1-3 DCs are potent, professional Ag-presenting cells (APCs) capable of priming and activating naive T cells.4 However, difficulties generating sufficient quantities of DCs for clinical use and identifying the optimal DC subtypes for tumor vaccines have hampered this strategy.5,6 In addition, T cell–based therapy has not met clinical expectation in the oncology field.7 In contrast, antitumor monoclonal Ab (mAb) therapy has reached fruition as an established standard care for cancer therapy.8 Therefore, it would be desirable if tumor vaccines could elicit antitumor T-cell responses as well as humoral responses
B cells are conventionally considered to be Ab-secreting cells; however, they can also serve as potent APCs to prime both Th1 and Th2 cells.9,10 A recent study demonstrates that B cells can cross-present Ags via MHC class I to naive CD8 T cells, thus activating CD8 T cells.11 Interestingly, previous studies also suggest that B cells can induce T-cell tolerance.12,13 The distinct T-cell outcomes may lie in the differential activation status of B cells14 as well as the Ag specificity of B cells.15 Studies from autoimmune diseases demonstrate that Ag-specific autoreactive B cells are also very important autoAg-presenting cells that prime and activate autoreactive T cells and break T-cell tolerance in autoimmunity.16-19 Indeed, anti-CD20 Ab B-cell depletion therapy in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) patients reveals that autoAb levels and T-cell activation were significantly decreased.20-22 B cells can be activated to secrete Abs and can also serve as APCs to prime T cells. As such, there is considerable interest in developing B cell–based tumor vaccines, such as a CD40-activated B-cell vaccine. Studies demonstrate that CD40-activated B cells express all the necessary machineries such as important T-cell attractants to induce T-cell chemotaxis and homing to secondary lymphoid organs.23 However, this strategy has not been tested in the immune-tolerant host, where its immune-suppressive network is dominant over effector mechanisms.
Immune regulation in the tumor-bearing host is a very complex network in which the tumor uses numerous active mechanisms to suppress host immunity.24,25 In addition, most tumor-associated Ags (TAAs) are characterized as nonmutated self-proteins, which pose the challenge of breaking immune tolerance to these TAAs. The epithelial mucin MUC1 is a large membrane glycoprotein that is overexpressed on a broad range of adenocarcinomas, including colon, cervical, breast, gastric, lung, and prostate carcinoma.26-28 In addition, studies have shown that the glycoforms of MUC1 expressed on the apical surface of normal ductal epithelia versus adenocarcinoma are different, and anti-MUC1 Ab and T cells can recognize the tumor-specific MUC1 glycoform.29-31 Previous preclinical studies demonstrated that robust anti-MUC1 responses could be elicited in wild-type mice.32 However, significantly reduced responses occurred in MUC1-transgenic (Tg) mice in which both T cells and B cells were tolerant to MUC1.32-34 It appears that a Th/T-regulatory cell imbalance in MUC1 Tg mice causes ineffective anti-MUC1 responses.35 In MUC1-based vaccine clinical trials in cancer patients, only weak cytotoxic T lymphocyte (CTL) responses and low-titer Abs, predominantly IgM, were elicited.36-38 These studies suggest that effective T-cell help is lacking in both MUC1 Tg mice and cancer patients.
Our previous study has shown that targeting Ag to B cells via CD19 could induce robust CD4 T-cell responses.39 In the current study, we found that this strategy elicits not only mounted CD4 T-cell responses but also CD8 T-cell responses. More importantly, high titers of anti-MUC1 Abs with different isotypes and MUC1-specific T-cell responses were elicited in MUC1 Tg mice with this B cell–based vaccine. These augmented anti-MUC1 responses could significantly prevent tumor progression in both prophylactic and therapeutic settings and achieve significant long-term, tumor-free survival.
Methods
Mice and tumor cell line
C57Bl/6, CD45.1+ B6-SJL, ovalbumin (OVA) T-cell receptor (TCR) Tg OT-I, and OT-II Rag-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Human MUC1 Tg mice on a C57Bl/6 background have been obtained from Dr S. Gendler (Mayo Clinic, Scottsdale, AZ). Polymerase chain reaction (PCR) was performed routinely to identify MUC1 Tg-positive mice in the colony.34 All experimental mice were housed under specific pathogen-free conditions in the animal facility of University of Louisville and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville.
Murine lymphoma RAM cells were stably transfected with a full-length human MUC1 cDNA (generously provided by Dr Olivera Finn, University of Pittsburgh, PA) as described previously.32 Cells were maintained in DMEM supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 μg/mL G418.
Abs, H-2Kb OVA pentamer, and MUC1 peptide
Fluorochrome-conjugated mAbs, including CD4, CD8, CD45.2, and IFN-γ, were purchased from eBioscience (San Diego, CA). H-2Kb/SIINFEKL (OVA 257-264)-R-PE MHC pentamer was purchased from ProImmune (Bradenton, FL). The 100-mer human MUC1 peptide was synthesized by the solid phase on a Pioneer peptide synthesizer (Applied Biosystems, Foster City, CA) using a FMOC synthesis protocol in the Peptide Synthesis Facility, University of Pittsburgh. The MUC1 100-mer peptide is corresponding to 5 tandem repeats of a 20–amino acid (AA) sequence from the extracellular tandem repeat domain of human MUC1. The AA sequence of one repeat is GVTSAPDTRPAPGSTAPPAH.
Conjugation of OVA or MUC1 to anti-CD19 mAb
Rat antimouse CD19 mAb (IgG2a) or isotype control mAb (rat IgG2a; eBioscience) was conjugated to LPS-free OVA (Pierce, Rockford, IL) or MUC1 100-mer peptide that had been activated with succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC; Pierce) according to the manufacturer's protocol. In brief, the mAb was reduced in 20 mM dithiothreitol (DTT; Bio-Rad, Hercules, CA) at room temperature for 30 minutes and separated from the reducing agent over a desalting column. The activated OVA or MUC1 peptide was then mixed with the reduced mAb for 1 hour at room temperature and then incubated overnight at 4°C. The unconjugated anti-CD19 mAb or free OVA or MUC1 peptide was removed by size-exclusive column. The conjugates were examined for B-cell binding as assessed by flow cytometry.
In vivo T-cell proliferation assay
OT-I (CD8+) or OT-II (CD4+) T cells purified from splenocytes of OT-I or OT-II Tg mice were labeled with 10 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Carlsbad, CA) for 10 minutes at 37°C as previously described.39 T cells (106/mouse) were then adoptively transferred into recipient mice. Isotype mAb-OVA or anti–CD19-OVA conjugates were injected into mice intravenously 24 hours after adoptive transfer. Recipient mice were killed at the indicated time points and the turnover of T cells from splenic cells or lymph node cells was examined by flow cytometry.
Flow cytometry
A single-cell suspension was prepared from spleens or lymph nodes. The cells were incubated with anti-CD16/CD32 Fc receptor blocker for 20 minutes on ice and then washed and stained with indicated fluorochrome-conjugated mAbs. Cells were collected with a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Intracellular IFN-γ staining
Intracellular cytokine staining was performed using BD Cytofix cytoperm kit with BD Golgiplug (BD Pharmingen, San Diego, CA) according to the manufacturer's protocol. The cells were stimulated with 10 μg/mL OVA or 20 μg/mL MUC1 peptide in the presence of Golgiplug and then stained with PE-Cy5–conjugated mAb against CD8 (clone 53-6.7; eBioscience) and PE-conjugated IFN-γ (clone XMG1.2; eBioscience).
Mouse immunization and tumor challenge
Six- to 8-week-old MUC1 Tg mice were immunized intravenously with anti–CD19-MUC1 conjugates or isotype mAb-MUC1 conjugates with or without CpG oligodeoxyribonucleotide (CpG ODN) 1826, TCCATGACGTTCCTGACGTT (Qiagen, Valencia, CA), at 50 μg/injection on days 0 and 14. A group of mice immunized with PBS was used as control. On day 21, the sera were collected for MUC1 Ab measurement. For tumor therapy in the prophylactic setting, immunized mice were then challenged by subcutaneous injection in the flank with 5 × 104 syngeneic RAM-MUC1 lymphoma cells. In the therapeutic setting, MUC1 Tg mice were first challenged with 5 × 105 syngeneic RAM-MUC1 lymphoma cells for 7 days and then immunized twice with different regimens as described in this section at 1-week intervals. Tumor diameter was measured by caliper twice per week. Mice were killed when tumors reached 15 mm in diameter. In some experiments, survival was monitored up to 50 or 100 days beyond tumor implantation.
Detection of anti-MUC1 Abs by enzyme-linked immunosorbent assay
Ninety-six-well plates were coated with MUC1 peptide overnight at 4°C and blocked by incubation with 0.5% BSA. Mouse sera were diluted 1:20 in PBS and applied to wells, followed by goat anti–mouse IgM, IgG1, IgG2a and IgG2b horseradish peroxidase (HRP) conjugates (Southern Biotech, Birmingham, AL) as the secondary Ab. The assays were subsequently developed by the addition of ABTS 1 Component Microwell Substrate (BioFX Laboratories, Owings Mills, MD) and the OD405 was determined. Ab concentrations were determined by generating a standard curve from serial dilution of the purified anti-MUC1 mAb BCP8.
In vivo cytotoxicity assay
MUC1-loaded or -unloaded purified B cells were used as target cells for in vivo cytotoxicity assay. In brief, splenic B cells were purified by negative selection with CD43 magnetic beads (Miltenyi Biotech, Auburn, CA). A fraction of B cells was pulsed with anti–CD19-MUC1 conjugates and then labeled with 10 μM CFSE (CFSEhigh). Uncoated B cells were labeled with 1 μM CFSE (CFSElow). The mixed B cells (2 × 106), at a ratio of 1:1, were adoptively transferred into the immunized MUC1 Tg mice. Mice were killed after 24 hours of target cell transfer. Specific cytotoxicity was determined by detecting the differentially labeled fluorescent target cell populations by flow cytometry. The percentage of cytotoxicity was determined as follows: (1-CFSEhigh/CFSElow) × 100%.
Statistical analysis
Unpaired t test analysis was used to determine whether the differences between T cell–mediated immune responses induced by anti–CD19-OVA/MUC1 conjugates versus isotype mAb-OVA/MUC1 conjugates were significant. In comparing statistical significance among multiple groups, one-way ANOVA was used. A 2-way ANOVA and Kaplan-Meier survival analysis were used to determine significant difference in an in vivo tumor therapy. P values less than .05 were considered significant.
Results
Anti–CD19-OVA conjugates stimulate augmented Ag-specific CD4 and CD8 T-cell proliferation
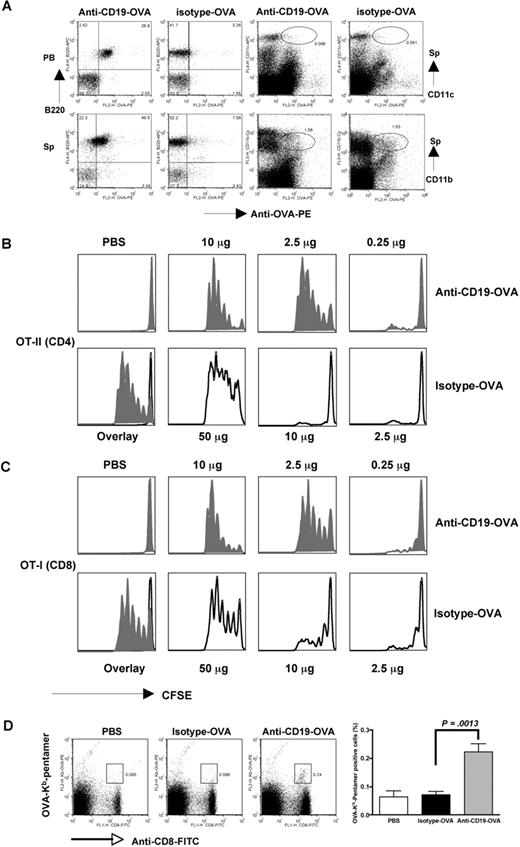
To determine whether targeting Ag to B cells could elicit both mounted CD4 and CD8 T-cell proliferation, we first chemically linked surrogate Ag OVA to anti-CD19 as a means to target the Ag to B lymphocytes for Ag presentation. In vivo targeting assay demonstrated that anti–CD19-OVA conjugates specifically bound to B cells but not DCs or macrophages (Figure 1A). Isotype mAb-OVA conjugates did not bind to any cell subsets, suggesting that anti–CD19-OVA specifically targets to B cells. For in vitro Ag presentation assay, OT-II CD4 T cells were used as a readout of OVA peptide presentation. Indeed, B cells with OVA conjugated to anti-CD19 stimulated responder CD4+ T cells more than 100-fold more efficiently compared with OVA protein alone or isotype mAb-OVA conjugates (data not shown). Next, we examined whether this strategy would lead to enhanced T-cell proliferation in vivo. To this end, mice were administered intravenously with106 CFSE-labeled naive OT-I or OT-II cells. The next day, mice were injected with anti–CD19-OVA conjugates, isotype mAb-OVA conjugates, soluble OVA, or anti-CD19 mAb. As additional controls, PBS or anti-CD19 mixed with OVA (without conjugation) was injected into another group of mice. Splenic cells or lymph node cells were obtained 3 days later. As shown in Figure 1B and C, both CD4 and CD8 T cells, respectively, underwent at least 5 divisions within the first 3 days of exposure to anti-CD19–conjugated Ag (2.5 μg/per mouse) in vivo. By contrast, OT-I or OT-II T cells did not respond to the same amount of isotype mAb-OVA conjugates or other controls (data not shown). A significant proliferation occurred when much higher isotype-OVA Ag (50 μg/mouse) was used. To further test whether we could prime the endogenous naive repertoire that contains low frequency of Ag-specific T cells, we used H-2Kb-OVA/SIINFEKL pentamer staining to track OVA-specific CD8 T cells after immunization. As shown in Figure 1D, CD8 T cells from unimmunized or isotype-OVA conjugate–immunized mice had almost no binding to pentamer, whereas CD8 T cells from mice immunized with anti–CD19-OVA conjugates had significant binding to OVA/SIINFEKL pentamer. These results indicate that direct in vivo delivery of protein Ag to B cells via CD19 markedly enhances the efficiency of Ag presentation leading to the augmented Ag-specific CD4 and CD8 T-cell responses.
In vivo targeting of conjugates and T-cell proliferation mediated by anti–CD19-OVA or isotype mAb-OVA conjugates. (A) Anti–CD19-OVA or isotype mAb-OVA conjugates (10 μg) were intravenously injected into mice. Peripheral blood (PB) was drawn at 30 minutes after injection. Cells were stained with B220 and anti-OVA Ab. Mice were then killed after 2 hours of injection. Splenocytes (Sp's) were stained with B220, CD11c, CD11b, and anti-OVA Ab. Data suggest that anti–CD19-OVA conjugates predominately bind to B cells. For in vivo T-cell proliferation assay, 106 CFSE-labeled naive CD4 OT-II T cells (B) or CD8 OT-I T cells (C) were intravenously adoptively transferred into naive C57Bl/6 mice. The next day, mice were injected with a single dose of anti–CD19-OVA or isotype mAb-OVA conjugates or PBS. Recipient mice were killed after 3 days and turnover of T cells from splenic cells was examined by flow cytometry. Cells were gated on CFSE-positive population. T-cell proliferation shown in overlay histogram was from PBS, 2.5 μg isotype-OVA, or 2.5 μg anti–CD19-OVA. (D) Naive C57Bl/6 mice were immunized with isotype mAb-OVA or anti–CD19-OVA conjugates (10 μg). Mice injected with PBS were used as controls. Five days after immunization, mice were killed and splenocytes were stained with anti-CD8, anti-CD19, and H-2Kb-OVA/SIINFEKL pentamer. Cells were gated on CD19− lymphocytes. Representative of 3 or more experiments. Error bars represent SD.
In vivo targeting of conjugates and T-cell proliferation mediated by anti–CD19-OVA or isotype mAb-OVA conjugates. (A) Anti–CD19-OVA or isotype mAb-OVA conjugates (10 μg) were intravenously injected into mice. Peripheral blood (PB) was drawn at 30 minutes after injection. Cells were stained with B220 and anti-OVA Ab. Mice were then killed after 2 hours of injection. Splenocytes (Sp's) were stained with B220, CD11c, CD11b, and anti-OVA Ab. Data suggest that anti–CD19-OVA conjugates predominately bind to B cells. For in vivo T-cell proliferation assay, 106 CFSE-labeled naive CD4 OT-II T cells (B) or CD8 OT-I T cells (C) were intravenously adoptively transferred into naive C57Bl/6 mice. The next day, mice were injected with a single dose of anti–CD19-OVA or isotype mAb-OVA conjugates or PBS. Recipient mice were killed after 3 days and turnover of T cells from splenic cells was examined by flow cytometry. Cells were gated on CFSE-positive population. T-cell proliferation shown in overlay histogram was from PBS, 2.5 μg isotype-OVA, or 2.5 μg anti–CD19-OVA. (D) Naive C57Bl/6 mice were immunized with isotype mAb-OVA or anti–CD19-OVA conjugates (10 μg). Mice injected with PBS were used as controls. Five days after immunization, mice were killed and splenocytes were stained with anti-CD8, anti-CD19, and H-2Kb-OVA/SIINFEKL pentamer. Cells were gated on CD19− lymphocytes. Representative of 3 or more experiments. Error bars represent SD.
The expanded Ag-specific T cells are not deleted or anergized in vivo
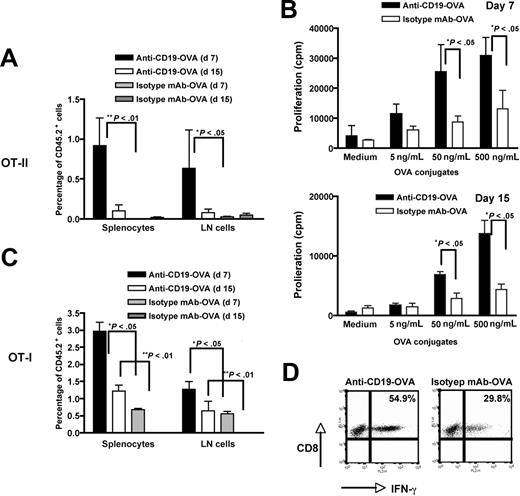
To further determine the frequency of Ag-specific T cells and their in vivo function, naive OT-I and OT-II T cells (CD45.2+) were administrated into SJL mice (CD45.1+). The next day, 2.5 μg anti–CD19-OVA conjugates or 50 μg isotype mAb-OVA alone was intravenously injected. Splenocytes or lymph node cells were harvested at day 7 or day 15, and the percentage of CD45.2+ cells was determined by flow cytometry. As shown in Figure 2A, the percentage of adoptively transferred OVA CD4 T cells at day 7 was significantly higher in anti–CD19-OVA conjugate–immunized mice compared with that in isotype mAb-OVA–immunized mice. Even on day 15, OVA CD4 T cells were still detectable in anti–D19-OVA–immunized mice, although at a lower level. Importantly, these expanded T cells were not anergized; the T cells were proliferated vigorously in response to OVA Ag restimulation (Figure 2B). Similarly, the percentage of adoptively transferred OVA CD8 T cells was also significantly higher both at day 7 and day 15 in anti–CD19-OVA conjugate–immunized mice versus mice immunized with isotype mAb-OVA, despite the OVA concentration in these mice being 25-fold higher (Figure 2C). In addition, CD8 T cells produced abundant amounts of IFN-γ (Figure 2D), suggesting that these T cells are functionally activated. Taken together, these data indicate that expanded Ag-specific T cells elicited by anti–CD19-OVA conjugates are not deleted or anergized in vivo and possess full function in response to Ag stimulation.
The frequency of in vivo expanded Ag-specific T cells and their functional activity. Three million naive CD4 OT-II (A) or CD8 OT-I (C) T cells (CD45.2+) were administrated intravenously into SJL mice (CD45.1+). The next day, recipient mice were injected with 2.5 μg anti–CD19-OVA conjugates or 50 μg isotype mAb-OVA. Splenocytes or lymph node cells were harvested at day 7 or day 15, and the percentage of CD45.2+ cells was enumerated by flow cytometry. Cells were gated on the CD4+ or CD8+ population. Splenocytes from mice receiving CD4 OT-II cells were harvested at day 7 or day 15 (B) and restimulated with various amounts of anti–CD19-OVA conjugates for 60 hours. Proliferation was measured by 3H-thymidine incorporation. Splenocytes from mice receiving CD8 OT-I cells were harvested at day 7 and restimulated with 10 μg/mL OVA overnight (D). The cells were stained for intracellular IFN-γ. Cells were gated on the CD8+CD45.2+ population. Mean plus or minus SE is shown (n = 3).
The frequency of in vivo expanded Ag-specific T cells and their functional activity. Three million naive CD4 OT-II (A) or CD8 OT-I (C) T cells (CD45.2+) were administrated intravenously into SJL mice (CD45.1+). The next day, recipient mice were injected with 2.5 μg anti–CD19-OVA conjugates or 50 μg isotype mAb-OVA. Splenocytes or lymph node cells were harvested at day 7 or day 15, and the percentage of CD45.2+ cells was enumerated by flow cytometry. Cells were gated on the CD4+ or CD8+ population. Splenocytes from mice receiving CD4 OT-II cells were harvested at day 7 or day 15 (B) and restimulated with various amounts of anti–CD19-OVA conjugates for 60 hours. Proliferation was measured by 3H-thymidine incorporation. Splenocytes from mice receiving CD8 OT-I cells were harvested at day 7 and restimulated with 10 μg/mL OVA overnight (D). The cells were stained for intracellular IFN-γ. Cells were gated on the CD8+CD45.2+ population. Mean plus or minus SE is shown (n = 3).
TLR9 agonist CpG ODN enhances Ag-specific T-cell survival and activation
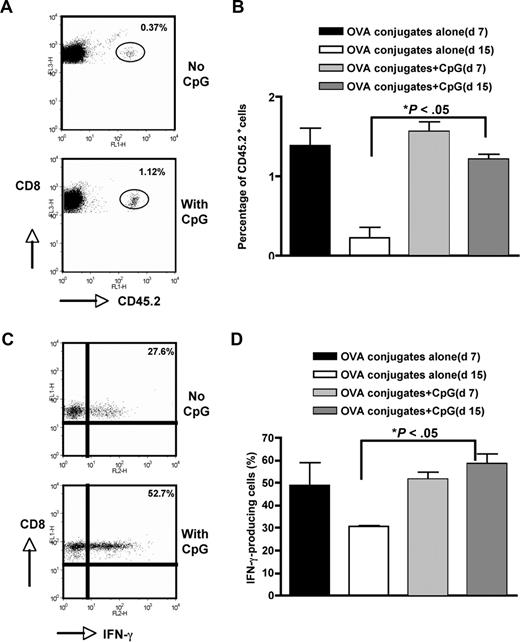
We showed that low dose of anti–CD19-OVA conjugates could significantly expand Ag-specific T cells in a short term (day 7). However, the long-term survival of Ag-specific T cells was not enhanced dramatically, particularly in CD4 T cells. This is not surprising, since after T-cell expansion most effector T cells undergo activation-induced cell death (ACID) to maintain homeostasis. Toll-like receptor 9 (TLR9) agonist CpG ODN is a potent adjuvant to stimulate activation of B cells, DCs, and macrophages both in vitro and in vivo. CpG ODN can also induce B-cell proliferation, Ig isotype switching, costimulatory molecule expression, and cytokine secretion.40,41 Therefore, we tested whether anti–CD19-OVA conjugates in combination with CpG ODN could significantly increase long-term Ag-specific T-cell survival. To this end, naive OT-I T cells (CD45.2+) were administrated into SJL mice (CD45.1+). The next day, a single dose of anti–CD19-OVA conjugates (2.5 μg) was injected intravenously with or without CpG ODN. As shown in Figure 3, the frequency of OVA CD8 T cells at day 7 was not significantly higher in CpG-immunized mice. However, the percentage of OVA CD8 T cells at day 15 was significantly increased in these mice, with the level equivalent to that at day 7. In addition, CpG could significantly increase IFN-γ–producing CD8 T cells at day 15 (Figure 3C,D). Similar dynamics were observed with CD4 OT-II T cells (data not shown). Therefore, Ag targeting to B cells via CD19 coupled with CpG adjuvant greatly increases long-term Ag-specific T-cell survival.
Prolonged T-cell survival by CpG. Similar to Figure 2C, 2 million CD8 OT-I T cells were administrated to SJL mice. Mice were immunized with 2.5 μg anti–CD19-OVA conjugates in the presence or absence of CpG (50 μg/mouse). Splenocytes were harvested at day 7 or day 15 and the percentage of CD45.2+ cells was determined by flow cytometry. Cells were gated on the CD8+ population. (A) Representative dot plots showing CD8+CD45.2+ cells at day 15. (B) Percentage of CD8+CD45.2+ cells (n = 5). (C) Splenocytes harvested on day 15 were restimulated with OVA (10 μg/mL) and intracellular IFN-γ staining was performed. Representative dot plots showing IFN-γ staining. Cells were gated on the CD8+CD45.2+ population. (D) Percentage of IFN-γ–producing CD8+ T cells. Cells were gated on the CD8+CD45.2+ population (n = 5). Error bars represent SEM.
Prolonged T-cell survival by CpG. Similar to Figure 2C, 2 million CD8 OT-I T cells were administrated to SJL mice. Mice were immunized with 2.5 μg anti–CD19-OVA conjugates in the presence or absence of CpG (50 μg/mouse). Splenocytes were harvested at day 7 or day 15 and the percentage of CD45.2+ cells was determined by flow cytometry. Cells were gated on the CD8+ population. (A) Representative dot plots showing CD8+CD45.2+ cells at day 15. (B) Percentage of CD8+CD45.2+ cells (n = 5). (C) Splenocytes harvested on day 15 were restimulated with OVA (10 μg/mL) and intracellular IFN-γ staining was performed. Representative dot plots showing IFN-γ staining. Cells were gated on the CD8+CD45.2+ population. (D) Percentage of IFN-γ–producing CD8+ T cells. Cells were gated on the CD8+CD45.2+ population (n = 5). Error bars represent SEM.
Targeting TAA MUC 1 to B cells via CD19 elicits large amounts of anti-MUC1 Ab with different isotypes and T-cell responses in MUC1 Tg mice
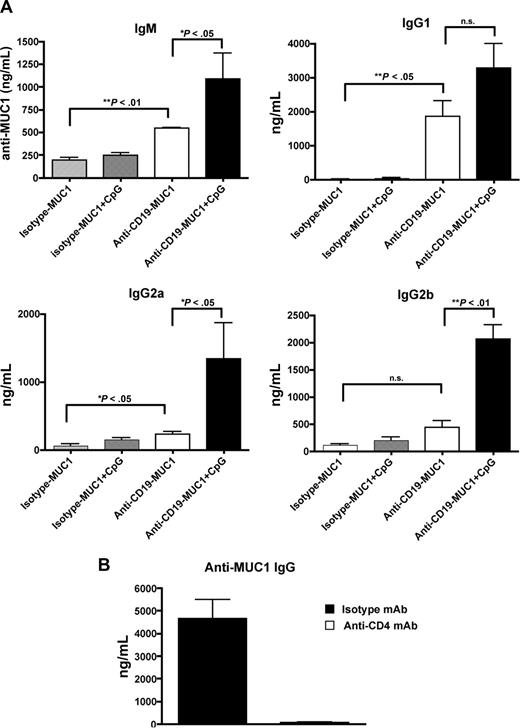
MUC1 Tg mice were previously shown to lack a critical T-cell help and elicit minimal levels of anti-MUC1 Ab levels (predominantly IgM) in the DC-based MUC1 vaccine.32,35 To test whether targeting MUC1 Ag to B cells can break T- and B-cell tolerance in MUC1 Tg mice, mice were immunized with anti–CD19-MUC1 conjugates with or without CpG ODN as an adjuvant. MUC1 peptide without conjugation or isotype mAb-MUC1 peptide was used as control. Anti–CD19-MUC1 conjugates readily bound to B cells, but not DCs or macrophages, both in vivo and in vitro (data not shown). As depicted in Figure 4, MUC1 Tg mice vaccinated with anti–CD19-MUC1 conjugates with CpG secreted large amounts of anti-MUC1 Abs with different subclasses and isotypes, predominately IgM, IgG1, IgG2a, and IgG2b. CpG adjuvant significantly increased anti-MUC1 Ab levels, particularly IgG2a and IgG2b. It was worth noting that mice vaccinated with anti–CD19-MUC1 conjugates alone also secreted higher levels of anti-MUC1 Abs compared with isotype mAb-MUC1–immunized mice. However, MUC1 peptide alone or isotype mAb-MUC1 did not elicit any appreciable level of anti-MUC1 Abs. To determine whether the anti-MUC1 Abs elicited by the synthetic MUC1 peptide were also capable of recognizing the native MUC1 molecule on tumor cells, we stained full-length MUC1-transfected RAM-MUC1 cells with immune serum. We found that the immune serum could specifically bind to RAM-MUC1 cells but not parental RMA cells, which indicates MUC1 protein–specific immune reactivity (data not shown).
MUC1-specific Abs elicited in MUC1 Tg mice. (A) MUC1 Tg mice (n = 5) were immunized intravenously with isotype mAb-MUC1 or anti–CD19-MUC1 conjugates (50 μg per mouse) with or without CpG (50 μg), 2 times at a 2-week interval. Unimmunized mice were used as controls. Mice were bled at day 21 and the sera were measured for MUC1-specific Abs with different isotype by enzyme-linked immunosorbent assay (ELISA). (B) MUC1 Tg mice were first injected with anti-CD4 mAb (250 μg, intraperitoneally) or isotype control mAb on day −1. Mice were then immunized with anti–CD19-MUC1 plus CpG. The depletion mAb or isotype control mAb was further injected 1 day before boost immunization. Mice were bled at day 21 and the sera were measured for IgG MUC1-specific Abs by ELISA. Data are representative of at least 3 experiments. Error bars show SEM.
MUC1-specific Abs elicited in MUC1 Tg mice. (A) MUC1 Tg mice (n = 5) were immunized intravenously with isotype mAb-MUC1 or anti–CD19-MUC1 conjugates (50 μg per mouse) with or without CpG (50 μg), 2 times at a 2-week interval. Unimmunized mice were used as controls. Mice were bled at day 21 and the sera were measured for MUC1-specific Abs with different isotype by enzyme-linked immunosorbent assay (ELISA). (B) MUC1 Tg mice were first injected with anti-CD4 mAb (250 μg, intraperitoneally) or isotype control mAb on day −1. Mice were then immunized with anti–CD19-MUC1 plus CpG. The depletion mAb or isotype control mAb was further injected 1 day before boost immunization. Mice were bled at day 21 and the sera were measured for IgG MUC1-specific Abs by ELISA. Data are representative of at least 3 experiments. Error bars show SEM.
Previous studies showed that CpG could regulate isotype switching through direct interaction with B cells rather than indirectly through Th help.42 To determine whether isotype switching elicited by targeting of MUC1 to B cells is Th dependent, CD4 T cells were first depleted before anti–CD19-MUC1 plus CpG immunization. Strikingly, no IgG anti-MUC1 Abs were detected in these mice (Figure 4B), suggesting that CD4 T cells are absolutely required for anti-MUC1 Ab isotype switching.
Next, we examined MUC1-specific CD8 T-cell responses in these immunized mice. As shown in Figure 5A, CD8 T cells from anti–CD19-MUC1 conjugate–immunized mice with or without CpG ODN secreted significant amounts of IFN-γ production. In contrast, minimal numbers of IFN-γ–producing CD8 T cells were found in isotype mAb-MUC1–immunized mice. To determine the cytolytic activity against MUC1-positive target cells, an in vivo cytotoxicity assay was performed. As shown in Figure 5C-D, anti–CD19-MUC1 conjugates in combination with CpG-immunized mice exhibited the highest cytolytic activity (mean = 66%) versus 40% cytotoxicity in mice immunized with anti–CD19-MUC1 conjugate alone (P < .05). However, when CD4 T cells were deleted before anti–CD19-MUC1 plus CpG immunization, the cytolytic activity was significantly decreased (Figure 5D). In addition, mice immunized with isotype-MUC1 with or without CpG ODN displayed limited cytolytic activity. These results indicate that targeting TAA MUC1 to B cells via CD19 could reverse immune tolerance to MUC1 and elicit MUC1-specific B-cell and T-cell responses.
Increased IFN-γ–producing cells and augmented in vivo cytolytic activity elicited by targeting of MUC1 to B cells in MUC1 Tg mice. MUC1 Tg mice (n = 3 or 4) were immunized with isotype-MUC1 or anti–CD19-MUC1 conjugates in the presence or absence of CpG adjuvant. Mice were immunized twice at a 2-week interval and were killed at day 21. One group of mice was first injected with anti-CD4 depleting mAb as described in Figure 4B before immunization. (A) Splenocytes from immunized mice were cultured with 20 μg/mL MUC1 peptide overnight and then stained for intracellular IFN-γ production. Representative dot plots are shown. Cells were gated on CD8+ T cells. (B) The percentage of IFN-γ–producing CD8+ T cells. Mean plus or minus SE is shown. ns indicates not significant. (C) In vivo cytotoxicity. CFSE-labeled syngeneic B cells pulsed with anti–CD19-MUC1 conjugates (CFSEhigh) or not (CFSElow) were injected intravenously into immunized mice. Mice were killed 24 hours after transfer and splenocytes were harvested and assessed by flow cytometry. Cells were gated on the CFSE-positive cells. Error bars show SEM. (D) Cytolytic activity in different regimen immunized mice. Data are representative of at least 3 experiments.
Increased IFN-γ–producing cells and augmented in vivo cytolytic activity elicited by targeting of MUC1 to B cells in MUC1 Tg mice. MUC1 Tg mice (n = 3 or 4) were immunized with isotype-MUC1 or anti–CD19-MUC1 conjugates in the presence or absence of CpG adjuvant. Mice were immunized twice at a 2-week interval and were killed at day 21. One group of mice was first injected with anti-CD4 depleting mAb as described in Figure 4B before immunization. (A) Splenocytes from immunized mice were cultured with 20 μg/mL MUC1 peptide overnight and then stained for intracellular IFN-γ production. Representative dot plots are shown. Cells were gated on CD8+ T cells. (B) The percentage of IFN-γ–producing CD8+ T cells. Mean plus or minus SE is shown. ns indicates not significant. (C) In vivo cytotoxicity. CFSE-labeled syngeneic B cells pulsed with anti–CD19-MUC1 conjugates (CFSEhigh) or not (CFSElow) were injected intravenously into immunized mice. Mice were killed 24 hours after transfer and splenocytes were harvested and assessed by flow cytometry. Cells were gated on the CFSE-positive cells. Error bars show SEM. (D) Cytolytic activity in different regimen immunized mice. Data are representative of at least 3 experiments.
Vaccination with anti–CD19-MUC1 conjugates plus CpG induces significant tumor protection and therapeutic efficacy in MUC1 Tg mice
Since augmented anti-MUC1 Ab responses as well as T-cell responses were elicited by anti–CD19-MUC1 conjugates in combination with CpG in MUC1 Tg mice, our next step was to determine whether antitumor immunity could be established by this new vaccination approach. For prophylactic experiments, MUC1 Tg mice were immunized twice at day 0 and day 14 with different regimens. Mice were then challenged with RAM-MUC1 lymphoma cells at day 21. As shown in Figure 6A, mice immunized with anti–CD19-MUC1 conjugates plus CpG ODN had a significantly delayed tumor progression compared with mice immunized with isotype mAb-MUC1 peptide with CpG ODN or PBS control mice. In addition, these immunized mice achieved approximately 50% greater long-term, tumor-free survival (Figure 6B). Furthermore, the protected mice were resistant to tumor rechallenge (data not shown). For therapeutic experiments, MUC1 Tg mice were first challenged with 5 × 105 RAM-MUC1 tumor cells. Seven days after tumor inoculation, MUC1 Tg mice were immunized with anti–CD19-MUC1 conjugates or isotype mAb-MUC1 in the presence of CpG twice at 1-week intervals. As shown in Figure 6C, the tumor-bearing mice treated with anti–CD19-MUC1 conjugates plus CpG had a significantly lower tumor burden compared with isotype-MUC1 plus CpG–immunized mice or PBS control mice. In addition, these mice achieved approximately 35% greater long-term, tumor-free survival at day 50.
Significantly reduced tumor burden and enhanced tumor-free survival after anti–CD19-MUC1 plus CpG immunization in MUC1 Tg mice. (A) MUC1 Tg mice were vaccinated with isotype mAb-MUC1 or anti–CD19-MUC1 conjugates in the presence or absence of CpG twice at a 2-week interval. PBS-immunized mice were used as control. At day 21, immunized mice were challenged with 5 × 104 RAM-MUC1 tumor cells subcutaneously. Tumor growth was recorded twice a week. (B) Data suggest that mice immunized with anti–CD19-MUC1 conjugates plus CpG achieved significant long-term tumor-free survival. (C) MUC1 Tg mice were inoculated subcutaneously with 5 × 105 RAM-MUC1 tumor cells. After 7 days, mice were immunized as described in panel A. Tumor growth was recorded twice a week. Error bars show SEM. (D) Long-term tumor-free survival at day 50.
Significantly reduced tumor burden and enhanced tumor-free survival after anti–CD19-MUC1 plus CpG immunization in MUC1 Tg mice. (A) MUC1 Tg mice were vaccinated with isotype mAb-MUC1 or anti–CD19-MUC1 conjugates in the presence or absence of CpG twice at a 2-week interval. PBS-immunized mice were used as control. At day 21, immunized mice were challenged with 5 × 104 RAM-MUC1 tumor cells subcutaneously. Tumor growth was recorded twice a week. (B) Data suggest that mice immunized with anti–CD19-MUC1 conjugates plus CpG achieved significant long-term tumor-free survival. (C) MUC1 Tg mice were inoculated subcutaneously with 5 × 105 RAM-MUC1 tumor cells. After 7 days, mice were immunized as described in panel A. Tumor growth was recorded twice a week. Error bars show SEM. (D) Long-term tumor-free survival at day 50.
Discussion
We recently showed that targeting Ag to B cells via CD19 potently induces CD4 T-cell activation both in vitro and in vivo.39 Here, we extended these findings by examining both Ag-specific CD4 and CD8 T-cell responses. Consistent with our previous findings, the OVA-specific CD4 T-cell response was significantly increased in anti–CD19-OVA conjugate–immunized mice with respect to that in isotype mAb-OVA–immunized counterparts both in vitro and in vivo. In addition, our study demonstrated for the first time that this strategy also elicited strong Ag-specific CD8 T-cell response. This enhancement of T-cell responses was not just transient, but also a long-term effect. Even on day 15, Ag-specific T cells were still detectable, although at lower levels. Further study suggested that TLR9 agonist CpG in conjunction with anti–CD19-OVA conjugates could significantly increase long-term survival of Ag-specific T cells. Of importance, these Ag-specific T cells were not anergized and had strong functional activity in response to Ag restimulation. These data suggest that targeting of Ag to B cells via CD19 could lead to more efficient Ag presentation and elicit potent CD4 and CD8 T-cell responses.
CD19 is a B cell–specific coreceptor expressed at almost every stage of B-cell development except after differentiation into plasma cells.43 It has been shown that CD19 can enhance B-cell receptor (BCR)–initiated signaling, particularly in a complex with CD21 (complement receptor 2 [CR2]).44 C3d-tagged Ags could bind to CD21, thus coligating BCR and CD19, which leads to the reduction of the B-cell activation threshold.45 In this case, CD19 functions as a membrane adaptor protein that recruits intracellular signaling molecules and activates phospholipase C-γ and the MAP kinase pathway. CD19 can also regulate B cells independently of other proteins on the surface,46 indicating that it may have other unique binding and/or signaling activity potential. A recent study also demonstrates the necessity of CD19 in B-cell activation through its stimulation of BCR membrane–bound Ag microcluster formation.47 Here, we showed that CD19 is important for B-cell Ag presentation. Targeting of Ags to B cells via CD19 led to more efficient Ag presentation by B cells. The binding appears to be specific for B cells since the anti-CD19 conjugates did not bind to DCs or macrophages in vivo, indicating that the binding is not through Fc receptors. The coligation of CD19 and BCR by this complex could potently activate B cells and present (or cross-present) Ags, thereby eliciting mounted Ag-specific T-cell responses. This notion is supported by the findings that anti–CD19-OVA conjugates specifically bind to B cells and subsequently cross-present SIINFEKL epitope, as readout in vitro (Figure 1D). However, it is still possible that B-cell activation mediated by anti–CD19-Ag conjugates renders B cells to undergo apoptosis and thus allows host DCs to present Ag through phagocytosis of apoptotic B cells or alternatively B cells can transfer Ag to DCs.31 Furthermore, CD19 could serve as an endocytosis receptor, such as DEC-205 expressed on DCs. Indeed, targeting Ags to mature DCs via DEC-205 elicits potent T-cell responses as well as Th-dependent Ab responses.48 Nevertheless, our data suggest that anti–CD19-Ag complex could specifically target Ag to B cells for efficient Ag presentation, thus leading to potent T-cell activation.
Although targeting of OVA Ag to B cells demonstrates the proof of concept, further testing was conducted with TAA MUC1 in MUC1 Tg mice where both T and B cells were tolerant to MUC1 Ag.34 Previous clinical trials with MUC1 peptide–based vaccines suggest that low titer of anti-MUC1 Ab with predominant IgM was elicited in cancer patients.36 Similar observations occurred in the MUC1 Tg mice.35 This ineffectiveness is seemingly ascribed to the lack of MUC1-specific Th responses. Therefore, we tested this strategy in the MUC1 Tg mouse setting to determine whether T- and B-cell tolerances to MUC1 could be broken. Strikingly, high titers of anti-MUC1 Abs with IgM, IgG1, IgG2a, and IgG2b were elicited in MUC1 Tg mice with this vaccination approach. CpG adjuvant significantly increased anti-MUC1 Ab levels, particularly IgG2a and IgG2b. The isotype switching of anti-MUC1 Abs suggests that MUC1-specific T-cell help is functional. It is possible that coligation of BCR and CD19 may be sufficient for B-cell activation and isotype switching. Indeed, TLR9 can regulate isotype switching directly by interacting with B cells rather than indirectly by inducing Th1 responses.42 However, no IgG anti-MUC1 Abs were elicited after depletion of CD4 T cells before immunization (Figure 4B), strongly suggesting that T-cell help is essential for anti-MUC1 Ab isotype switching. Using this strategy, we also demonstrated that IFN-γ–producing CD8 T cells were significantly increased in anti–CD19-MUC1 conjugate–immunized mice. However, CpG adjuvant did not significantly increase IFN-γ–producing cells. In addition, MUC1 Tg mice immunized with anti–CD19-MUC1 conjugates in either the presence or absence of CpG achieved significant cytolytic activity against target cells loaded with MUC1 peptide. The in vivo cytotoxicity was greatly enhanced when CpG adjuvant was used simultaneously. It appears that both T cell–mediated cytotoxicity and Ab-mediated cytotoxicity are contributed to the in vivo cytolytic activity elicited by this vaccine strategy. In CD4-depleted anti–CD19-MUC1 conjugate–immunized MUC1 Tg mice, no IgG anti-MUC1 Abs were elicited. Accordingly, significantly lower levels of cytotoxicity were observed in these mice compared with CD4-intact MUC1 Tg mice immunized with the same regimen (Figure 5). These data suggest that CD4 T-cell help indeed is critical for breaking tolerance in MUC1 Tg mice.
Compared with DC-based MUC1 peptide vaccines,30,32,49,50 the unique feature of this approach is the elicitation of high titers of anti-MUC1 Abs with different isotypes. These Abs appear to be protective for MUC1-positive tumor challenge. In both prophylactic and therapeutic settings, significant delayed tumor progression and long-term tumor-free survival were achieved when MUC1 Tg mice were immunized with anti–CD19-MUC1 in conjunction with CpG. Surprisingly, anti–CD19-MUC1 immunization alone induced similar levels of IFN-γ–producing CD8 T cells, and lower but significant amounts of anti-MUC1 Ab production. This also reduced tumor burden but did not reach statistical significance compared with untreated or isotype mAb-MUC1–immunized animals. This may be due to lower titer of anti-MUC1 Abs and lower levels of in vivo cytolytic activity compared with those elicited by anti–CD19-MUC1 conjugates with CpG adjuvant. It is worth noting that although high titers of anti-MUC1 Ab production and T-cell responses were elicited in MUC1 Tg mice, no apparent signs of autoimmunity in MUC1-expressing tissues were detected, including lung, pancreas, liver, and mammary duct (data not shown). This would suggest that the MUC1-specific immune responses did not cause autoimmune damage to normal MUC1-expressing tissues.
In summary, we have demonstrated that targeting of Ags to B cells elicits robust Ag-specific T-cell responses as well as Ab production both in naive mice and in an immunotolerant host. Given the importance and considerable interest in MUC1 as a targeted therapy, this strategy may open a new avenue for developing the MUC1 vaccine for cancer treatment. This may also be critical for pathogen vaccine development because both cellular and humoral immunity are required for optimal vaccination.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from National Institutes of Health (Bethesda, MD) RO1 CA86412 and the Kentucky Lung Cancer Research Board (Louisville, KY). J.Y. is a recipient of an Investigator Award from the American College of Rheumatology and Arthritis Foundation (Atlanta, GA).
National Institutes of Health
Authorship
Contribution: C.D. helped design experiments, performed experiments, analyzed data, and wrote the paper; L.W. and J.M. performed supporting experiments; and J.Y. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Yan, Tumor Immunobiology Program, James Graham Brown Cancer Center, Delia D. Baxter Research Building, Room 119A, University of Louisville, 580 South Preston Street, Louisville, KY 40202; e-mail: jun.yan@louisville.edu.