Abstract
Cancer patients are at high risk for venous thromboembolism (VTE). Laboratory parameters with a predictive value for VTE could help stratify patients into high- or low-risk groups. The cell adhesion molecule P-selectin was recently identified as risk factor for VTE. To investigate soluble P-selectin (sP-selectin) in cancer patients as risk predictor for VTE, we performed a prospective cohort study of 687 cancer patients and followed them for a median (IQR) of 415 (221-722) days. Main tumor entities were malignancies of the breast (n = 125), lung (n = 86), gastrointestinal tract (n = 130), pancreas (n = 42), kidney (n = 19), prostate (n = 72), and brain (n = 80); 91 had hematologic malignancies; 42 had other tumors. VTE occurred in 44 (6.4%) patients. In multivariable analysis, elevated sP-selectin (cutoff level, 53.1 ng/mL, 75th percentile of study population) was a statistically significant risk factor for VTE after adjustment for age, sex, surgery, chemotherapy, and radiotherapy (hazard ratio = 2.6, 95% confidence interval, 1.4-4.9, P = .003). The cumulative probability of VTE after 6 months was 11.9% in patients with sP-selectin above and 3.7% in those below the 75th percentile (P = .002). High sP-selectin plasma levels independently predict VTE in cancer patients. Measurement of sP-selectin at diagnosis of cancer could help identify patients at increased risk for VTE.
Introduction
Venous thromboembolism (VTE) is a well-recognized complication of cancer, which aggravates the clinical course and leads to additional morbidity and increased mortality of cancer patients.1-6
The pathogenesis of VTE in cancer patients appears to be multifactorial. The most important clinical determinants for the risk of VTE in patients with a malignant disease are the tumor site, tumor stage at the time of diagnosis, anticancer therapy (including chemotherapy, radiotherapy, and hormonal therapy) and surgery.7 A relevant role is attributed to the tumor cells' capacity to interact with and activate the hemostatic system.8-10 Activated coagulation again favors tumor angiogenesis, progression, growth, and metastasis.11-13
Accumulating data suggest that P-selectin, which is a member of the selectin family of cell adhesion molecules, might play an important role in the interrelation between cancer and thrombosis.7,14 P-selectin, which is found in the α granules of platelets and the Weibel-Palade bodies of endothelial cells and is expressed on the cell surface on activation, mediates the adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis.15 The interaction between P-selectin and its main counter-receptor P-selectin glycoprotein ligand-1 (PSGL-1), which is mainly present on leukocytes, triggers, in cooperation with other mediators, the release of procoagulant microparticles from leukocytes16 and supports fibrin formation and thrombus growth.17,18 Furthermore, P-selectin increases the expression of tissue factor on monocytes19 and was also reported to induce phosphatidylserine exposure and to increase surface-dependent thrombin generation on monocytes.20 Interestingly, cancer cells are able to enhance P-selectin expression on monocytes, macrophages, endothelial cells, and platelets.21 In addition, neoplastic cells express on their surface the ligand CD24, which was identified to be a receptor for P-selectin.22-24 The interaction of P-selectin with CD24 on neoplastic cancer cells allows their interaction with platelets and their adherence to endothelium in the process of metastatic spread.25
Recent studies have demonstrated that high plasma levels of soluble P-selectin (sP-selectin) are strongly associated with VTE.26-28 In a prospective cohort study, sP-selectin was also shown to be a risk factor for recurrent VTE.29 The clinical relevance of sP-selectin in cancer-associated VTE is yet unknown.
Because a remarkable number of patients with malignancy develop VTE during the course of their disease and because VTE has a negative impact on the prognosis, an effective thrombosis prophylaxis is desirable. However, the prophylaxis and treatment of VTE in cancer patients are challenging because of the high rates of bleeding complications.30-32 It would be a major advantage to have knowledge on predictive parameters for the development of VTE and to be able to select patients individually according to their risk profile. Therefore, we hypothesize that, by measurement of sP-selectin plasma levels, patients with cancer could be stratified into high- and low-risk categories for the occurrence of VTE. To test this hypothesis, we prospectively followed 687 patients with active cancer and assessed the relationship between sP-selectin plasma levels and the risk of VTE.26
Methods
Patients and study design
This study was approved by the ethics committee of the Medical University of Vienna, in accordance with the Declaration of Helsinki. The Vienna Cancer and Thrombosis Study (CATS) is an ongoing, prospective cohort study involving cancer patients from the Medical University of Vienna. The aim of the study is to investigate predictive parameters for the occurrence of VTE in cancer patients. Between October 2003 and May 2007, 812 patients with active malignant disease were enrolled. Every patient was informed of the details of the study in individual interviews, and all patients gave written informed consent. The inclusion criteria for the study were as follows: (1) patients with newly diagnosed cancer of the brain, breast, lung, upper or lower gastrointestinal tract, pancreas, kidney, prostate or gynecologic system; sarcoma; hematologic malignancies (myeloma, high- and low-grade lymphoma); or progression of disease after complete or partial remission; (2) histologic confirmation of diagnosis; (3) age more than 18 years; (4) willingness to participate; and (5) written informed consent. Exclusion criteria were overt bacterial or viral infection within the last 2 weeks, venous or arterial thromboembolism within the last 3 months, and continuous anticoagulation with vitamin K antagonists or low molecular weight heparin (LMWH). Patients were allowed to take aspirin, ticlopidine, or clopidogrel, and immobilized patients were treated with LMWH as thrombosis prophylaxis during their hospital stay. Further exclusion criteria were surgery or radiotherapy within the last 2 weeks and chemotherapy within the last 3 months to exclude a transient influence of these interventions on the hemostatic system. For inclusion in the study, all inclusion criteria had to be fulfilled; one exclusion criterion sufficed for exclusion. Patients underwent a structured interview on their medical history, and data on the tumor site, tumor histology, and tumor stage were documented, drawing on medical records and diagnostic findings. Patients were given detailed written information on symptoms of VTE and were asked to report immediately to our center if such symptoms occurred. A blood sample for determination of laboratory parameters was drawn.
The observation period started at the time of blood sampling. Patients were contacted every 3 months via postal mail or phone to get information about the clinical course of their disease regarding occurrence of VTE and anticancer treatment. If they did not respond, their family doctors or relatives were contacted. The observation period ended after a 2-year period, until the occurrence of VTE or death, loss of follow-up, or withdrawal of consent, whichever came first.
Of the 812 enrolled patients, 135 were excluded for the following reasons: 52 patients did not match the exact inclusion and exclusion criteria of the study after reevaluation (eg, long-term prophylaxis with LMWH or tumor not confirmed by histology), in 61 patients no complete information on follow-up, and in 12 patients no adequate material for laboratory testing was available.
Diagnosis of VTE
No routine screening for VTE was performed. When a patient presented with symptoms of VTE, objective methods were applied to confirm or exclude the diagnosis. Positive findings in duplex sonography or venography established the diagnosis of deep vein thrombosis (DVT) and positive findings in computerized tomography or ventilation/perfusion lung scan that of pulmonary embolism (PE), respectively. When patients had died, available death certificates and autopsy protocols were reviewed with regard to the presence of VTE. Whenever possible, physicians were contacted and interviewed that had cared for the patients shortly before their death. In patients who died of fatal PE, autopsy findings were used to establish the diagnosis. About once a year, an independent committee consisting of experts in the fields of angiology, radiology, and nuclear medicine met to discuss every single case of VTE and confirm the events. The members of this committee were informed of patients' medical history but unaware of laboratory results. Accidentally detected VTE (eg, PE detected in a routine computerized tomography) was counted as an event if the adjudication committee decided that it was of clinical significance and requested continuous anticoagulant treatment. When a patient died and an autopsy was performed, the written information, including autopsy protocols, if available, was reviewed with regard to the presence of VTE.
Outcome measures
The endpoint of the study was occurrence of VTE, either symptomatic or fatal VTE, confirmed by duplex sonography, venography, and/or computerized tomography or autopsy.
Blood sampling and laboratory analysis
Venous blood samples were drawn into plasma vacuum tubes (Vacuette; Greiner Bio One, Kremsmuenster, Austria) containing one-tenth volume sodium citrate stock solution at 0.129 mM by atraumatic and sterile antecubital venipuncture at the study entry. To obtain platelet-poor plasma, the citrated blood was centrifuged (ROTANTA/TRC; Hettich, Tuttlingen, Germany) at 1500g for 15 minutes, and to obtain platelet-free plasma a second centrifugation step (Eppendorf) at 13 400g for 2 minutes was performed. Plasma aliquots were stored at −80°C until they were assayed for the determination of sP-selectin plasma levels in series. Samples were coded before laboratory analysis. The technicians were unaware of the patients' characteristics at all times. sP-selectin levels were measured using a human sP-selectin Immunoassay (R&D Systems, Minneapolis, MN) following the manufacturer's instructions as described previously.26 To provide validation of the commercial assay the intraassay and interassay variability was estimated. The intraassay variability (10 determinations in 1 run) was 2.25% and the interassay variability 6.17% (1 determination in 10 runs).
Statistical analysis
Continuous variables were described with the median and the interquartile range (IQR). The median follow-up time was calculated with the reverse Kaplan-Meier method.33 The correlation between continuous variables is described by the Spearman correlation coefficient. The Kruskal-Wallis test was applied to test the equality of sP-selectin levels among the different tumor sites.
Univariate and multivariable Cox regression analyses were used for calculating the risk of VTE from study inclusion until first thrombosis, the last follow-up, patient's death, or the end of the study. The multivariable Cox regression analysis comprised the following parameters. The covariate of main interest was sP-selectin. We assumed that surgery, chemotherapy, and radiotherapy would confer a modified risk for VTE not only on the exact time point of the procedure but even for a certain time period immediately following the procedure. Therefore, 3 time-dependent binary variables were included into the statistical model that indicated times of possible influence on the VTE risk by surgery (from the day of surgery plus 6 consecutive weeks), chemotherapy (from the first day of a treatment cycle until the last day plus 4 weeks), or radiotherapy (from the first day of a treatment until the last day plus 4 weeks). Furthermore, the analysis was adjusted for age at study inclusion and sex. In addition, we adjusted in a second multivariable Cox regression analysis for platelet count and for tumor site and stage by introducing 4 groups of patients into the statistical model: (1) brain tumors, (2) hematologic malignancies, (3) solid tumors without distant metastasis, and (4) solid tumors with distant metastasis. We tested for all pairwise interactions and interactions with log(time) by candidate variables within the multivariable statistical model. Because no significant interaction was found (P < .01), no interaction was added to the multivariable Cox regression model.
To make further analyses with Kaplan-Meier curves possible, we defined 2 groups of patients with elevated or nonelevated sP-selectin. The cutoff for this categorized variable was set at the 75th percentile of sP-selectin of the total group. Alternatively to the inclusion of sP-selectin as a metric variable into the statistical models, we included sP-selectin as categorized variable.
The cumulative probability of VTE was illustrated in a Kaplan-Meier plot applying the aforementioned cut-off for sP-selectin. A log rank test was used to compare the time until first thrombosis in these 2 groups.
A P value less than .05 was regarded as statistically significant.
Results
Study population
Baseline characteristics of our study population are shown in Table 1. A total of 687 patients (320 women and 367 men) with malignant disease were studied. The median age of patients at inclusion in the study was (median [IQR], 62 [54-68] years). They were enrolled after a newly diagnosed malignant disease (n = 365) or progression of disease after complete or partial remission (n = 322) and were prospectively followed for a median of 415 days (IQR, 221-722 days). Main tumor entities were malignancies of the breast (n = 125), lung (n = 86), upper (n = 30) and lower gastrointestinal tract (n = 100), pancreas (n = 42), kidney (n = 19), and prostate (n = 72). Furthermore, 80 patients had a brain tumor (high-grade glioma), 73 lymphomas, 18 multiple myeloma, and 42 other tumor types (mainly tumors of the gynecologic system and sarcomas). At the time of recruitment, distant metastases were found in 268 patients with solid tumors. A total of 233 patients died during the observation period without a clear evidence for a fatal VTE.
Baseline characteristics of patients, n = 687
| Characteristic . | Value . |
|---|---|
| Age at study entry, y [median (IQR)] | 62 (54-68) |
| Sex, n (%) | |
| Female | 320 (47) |
| Male | 367 (53) |
| Classification of tumor, n (%) | |
| Localized | 224 (35.5) |
| Distant metastasis | 268 (39.0) |
| Not classifiable (brain tumors and hematologic malignancies) | 171 (24.9) |
| Classification unclear | 4 (0.6) |
| Site of cancer, n (%) | |
| Breast | 125 (18.2) |
| Lung | 86 (12.5) |
| Upper gastrointestinal | 30 (4.4) |
| Colorectal | 100 (14.6) |
| Pancreas | 42 (6.1) |
| Kidney | 19 (2.8) |
| Prostate | 72 (10.5) |
| Brain | 80 (11.6) |
| Lymphoma | 73 (10.6) |
| Multiple myeloma | 18 (2.6) |
| Others | 42 (6.1) |
| Cancer treatment during observation period, n (%) | |
| Chemotherapy | 465 (67.7) |
| Surgery | 295 (43.0) |
| Radiotherapy | 337 (49.1) |
| Combination of treatments during observation period, n (%) | |
| Chemotherapy and radiotherapy | 119 (17.3) |
| Chemotherapy and surgery | 77 (11.2) |
| Surgery and radiotherapy | 69 (10) |
| Chemotherapy, surgery, and radiotherapy | 91 (13.3) |
| Observation time, d [median (IQR)] | 415 (221-722) |
| Characteristic . | Value . |
|---|---|
| Age at study entry, y [median (IQR)] | 62 (54-68) |
| Sex, n (%) | |
| Female | 320 (47) |
| Male | 367 (53) |
| Classification of tumor, n (%) | |
| Localized | 224 (35.5) |
| Distant metastasis | 268 (39.0) |
| Not classifiable (brain tumors and hematologic malignancies) | 171 (24.9) |
| Classification unclear | 4 (0.6) |
| Site of cancer, n (%) | |
| Breast | 125 (18.2) |
| Lung | 86 (12.5) |
| Upper gastrointestinal | 30 (4.4) |
| Colorectal | 100 (14.6) |
| Pancreas | 42 (6.1) |
| Kidney | 19 (2.8) |
| Prostate | 72 (10.5) |
| Brain | 80 (11.6) |
| Lymphoma | 73 (10.6) |
| Multiple myeloma | 18 (2.6) |
| Others | 42 (6.1) |
| Cancer treatment during observation period, n (%) | |
| Chemotherapy | 465 (67.7) |
| Surgery | 295 (43.0) |
| Radiotherapy | 337 (49.1) |
| Combination of treatments during observation period, n (%) | |
| Chemotherapy and radiotherapy | 119 (17.3) |
| Chemotherapy and surgery | 77 (11.2) |
| Surgery and radiotherapy | 69 (10) |
| Chemotherapy, surgery, and radiotherapy | 91 (13.3) |
| Observation time, d [median (IQR)] | 415 (221-722) |
During the observation period, 465 (67.7%) patients received chemotherapy, 337 (49.1%) patients radiotherapy, and 295 (43.0%) patients underwent a tumor surgery. A total of 119 (17.3%) patients were treated by both chemotherapy and radiotherapy, 77 (11.2%) patients by both surgery and chemotherapy, 69 (10.0%) patients by both surgery and radiotherapy, and 91 (13.3%) patients by chemotherapy, radiotherapy, and surgery.
Thromboembolic events
During the follow-up period, VTE occurred in 44 (6.4%) patients (20 female and 24 male; median [IQR] age, 62 [48-66] years). An isolated DVT was diagnosed in 19, an isolated PE in 18, and a combined DVT and PE in 2 patients. Furthermore, we found each of the following thrombotic events in one patient, respectively: thrombosis of the portal vein, sinus vein thrombosis, inferior vein cava thrombosis, combined DVT and thrombosis of the portal vein, combined deep venous thrombosis of the upper extremity, and PE. PE was fatal in 3 patients (6.8% of VTE events). Four of the events, all of which were PE, were detected incidentally on CT scan; they were, however, considered as clinically significant by the adjudication committee and were therefore classified as an event. The cumulative probability of VTE in the total study population was 5.5% after 6 months and 6.8% after one year. Detailed information on patients with VTE, including site of tumor, is given in Table 2.
Characteristics of cancer patients with VTE, n = 44
| Characteristic . | Value . |
|---|---|
| Age at study entry, y [median (IQR)] | 62 (48-66) |
| Sex, n (%) | |
| Female | 20 (45.5) |
| Male | 24 (54.5) |
| Site of thrombotic event, n (%) | |
| Isolated deep venous thrombosis (DVT) | 19 (45.5) |
| Isolated pulmonary embolism (PE) | 18 (38.6) |
| Combined DVT and PE | 2 (4.5) |
| Thrombosis of the portal vein | 1 (2.3) |
| Sinus vein thrombosis | 1 (2.3) |
| Inferior vein cava thrombosis | 1 (2.3) |
| Combined DVT and thrombosis of the portal vein | 1 (2.3) |
| Combined brachial vein thrombosis and PE | 1 (2.3) |
| Classification of tumor, n (%) | |
| Localized | 10 (22.7) |
| Distant metastasis | 17 (38.6) |
| Not classifiable (brain tumors and hematologic malignancies) | 17 (38.6) |
| Classification unclear | 0 (0) |
| Site of cancer, n (%) | |
| Breast | 2 (4.5) |
| Lung | 3 (6.8) |
| Upper gastrointestinal | 5 (11.4) |
| Colorectal | 8 (18.2) |
| Pancreas | 6 (13.6) |
| Kidney | 0 (0) |
| Prostate | 0 (0) |
| Brain | 13 (29.5) |
| Lymphoma | 3 (6.8) |
| Multiple myeloma | 1 (2.3) |
| Others | 3 (6.8) |
| Characteristic . | Value . |
|---|---|
| Age at study entry, y [median (IQR)] | 62 (48-66) |
| Sex, n (%) | |
| Female | 20 (45.5) |
| Male | 24 (54.5) |
| Site of thrombotic event, n (%) | |
| Isolated deep venous thrombosis (DVT) | 19 (45.5) |
| Isolated pulmonary embolism (PE) | 18 (38.6) |
| Combined DVT and PE | 2 (4.5) |
| Thrombosis of the portal vein | 1 (2.3) |
| Sinus vein thrombosis | 1 (2.3) |
| Inferior vein cava thrombosis | 1 (2.3) |
| Combined DVT and thrombosis of the portal vein | 1 (2.3) |
| Combined brachial vein thrombosis and PE | 1 (2.3) |
| Classification of tumor, n (%) | |
| Localized | 10 (22.7) |
| Distant metastasis | 17 (38.6) |
| Not classifiable (brain tumors and hematologic malignancies) | 17 (38.6) |
| Classification unclear | 0 (0) |
| Site of cancer, n (%) | |
| Breast | 2 (4.5) |
| Lung | 3 (6.8) |
| Upper gastrointestinal | 5 (11.4) |
| Colorectal | 8 (18.2) |
| Pancreas | 6 (13.6) |
| Kidney | 0 (0) |
| Prostate | 0 (0) |
| Brain | 13 (29.5) |
| Lymphoma | 3 (6.8) |
| Multiple myeloma | 1 (2.3) |
| Others | 3 (6.8) |
sP-selectin and risk of VTE
sP-selectin levels were statistically significantly higher among cancer patients with VTE than among those without VTE (median [IQR], 45.9 [35.4-62.8] ng/mL vs 42.1 [32.9-52.2] ng/mL, P = .025). No statistically significant difference was observed between sP-selectin levels in study participants with newly diagnosed cancer and those with progression of cancer disease after complete or partial remission (42.1 [33.1-52.8] ng/mL vs 42.5 [33.4-54.8] ng/mL, P = .80). We evaluated the correlation coefficient between sP-selectin levels and platelet count and found no relevant correlation (r = 0.19). When sP-selectin was analyzed as a continuous variable in a Cox proportional hazard model, hazard ratio (HR) of VTE was 1.2 (95% confidence interval [CI], 1.1-1.4, P = .007) per 10 ng/mL increase in sP-selectin levels. After adjustment for age, sex, chemotherapy, surgery, and radiotherapy, HR per 10 ng/mL sP-selectin increase remained unchanged at 1.2 (95% CI, 1.1-1.4, P = .005). We stratified patients into 2 groups according to sP-selectin levels (75th percentile or higher and lower than the 75th percentile). A total of 173 patients (25%) had sP-selectin levels higher than the 53.1 ng/mL cutoff. The HR of VTE in patients with sP-selectin levels higher than the 75th percentile compared with patients with levels lower than the 75th percentile was 2.5 (95% CI, 1.3-4.8, P = .008). In multivariable analysis (Table 3), including elevated sP-selectin levels (≥ 53.1 ng/mL), age, sex, chemotherapy, surgery, and radiotherapy, the HR of VTE was statistically significantly increased for elevated sP-selectin (HR = 2.6; 95% CI, 1.4-4.9, P = .003), for surgery (HR = 3.9, 95% CI, 1.8-8.5, P < .001), and for radiotherapy (HR = 2.9; 95% CI, 1.4-5.8, P = .003). A very strong association of elevated sP-selectin levels with the occurrence of VTE (HR = 2.3; 95% CI, 1.2-4.5, P = .009) was still revealed after additional adjustment for platelet count and different tumor groups (considering tumor site and tumor stage).
Multivariable Cox proportional hazards model, including elevated sP-selectin levels (≥ 53.1 ng/mL), surgery, radiotherapy, chemotherapy, sex, and age
| . | Multivariable HR (95% CI) . | P . |
|---|---|---|
| sP-selectin (≥ 75th percentile) | 2.6 (1.4-4.9) | .003 |
| Surgery | 3.9 (1.8-8.5) | <.001 |
| Radiotherapy | 2.9 (1.4-5.8) | .003 |
| Chemotherapy | 1.3 (0.7-2.6) | .377 |
| Sex (male) | 1.1 (0.6-2.0) | .828 |
| Age | 1.0 (1.0-1.0) | .332 |
| . | Multivariable HR (95% CI) . | P . |
|---|---|---|
| sP-selectin (≥ 75th percentile) | 2.6 (1.4-4.9) | .003 |
| Surgery | 3.9 (1.8-8.5) | <.001 |
| Radiotherapy | 2.9 (1.4-5.8) | .003 |
| Chemotherapy | 1.3 (0.7-2.6) | .377 |
| Sex (male) | 1.1 (0.6-2.0) | .828 |
| Age | 1.0 (1.0-1.0) | .332 |
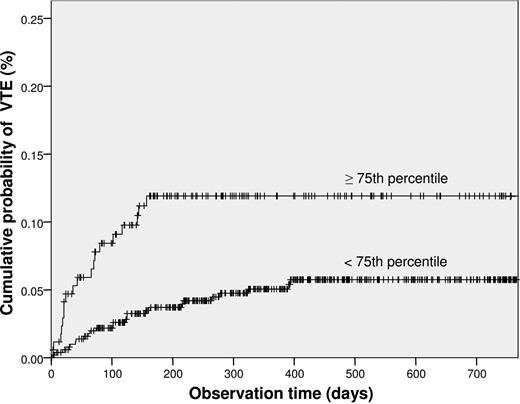
The cumulative probability of developing VTE after 6 months was 11.9% plus or minus 0.02% among patients with high sP-selectin levels compared with 3.7% plus or minus 0.04% in those with lower levels (log-rank test, P = .002; Figure 1).
Kaplan-Meier method estimates of the risk of venous thromboembolism (VTE) among cancer patients according to sP-selectin levels (75th percentile or higher and lower than the 75th percentile). The probability of VTE was statistically significantly higher among patients with sP-selectin levels more than or equal to the 75th percentile compared with patients with levels less than the 75th percentile (P = .002).
Kaplan-Meier method estimates of the risk of venous thromboembolism (VTE) among cancer patients according to sP-selectin levels (75th percentile or higher and lower than the 75th percentile). The probability of VTE was statistically significantly higher among patients with sP-selectin levels more than or equal to the 75th percentile compared with patients with levels less than the 75th percentile (P = .002).
sP-selectin levels and tumor site
Table 4 shows sP-selectin levels for each tumor site included in the study. There was no statistically significant difference in sP-selectin levels between the different tumor sites (Kruskal-Wallis test, P = .25).
sP-selectin levels associated with site of cancer
| Site of cancer . | sP-selectin level, ng/mL [median (IQR)] . |
|---|---|
| Breast (n = 125) | 40.9 (32.5-49.5) |
| Lung (n = 86) | 43.9 (35.5-55.5) |
| Upper gastrointestinal (n = 30) | 44.9 (40.0-59.5) |
| Colorectal (n = 100) | 43.2 (32.7-52.8) |
| Pancreas (n = 42) | 40.2 (32.7-52.4) |
| Kidney (n = 19) | 44.6 (35.3-58.6) |
| Prostate (n = 72) | 45.2 (35.9-53.3) |
| Brain (n = 80) | 41.1 (32.2-55.3) |
| Lymphoma (n = 73) | 40.6 (31.2-53.9) |
| Multiple myeloma (n = 18) | 33.3 (27.2-50.7) |
| Others (n = 42) | 41.3 (34.1-58.0) |
| P | .25* |
| Site of cancer . | sP-selectin level, ng/mL [median (IQR)] . |
|---|---|
| Breast (n = 125) | 40.9 (32.5-49.5) |
| Lung (n = 86) | 43.9 (35.5-55.5) |
| Upper gastrointestinal (n = 30) | 44.9 (40.0-59.5) |
| Colorectal (n = 100) | 43.2 (32.7-52.8) |
| Pancreas (n = 42) | 40.2 (32.7-52.4) |
| Kidney (n = 19) | 44.6 (35.3-58.6) |
| Prostate (n = 72) | 45.2 (35.9-53.3) |
| Brain (n = 80) | 41.1 (32.2-55.3) |
| Lymphoma (n = 73) | 40.6 (31.2-53.9) |
| Multiple myeloma (n = 18) | 33.3 (27.2-50.7) |
| Others (n = 42) | 41.3 (34.1-58.0) |
| P | .25* |
P value in Kruskal-Wallis test for association of sP-selectin levels and the site of cancer in the total study population.
Discussion
In this prospective cohort study, we demonstrated for the first time that high plasma levels of sP-selectin are a predictive parameter for development of VTE in cancer patients. The risk of VTE was 2.6 (95% CI, 1.4-4.8) times higher among those with levels higher than the 75th percentile compared with cancer patients with levels below the upper quartile. In Kaplan-Meier analysis, the probability of developing VTE 6 months after study inclusion was 11.9% in patients with elevated sP-selectin levels and 3.7% in those with lower levels.
It is commonly known that cancer leads to a 4- to 7-fold increase in the risk of VTE.34,35 Occurrence of VTE in cancer has important clinical implications, as it is the second leading cause of death in cancer patients.9,36 Currently, international guidelines do not support routine VTE prophylaxis for cancer patients37 because only a subgroup of cancer patients develops VTE during the course of their disease and because the use of anticoagulants in cancer patients is associated with significantly increased bleeding complications.30,32 The ability to stratify patients into risk groups would allow appropriate use of VTE prophylaxis and selective treatment of high-risk patients. However, presently, data on predictive parameters are too scarce to attempt risk stratification of cancer patients for VTE. In this context, we think that our findings are of major clinical relevance. Using a simple, commercially available enzyme-linked immunosorbent assay developed to measure sP-selectin levels, we were able to identify patients with a malignant disease in whom the risk of VTE is considerably elevated. Anticoagulants, such as LMWH, are effective and widely used with respect to primary and secondary prophylaxis. With regard to cancer patients, those with high levels of sP-selectin would probably benefit most from prophylactic anticoagulant therapy, specifically in the first months after cancer diagnosis, when the risk of developing VTE is highest, as well as those with tumor sites with a high incidence of VTE.38,39
In one prospective observational study, which investigated laboratory risk factors for prediction of chemotherapy-associated VTE,40 a high prechemotherapy platelet count was a risk factor for chemotherapy-associated VTE. In our study, we found no relevant correlation of sP-selectin level and platelet count. The association of sP-selectin with cancer-associated VTE was also adjusted for platelet count in multivariable analyses and remained statistically significant. These data again suggest that high levels of sP-selectin are independently related to a patient's propensity to develop VTE and support the hypothesis of a strong link between P-selectin, cancer, and thrombosis.
Interestingly, in several experimental studies, sP-selectin was considered a target for pharmacologic control of thrombosis as well as the process of metastasis.41-46 It is discussed that the antimetastatic effects of heparins are mediated primarily through interference with P-selectin binding and mechanisms,7,44-46 which could be a possible explanation for the survival benefit of specific subgroups of cancer patients treated with LMWH in recent prospective clinical trials.32,47-50 Our findings that high levels of sP-selectin entail an increased risk for VTE provide a rationale for further research on the inhibition of P-selectin in human subjects.
Some limitations of this study need to be addressed. The endpoint ascertainment was done remotely from the thrombotic event. An independent adjudication committee reviewed all available original clinical reports and images, discussed every single case of VTE, and confirmed the events using standardized criteria. Our data can only be applied to patients similar to those included in our study. Patients with particular cancer sites, specifically brain, upper gastrointestinal tract, colorectal, and pancreas, contributed disproportionately to the incidence of VTE in this study population and certain cancer types, which are known to be strongly associated with VTE, such as tumors of the brain, were overrepresented in this population. In this respect, it is of interest that in our study sP-selectin levels were not statistically significantly different among patients with different tumor sites. In multivariable analysis, the association of elevated sP-selectin levels with occurrence of VTE was still high and statistically significant even after adjustment for different tumor groups, reflecting the different tumor sites and tumor stages. The general cancer population is heterogenous, comprising not only different sites of cancer but also comprises newly diagnosed patients or patients with progression of disease after remission, those receiving different anticancer treatments and those receiving palliative care. Our study population reflects this heterogeneity, and we found that sP-selectin remained significant for prediction of VTE in the multivariable model, including various parameters, such as different treatment strategies (surgery, radiotherapy, chemotherapy). Therefore, we think that sP-selectin could be a valuable tool for an improved risk stratification of individual patients.
In conclusion, the findings of our study could improve the stratification of patients with a malignant disease with regard to their risk for VTE. High plasma levels of sP-selectin independently predict VTE in cancer patients. Measurement of sP-selectin at diagnosis of cancer would help to identify patients at increased risk for VTE. Whether prophylactic anticoagulant treatment of cancer patients with high levels of sP-selectin might be beneficial, especially in tumor sites with a high incidence of VTE, needs to be cautiously evaluated in future, well-designed randomized controlled trials.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all persons who supported us in patient recruitment for the Vienna Cancer and Thrombosis Study (CATS): Prof Dr Thomas Brodowicz, Prof Dr Johannes Drach, Prof Dr Heinz Gisslinger, Prof Dr Michael Hejna, Prof Dr Ulrich Jäger, Prof Dr Michael Krainer, Prof Dr Leopold Öhler, Prof Dr Robert Pirker, Prof Dr Markus Raderer, Prof Dr Werner Scheithauer, Prof Dr Manuela Schmidinger, Prof Dr Günther Steger, Prof Dr Peter Valent, Prof Dr Herbert Watzke, Prof Dr Christoph Wiltschke, Prof Dr Sabine Zöchbauer-Müller, Dr Marco Hassler, Prof Dr Irene Kührer, Dr Axel Eisenhut, Prof Dr Michael Gnant, Prof Dr Bela Teleky, Dr Gregor Goldner, Prof Dr Ute Dieckmann, Prof Dr Gerda Hohenberg, Prof Dr Petra Munda, Prof Dr Ernst Kubista, Prof Dr Martin Schwarz, Magdalena Pabinger and many other colleagues (all from the Medical University Vienna); Richard Rataj, Heidi Dude, and Judith Raglhofer (Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University Vienna) for management of blood samples and technical assistance; and members of the adjudication committee: Prof Dr Renate Koppensteiner, Dr Markus Haumer, Prof Dr Andrea Willfort-Ehringer (all from the Department of Angiology, Medical University Vienna), Prof Dr Sylvia Metz-Schimmerl (Department of Diagnostic Radiology, Medical University of Vienna), and Prof Dr Robert Dudczak (Department of Nuclear Medicine, Medical University of Vienna). The authors also thank Tanja Altreiter, MA (Clinical Division of Hematology and Haemostaseology, Department of Medicine I, Medical University Vienna) for proofreading the manuscript.
This work was supported by grants from the Jubiläumsfond of the Austrian National Bank (Vienna, Austria; project number 10 935), by an unrestricted grant from Pfizer Austria (Vienna, Austria), and by the Fellinger Krebsforschung (Vienna, Austria).
Authorship
Contribution: I.P. and R.V. were responsible for study concept and design; R.S., C.A., G.A., S.K., C.M., and G.K. acquired the data; I.P., C.A., D.D., C.Z., and O.W. analyzed and interpreted the data; C.A., I.P., R.V., R.S., and G.A. drafted the manuscript; C.Z., O.W., C.M., G.K., D.D., S.K., and I.P. critically revisiewed the manuscript for important intellectual content; I.P., R.V., and C.A. obtained funding; D.D., R.V., and C.A. provided statistical analysis; S.K., O.W., C.A., G.A., and R.S. provided administrative, technical, or material support; I.P. was the study supervisor; all authors took part in reviewing and editing the entire manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingrid Pabinger, Clinical Division of Haematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: ingrid.pabinger@meduniwien.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal