Abstract
Mantle cell lymphoma (MCL) is considered incurable. Intensive immunochemotherapy with stem cell support has not been tested in large, prospective series. In the 2nd Nordic MCL trial, we treated 160 consecutive, untreated patients younger than 66 years in a phase 2 protocol with dose-intensified induction immunochemotherapy with rituximab (R) + cyclophosphamide, vincristine, doxorubicin, prednisone (maxi-CHOP), alternating with R + high-dose cytarabine. Responders received high-dose chemotherapy with BEAM or BEAC (carmustine, etoposide, cytarabine, and melphalan/cyclophosphamide) with R-in vivo purged autologous stem cell support. Overall and complete response was achieved in 96% and 54%, respectively. The 6-year overall, event-free, and progression-free survival were 70%, 56%, and 66%, respectively, with no relapses occurring after 5 years. Multivariate analysis showed Ki-67 to be the sole independent predictor of event-free survival. The nonrelapse mortality was 5%. The majority of stem cell products and patients assessed with polymerase chain reaction (PCR) after transplantation were negative. Compared with our historical control, the Nordic MCL-1 trial, the event-free, overall, and progression-free survival, the duration of molecular remission, and the proportion of PCR-negative stem cell products were significantly increased (P < .001). Intensive immunochemotherapy with in vivo purged stem cell support can lead to long-term progression-free survival of MCL and perhaps cure. Registered at www.isrctn.org as #ISRCTN 87866680.
Introduction
Mantle cell lymphoma (MCL) is considered incurable, with a reported median survival of 3 years after conventional chemotherapy based on COP- (cyclophosphamide, vincristine, prednisone) or CHOP- (COP + doxorubicin) like regimens.1-4 CHOP followed by consolidation with high-dose chemoradiotherapy plus stem cell support improved the progression-free survival (PFS) in younger patients compared with maintenance with interferon-α, but most patients relapsed with a median PFS of 3 to 4 years.5 The Nordic Lymphoma Group (NLG) conducted its first MCL phase 2 protocol (NLG MCL-1) in 1996 to 2000 with an induction treatment of 4 cycles of dose-intensified CHOP without rituximab, followed by etoposide, cytarabine, and melphalan (BEAM) or the same regimen with cyclophosphamide instead of melphalan (BEAC), high-dose chemotherapy with unpurged or ex vivo–purged autologous stem cell support (ASCT). The results were disappointing, as 85% of the patients failed therapy and most of the evaluable patients did have demonstrable minimal residual disease after transplantation, as did most of the evaluable stem cell products.6 Based on smaller series reporting a high efficacy in MCL of regimens containing cytarabine, both in terms of prolonged event-free survival7,8 and, in combination with rituximab, in terms of a high rate of clinical and molecular remission and tumor-cell free stem cell products,8 we launched the second Nordic MCL protocol (NLG MCL-2) in 2000, with both high-dose cytarabine and rituximab added to the dose-intensified CHOP induction treatment, with the aim of increasing the rates of event-free survival (EFS), PFS, and overall survival (OS), and of molecular remission and polymerase chain reaction (PCR)-negative stem cell products. We present here the outcome of all 160 patients with a median observation time of 3.8 years from entry onto the study, and the projected median EFS on intent to treat not reached at 7 years.
Methods
Patients
Since 1996, the NLG has completed 2 successive phase 2 trials of primary treatment of MCL: the MCL-1 trial in 1996 to 20006 and the present MCL-2 trial 2000 to 2006 (Table 1). Eligible for the MCL-2 protocol were newly diagnosed, stage II to stage IV MCL patients who were previously untreated or had just initiated first-line treatment according to local routine before a conclusive MCL diagnosis was reached, or because of immediate need of treatment. Exclusion criteria were major organ dysfunction, seropositivity for HIV, or any uncontrolled infections. The diagnostic specimens of all patients underwent central pathology review, including examination of immunophenotype, cyclinD1 expression, cytologic variant, and lymphoma growth pattern. Patients whose specimens did not fulfill the diagnostic WHO criteria of MCL,9 with a clear overexpression of cyclinD1 or t(11;14) positive lymphoma, were excluded. When possible, Ki-67 was analyzed by a semiquantitative assessment of the proportion of MIB1-α–positive cells. The characteristics of the patients of the MCL-2 protocol are given in Table 2. The Nordic MCL-2 protocol was approved by the following institutions: Denmark, the Danish Medicines Agency and the Science Ethics Committee of Copenhagen and Frederiksberg, valid for all Danish centers; Finland, the Ethics Committee of the Northern Ostrobothnia Hospital District, Oulu University Hospital, Finland, valid for all Finnish centers; Norway, the regional Committee for Medical Reearch Ethics, Region Northern Norway, Tromsø University, valid for all Norwegian centers; and Sweden, the Research Ethics Committee of Uppsala University, valid for all Swedish centers. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Treatment regimens
| Regimen . | Trial no. . | |
|---|---|---|
| MCL1: 1996-2000 . | MCL2: 2000-2006 . | |
| Induction | Maxi-CHOP21* × 3 | Maxi-CHOP21 × 3 alternating with HD-cytarabine × 2† |
| Stem cell mobilization | Maxi-CHOP21 | Rituximab day 1 of each cycle‡ |
| HD-cytarabine | ||
| Stem cell purging | In vitro: CD34+ cell selection when possible | In vivo: rituximab × 2 (days 1 and 9 of the HD-cytarabine) |
| Consolidation§ | Allowed (1-2 series) | Allowed (1-2 series) |
| High-dose therapy | BEAM¶/BEAC‖ | BEAM: 90 patients |
| BEAC 55 patients | ||
| Preemptive treatment at molecular relapse | No | Rituximab 375 mg/m2 weekly × 4 |
| Regimen . | Trial no. . | |
|---|---|---|
| MCL1: 1996-2000 . | MCL2: 2000-2006 . | |
| Induction | Maxi-CHOP21* × 3 | Maxi-CHOP21 × 3 alternating with HD-cytarabine × 2† |
| Stem cell mobilization | Maxi-CHOP21 | Rituximab day 1 of each cycle‡ |
| HD-cytarabine | ||
| Stem cell purging | In vitro: CD34+ cell selection when possible | In vivo: rituximab × 2 (days 1 and 9 of the HD-cytarabine) |
| Consolidation§ | Allowed (1-2 series) | Allowed (1-2 series) |
| High-dose therapy | BEAM¶/BEAC‖ | BEAM: 90 patients |
| BEAC 55 patients | ||
| Preemptive treatment at molecular relapse | No | Rituximab 375 mg/m2 weekly × 4 |
Maxi-CHOP-21: cyclophosphamide 1200 mg/m2 intravenously, doxorubicin 75 mg/m2 intravenously, vincristine 2 mg total intravenously day 1; prednisone 100 mg days 1-5 orally.
Cytarabine: 3 g/m2 (3-hour infusion intravenously) every 12 hours for a total of 4 doses; patients older than 60 years, cytarabine 2 g/m2.
Rituximab 375 mg/m2 intravenously on day 1 of each cycle from cycle 4.
In case of delay in the transplant unit, 1 extra immunochemotherapy cycle of Maxi-CHOP21, HD-cytarabine, or both were allowed.
BEAM: BCNU 300 mg/m2 day 1, etoposide 100 mg/m2 × 2 days 2-5, cytarabine 400 mg/m2 days 2-5, melphalan 140 mg/m2 day 6, all intravenously.
BEAC: As BEAM, cyclophosphamide 1.5 g/m2 days 2-5 instead of melphalan.
Characteristics of the 160 patients
| Variable . | No. (%) . |
|---|---|
| Patient-specific | |
| Male sex | 113 (70.6) |
| Median age, y (range) | 56 (32-65) |
| Stage IV | 136 (85.0) |
| Splenomegaly | 72 (45.0) |
| Extranodal disease other than BM | 51 (31.9) |
| International Prognostic Index (IPI) | |
| 0-1 | 35 (21.9) |
| 2 | 69 (43.1) |
| 3 | 42 (26.3) |
| 4-5 | 14 (8.8) |
| Disease-specific | |
| Cytologic variant | |
| Blastoid/pleomorphic | 31 (19.4) |
| Common | 129 (80.6) |
| Growth pattern (assessed in 151) | |
| Nodular | 26 (17.2) |
| Mantle | 5 (3.3) |
| Mixed | 39 (25.8) |
| Diffuse | 81 (53.6) |
| Ki-67 expression (assessed in 120) | |
| 0-9 | 10 (8.3) |
| 10-29 | 60 (50.0) |
| > 29 | 50 (41.7) |
| Variable . | No. (%) . |
|---|---|
| Patient-specific | |
| Male sex | 113 (70.6) |
| Median age, y (range) | 56 (32-65) |
| Stage IV | 136 (85.0) |
| Splenomegaly | 72 (45.0) |
| Extranodal disease other than BM | 51 (31.9) |
| International Prognostic Index (IPI) | |
| 0-1 | 35 (21.9) |
| 2 | 69 (43.1) |
| 3 | 42 (26.3) |
| 4-5 | 14 (8.8) |
| Disease-specific | |
| Cytologic variant | |
| Blastoid/pleomorphic | 31 (19.4) |
| Common | 129 (80.6) |
| Growth pattern (assessed in 151) | |
| Nodular | 26 (17.2) |
| Mantle | 5 (3.3) |
| Mixed | 39 (25.8) |
| Diffuse | 81 (53.6) |
| Ki-67 expression (assessed in 120) | |
| 0-9 | 10 (8.3) |
| 10-29 | 60 (50.0) |
| > 29 | 50 (41.7) |
At study entry, patients underwent full clinical and laboratory workup, including CT scans of the chest and abdomen, bone marrow trephine biopsies, and aspirates with histologic, cytologic, and flow cytometric assessment. In addition to the pathology review, samples of blood and bone marrow aspirates were sent to the central laboratory of the study (the Leukemia Marker Laboratory at the Department of Hematology, Rigshospitalet, Copenhagen, Denmark) to identify a disease- or patient-specific molecular marker. If clinically indicated, further staging procedures, including ear-nose-throat examination and gastrointestinal endoscopy, were done. The International Prognostic Index (IPI)10 was assessed at the time of diagnosis. The complete workup was repeated at response evaluation after the fifth cycle of induction chemotherapy, 2 months after transplantation, and subsequently every 4 months the first 2 years, and every 6 months for another 3 years. Patients still in remission 5 years after the high-dose therapy are subsequently followed twice annually with at least physical examination and blood tests.
Polymerase chain reaction analysis for minimal residual disease
DNA was extracted from fresh patient specimens (bone marrow and/or peripheral blood shipped overnight) using minipreps (Qiagen, Valencia, CA). Diagnostic samples of blood and bone marrow were used for PCR primer design and standard nested PCR amplification of patient specific clonally rearranged immunoglobulin heavy chain genes (IgH) and/or Bcl-1/IgH rearrangement (translocation 11;14). These analyses were done on consecutive posttransplantation bone marrow and/or peripheral blood samples, as previously described.11 In the present study, however, we substituted standard agarose gel electrophoresis with gene scan (Applied Biosystems, Foster City, CA) clonal analysis. The sensitivity of the standard nested PCR analysis using gene scan ranged between 10−4 and 10−6. Samples of stem-cell products were sent from selected centers for minimal residual disease assessment, also in Copenhagen.
Treatment
Details of the treatment regimens of the 2 trials are given in Table 1. Briefly, the induction treatment of the MCL-1 trial consisted of 4 cycles of dose-intensified CHOP (“maxi-CHOP”), the fourth cycle plus granulocyte-colony stimulating factor (G-CSF) serving as stem cell mobilization. In the MCL-2 trial, the induction treatment was increased to a total of 6 cycles, with maxi-CHOP-21 alternating with high-dose cytarabine-21, 3 cycles of each, all cycles given with 3-week intervals. The sixth cycle (cytarabine) plus G-CSF served as stem cell mobilization. Rituximab was given on the first day of cycles 4 and 5 and on the first and ninth day of cycle 6, the cycle of stem cell mobilization, for in vivo purging to a total of 4 doses. As an increased use of rituximab became economically more feasible, and no toxicity had been observed, an amendment in 2003 allowed rituximab to be given already from cycle 2, to a total of 6 infusions in 43 patients. After a sufficient stem cell harvest of at least 2 million CD34+ cells per kilogram of body weight, high-dose chemotherapy with BEAM or BEAC was started 1 to 2 weeks later followed by stem cell infusion. If this phase of the therapy was delayed for logistical reasons, additional therapy with 1 or 2 cycles of immunochemotherapy (R-maxi-CHOP or R-cytarabine) was allowed. Supportive care during the high-dose/stem cell support period, including G-CSF and prophylactic antibiotics, was given according to local routine. At follow-up, patients in clinical response who converted from PCR-negative to PCR-positive bone marrow or blood without signs of clinical relapse were offered preemptive therapy with rituximab 375 mg/m2 weekly for 4 weeks, to prevent clinical relapse. The detailed results hereof are presented elsewhere.12
Response criteria and end points
The response criteria of the International Workshop13 were used. The endpoints were EFS, OS, and PFS as well as molecular remission duration. EFS was calculated for all included patients on intent-to-treat basis, from entry onto the trial to the date of last follow-up or failure resulting from any of the following events: death from any cause, nonresponse to induction treatment, lymphoma relapse or progression, any toxic event that prohibited treatment according to protocol, failure to harvest stem cells from peripheral blood or bone marrow, failure to engraft, or patient refusal. OS was calculated for all patients on an intent-to-treat basis from entry onto the trial to the date of last follow-up or death from any cause. PFS was calculated for all responding patients who completed the treatment program, including BEAM/BEAC, from the time of first documentation of response to the date of last follow-up or to the date of relapse, progression, or death of lymphoma. Molecular PFS was calculated for responding patients with an available clonal disease marker, from the date of stem cell infusion to the date a standard nested PCR becoming positive or the date of the last molecular follow-up. In patients where PCR-detectable minimal residual disease remained present in bone marrow or peripheral blood at the first molecular follow-up 2 months after transplantation, the duration of the molecular remission was defined as zero days from the date of stem cell infusion.
Statistics
EFS, OS, and PFS were calculated according to the Kaplan-Meier method,14 and differences between subgroups were analyzed by the log-rank test. A multivariate Cox regression analysis was performed to assess the effect on the outcome of pretreatment prognostic factors (age, sex, Ki-67 expression, cytologic variant, IPI, and lymphoma growth pattern) as well as of the response to induction treatment, in terms of EFS, OS, and PFS. All P values were 2-tailed. Statistical analyses were performed with SAS software (SAS Institute, Cary, NC). For the present analyses, the database was closed March 12, 2008.
Results
In the period from 2000 to 2006, a total of 176 patients were registered for inclusion in the MCL-2 protocol. Sixteen patients were excluded: 12 because of change of diagnosis at the central pathology review (to CLL in 4 cases and to cyclinD1-negative lymphoma in 8 cases) and 4 because of previous treatment for MCL. Thus, 160 patients were included in the protocol. The patient- and disease-specific characteristics of the MCL-2 patients are given in Table 2. The patients of the MCL-1 trial6 were similar to the present cohort and typical for MCL in all aspects, including male predominance, stage IV disease with bone marrow infiltration, and frequent other extranodal disease.
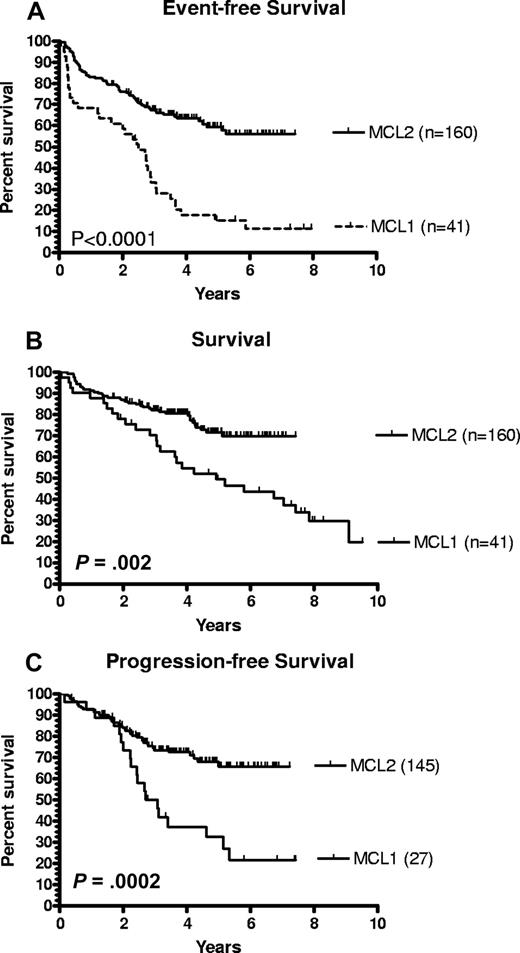
The outcome according to patient- and disease-specific characteristics is given in Table 3. Study-terminating events have occurred in 61 patients (37.5%), in 48 (30%) because of nonresponse, relapse, or progression of MCL, in 13 (8.1%) because of nonrelapse events: toxicity 7 (4.4%), harvest failure 4 (2.5%), and graft failure and late pulmonary embolism each 1 case. With a median observation time of 3.8 years, the 4-year EFS rate of the MCL-2 cohort was 63% on intention-to-treat basis, significantly higher than the 18% 4-year EFS of the MCL-1 trial (P < .001), mainly because of halving the rate of events caused by lymphoma from 76% in MCL-1 trial to 37.5% to date in the MCL-2 trial (Figure 1A). The latest event (a fatal pulmonary embolism) took place 5.2 years after entry into the trial. Twenty-eight percent of the patients at risk of an event are observed longer than that.
Characteristics of the 160 patients in the MCL2 study according to outcome
| Variable . | Score . | End point . | No. evaluable . | P . |
|---|---|---|---|---|
| Ki-67 expression | 0-9 vs 10-29 vs ≥29 | EFS | 120 (10 vs 60 vs 50) | .008 |
| Age | ≤ 60 vs > 60 | EFS | 160 (119 vs 41) | .29 |
| Sex | Male vs female | EFS | 160 (113 vs 47) | .1 |
| International Prognostic Index | 0-2 vs 3-5 | EFS | 160 (104 vs 56) | .046 |
| BEAM vs BEAC | EFS | 145 (90 vs 55) | .305 | |
| Rituximab | 4 vs 6 infusions | EFS | 160 (116 vs 44) | .790 |
| Cytologic variant | Common vs blastoid/pleomorphic | EFS | 160 (129 vs 31) | .069 |
| Lymphoma growth pattern | Nondiffuse vs diffuse | EFS | 70 vs 81 | .012 |
| Response to induction | CR vs PR | PFS | 145 (81 vs 64) | .035 |
| Variable . | Score . | End point . | No. evaluable . | P . |
|---|---|---|---|---|
| Ki-67 expression | 0-9 vs 10-29 vs ≥29 | EFS | 120 (10 vs 60 vs 50) | .008 |
| Age | ≤ 60 vs > 60 | EFS | 160 (119 vs 41) | .29 |
| Sex | Male vs female | EFS | 160 (113 vs 47) | .1 |
| International Prognostic Index | 0-2 vs 3-5 | EFS | 160 (104 vs 56) | .046 |
| BEAM vs BEAC | EFS | 145 (90 vs 55) | .305 | |
| Rituximab | 4 vs 6 infusions | EFS | 160 (116 vs 44) | .790 |
| Cytologic variant | Common vs blastoid/pleomorphic | EFS | 160 (129 vs 31) | .069 |
| Lymphoma growth pattern | Nondiffuse vs diffuse | EFS | 70 vs 81 | .012 |
| Response to induction | CR vs PR | PFS | 145 (81 vs 64) | .035 |
CR indicates complete disappearance of all detectable disease manifestations; PR indicates at least 50% reduction of all evaluable disease manifestations.
Survival. Event-free survival (A) and overall survival (B) of patients of NLG protocols MCL-1 and MCL-2, respectively, based on intention-to-treat of all included patients. (C) Progression-free survival of protocols MCL-1 and MCL-2, respectively, of responders who completed treatment.
Survival. Event-free survival (A) and overall survival (B) of patients of NLG protocols MCL-1 and MCL-2, respectively, based on intention-to-treat of all included patients. (C) Progression-free survival of protocols MCL-1 and MCL-2, respectively, of responders who completed treatment.
With regard to OS, 39 patients have died: 31 of lymphoma and 8 of nonlymphoma causes. With a median observation of 3.9 years, the 4-year OS rate on intention-to-treat basis was 81%, significantly higher than the 55% 4-year OS of the MCL-1 trial (P = .002; Figure 1B). Of the MCL-2 cohort, no deaths have been reported after 5.2 years, and 33 patients are presently observed alive after that time point.
With regard to response and PFS, 154 patients (96.3%) responded to the induction treatment, 73 (45.6%) with complete response (CR) and 14 (8.8%) with CR/unconfirmed (CRu), for a total of 54.4% CR/CRu. Because the outcome of patients with CR and CRu was identical (P = .83), these are henceforth designated CR. Sixty-seven (41.9%) had a partial response and 6 (3.8%) did not respond. The overall response rate of 96% and the CR rate of 54% were significantly higher than those of the MCL-1 study: 76% overall response (P = .001) and 27% CR (P < .001), respectively. A total of 145 responders, 81 (55.8%) in CR and 64 (44.1%) in partial response, proceeded to high-dose therapy with BEAM/BEAC, whereas 9 did not (4 because of harvest failure, 5 because of toxic events). After the high-dose therapy, the number of patients in CR had increased to 130 patients (89.7%, P < .001), whereas 11 (7.6%) remained in partial response, 3 (2.1%) had died, and 1 (0.7%) had progressed. During follow-up, 40 patients have relapsed or progressed, resulting in a projected 6-year PFS of 65.9% (Figure 1C), with a median observation time of 3.2 years from the time of response. The 4-year PFS of 73% of the MCL-2 cohort is significantly higher than the corresponding 37% of the MCL-1 cohort (P = .001). The latest relapse occurred at 5 years from the time of response with 27 patients observed in remission beyond that time point, suggesting a plateau, whereas 78 patients are still at risk of relapse on the sloping part of the curve. Complete responders had a significantly longer PFS than partial responders (P = .035).
Of the patients who received 4 and 6 rituximab infusions, respectively, there was no difference in EFS (P = .31) or PFS (P = .5).
With regard to molecular assessment of patients and stem cell products, of the 145 responders who completed the treatment program, 84 had an available molecular marker (in 32 for translocation (11;14) and in 52 for clonal IgH rearrangement) and 61 had no marker. The PFS of the 84 patients with a marker did not differ from that of the 47 patients in whom the submitted samples did not lead to an available marker (P = .1). Follow-up samples were received from 79 patients with a marker. At assessment of molecular response 2 months after the high-dose therapy, 73 (92%) were PCR-negative, a significantly higher proportion than the 38% in the MCL-1 trial (P < .001). Forty-three patients remained or became PCR-positive during follow-up, 19 within the first year after the high-dose therapy and 24 later. The PFS differed significantly between patients with PCR-positive samples within the first year of follow-up, (median PFS, 1.5 years), later (median PFS, 5 years), and not at all (median survival not reached) (P < .001). Twenty-six patients received retreatment with rituximab, resulting in a median molecular and clinical remission duration of 15 and 19 months, respectively. Forty-two stem cell products were assessed for tumor cells; 36 (88%) were PCR-negative, a significantly higher proportion than the 12% of the MCL-1 trial (P < .001).
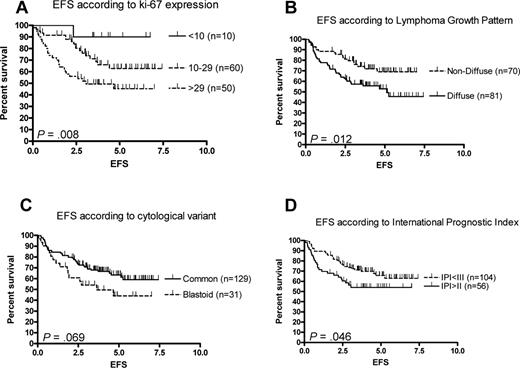
With regard to patient- and disease-specific characteristics, in univariate analyses, significantly inferior EFS rates were found for high Ki-67 expression, diffuse growth pattern, and high IPI (Figure 2A,B,D), whereas cytologic variant (Figure 2C), age, or sex did not have significant influence. In multivariate analyses of the relative influence of age, sex, stage, IPI, response to induction treatment, cytologic variant, growth pattern, and Ki-67 expression, solely Ki-67 had independent significance for EFS, whereas for PFS Ki-67 was only borderline significant, surpassed by sex (favoring females). For OS, only cytologic variant and IPI had independent significance (Table 4). Of note, even the patients in the poorest prognostic subgroups (high Ki-67, diffuse growth pattern, blastoid/pleomorphic variant, high IPI) had EFS plateaus at approximately 50% (Figure 2A-D).
Event-free survival. EFS according to the proportion of lymphoma cells that expressed Ki-67 (A), lymphoma growth pattern (B), cytologic variant (C), and IPI 0-II versus IPI III-5 (D).
Event-free survival. EFS according to the proportion of lymphoma cells that expressed Ki-67 (A), lymphoma growth pattern (B), cytologic variant (C), and IPI 0-II versus IPI III-5 (D).
Multivariate analyses of 120 patients with available Ki-67 value according to outcome
| Variable . | Score . | Event-free survival, n = 115 . | Progression-free survival, n = 105 . | Survival, n = 115 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | ||
| Ki-67 | 0-9/10-29/30+ | 1.96 | 1.19-3.23 | .008 | 1.77 | 0.95-3.30 | .074 | 1.17 | 0.57-2.43 | .663 |
| Cytologic variant | Common/blastoid | 1.19 | 0.59-2.40 | .631 | 0.85 | 0.33-2.19 | .735 | 2.70 | 1.31-5.50 | .014 |
| IPI | 0-2/3-5 | 1.28 | 0.67-2.42 | .451 | 0.81 | 0.35-1.88 | .624 | 2.62 | 1.29-5.32 | .007 |
| Growth pattern | Diffuse/nondiffuse | 1.61 | 0.84-3.08 | .149 | 1.42 | 0.65-3.11 | .381 | 2.07 | 0.97-4.41 | .059 |
| Sex | M/F | 0.74 | 0.36-1.5 | .429 | 0.23 | 0.05-0.95 | .041 | 0.58 | 0.23-1.44 | .243 |
| Age | ≤ 60/≥ 61 | 1.49 | 0.80-2.76 | .213 | 0.94 | 0.36-2.49 | .905 | 1.08 | 0.49-2.38 | .851 |
| Response to induction treatment | CR/PR | ND | 1.74 | 0.83-3.66 | .142 | ND | ||||
| Variable . | Score . | Event-free survival, n = 115 . | Progression-free survival, n = 105 . | Survival, n = 115 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | ||
| Ki-67 | 0-9/10-29/30+ | 1.96 | 1.19-3.23 | .008 | 1.77 | 0.95-3.30 | .074 | 1.17 | 0.57-2.43 | .663 |
| Cytologic variant | Common/blastoid | 1.19 | 0.59-2.40 | .631 | 0.85 | 0.33-2.19 | .735 | 2.70 | 1.31-5.50 | .014 |
| IPI | 0-2/3-5 | 1.28 | 0.67-2.42 | .451 | 0.81 | 0.35-1.88 | .624 | 2.62 | 1.29-5.32 | .007 |
| Growth pattern | Diffuse/nondiffuse | 1.61 | 0.84-3.08 | .149 | 1.42 | 0.65-3.11 | .381 | 2.07 | 0.97-4.41 | .059 |
| Sex | M/F | 0.74 | 0.36-1.5 | .429 | 0.23 | 0.05-0.95 | .041 | 0.58 | 0.23-1.44 | .243 |
| Age | ≤ 60/≥ 61 | 1.49 | 0.80-2.76 | .213 | 0.94 | 0.36-2.49 | .905 | 1.08 | 0.49-2.38 | .851 |
| Response to induction treatment | CR/PR | ND | 1.74 | 0.83-3.66 | .142 | ND | ||||
CR indicates complete disappearance of all detectable disease manifestations; PR, at least 50% reduction of all evaluable disease manifestations; and ND, not determined.
With regard to toxicity, of 465 cycles of maxi-CHOP and 464 cycles of high-dose cytarabine, 80 (17%) and 57 (12%), respectively, led to hospitalization for grade 3 or 4 adverse events, and 80% of these because of neutropenic fever. Of the 15 patients who failed to proceed to high-dose therapy, 5 did so because of toxicity: one because of heart insufficiency based on aortic stenosis, one because of perforated gastric ulcer needing surgery, and 3 because of recurrent, severe infection. The remaining 10 failed because of insufficient harvest in 4 cases and nonresponsive disease in 6 cases. A total of 8 nonrelapse deaths (5%) were recorded: 4 occurred during the high-dose therapy: one because of graft failure and 3 because of infections (septic shock, 2; pneumonitis, 1). Four deaths occurred later, 3 of heart failure (8, 20, and 28 months after the high-dose therapy) and 1 because of pulmonary embolism in a patient in complete remission 45 months after the high-dose therapy. A number of severe infections occurred that resolved on adequate treatment: 3 cases of Pneumocystis jiroveci pneumonia, 2 during induction and 1 during the transplantation; 1 patient had a respiratory syncytial virus pneumonia during induction and later cytomegalovirus reactivation during the high-dose procedure. There were 2 cases of other cancer (1 myelodysplasia and 1 breast cancer). The 3 deaths from heart failure all occurred among the 39 patients who received more than the 6 immunochemotherapy cycles before the BEAM/BEAC versus none in the remaining 115 who did not (P = .03). Five cases of late neutropenia occurred after recovery from the high-dose therapy with stem cell support. They all resolved after G-CSF treatment. Two patients developed hypogammaglobulinemia and recurrent respiratory tract infections after the high-dose therapy, who both responded to repeated series of intravenous immunoglobulin infusions.
Discussion
Almost since MCL was recognized as a disease entity,15 CHOP has been more or less accepted as the standard treatment, although retrospective studies did not suggest that anthracyclin-based therapy of MCL conferred any long-term advantage.1,2 CHOP as induction therapy yields response rates of approximately 75%, with CR rates ranging from 7% to 27%.6,16 The addition of rituximab on day 1 of each CHOP cycle increased the CR rate to 34% to 48% and the overall response rate to more than 90% but did not give any long-term survival advantage.16,17 After CHOP, the superiority of myeloablative therapy with stem cell support compared with maintenance with interferon-α was clearly demonstrated by the phase 3 study of the European MCL Network.5 The PFS of the high-dose arm, however, was identical to that of our MCL-1 phase 2 study of maxi-CHOP + BEAM/BEAC and ASCT,6 with 3-year PFS rates of approximately 50% and a continuous pattern of relapse. These data indicate that CHOP, with or without rituximab, is not sufficient as induction chemotherapy before high-dose therapy with stem cell support.
The 2 factors leading to the improvement in the MCL-2 trial compared with the MCL-1 trial was the addition of high-dose cytarabine and of rituximab, based on the reports of 2 phase 2 studies of cytarabine containing regimens followed by ASCT.7,8 An update of the high-dose sequential immunochemotherapy regimen18 reported a 5-year projected EFS of 61% and the stem cell mobilization/in vivo purging step of this regimen, consisting of cytarabine and rituximab, rendered the majority of assessed stem cell products PCR-negative.19 Khouri et al, in an update20 of their results of first-line treatment with hyperCVAD/MTX-cytarabine regimen without rituximab followed by high-dose therapy in 36 patients, reported before transplantation complete and overall response rates of 42%/100% and EFS/OS rates at 5 years of 43%/77%, and that the subsequent high-dose therapy induced CR in all the partial response patients.
The same induction regimen, now with rituximab added to every cycle and no high-dose therapy, induced CR/overall response rates of 87%/97% in a single-center study of 97 patients21 and 58%/88% in a multicenter study of 40 evaluable patients.22 Thus, at least in the multicenter setting, the R-hyperCVAD/MTX/cytarabine regimen and our MCL-2 regimen, both containing the CHOP components, high-dose cytarabine, and rituximab, appear to result in the same response rates, with the important difference that the omission of the high-dose therapy after the R-hyperCVAD/MTX/cytarabine regimen, permits the inclusion of patients over the age of 65. In both the R-hyperCVAD cohorts without final high-dose therapy, however, a continuous pattern of relapse was observed21,22 even after follow-up for a long time,21 in contrast to the MCL-2 cohort, where the emerging EFS and PFS plateaus as well as the markedly increased CR rate after the high-dose therapy strongly support the value of the final high-dose therapy. Only a randomized trial can show whether high-dose therapy is still necessary after this, much more intensive, induction therapy.
For MCL patients older than 65 years, who comprise more than half of all patients,4 and are not eligible for high-dose therapy, the R-hyperVCAD/MTX/cytarabine regimen remains an effective, but toxic, regimen.21,22 A randomized study of less intensive approaches, R-CHOP versus R-fludarabine + cyclophosphamide in elderly or frail patients,23 is presently ongoing.
Rituximab retreatment was offered to patients with solely molecular relapse after transplantation as an attempt of preemptive therapy aiming to prolong clinical PFS. The approach was feasible, did indeed reinduce molecular remission in most patients treated, and may have prolonged their clinical PFS.12 It did not, however, translate into a PFS advantage for the group of patients with an available molecular marker, compared with the group patients with samples that did not lead to identification of a marker. Reinduction of molecular remission by rituximab has been documented previously,24 and one randomized study has suggested a prolongation of second CR after rituximab maintenance.25 Randomized studies of this untoxic treatment modality are ongoing.23
Total body irradiation (TBI) has been claimed an important part of the myeloablative regimen for MCL.26 After induction therapy with CHOP, however, high-dose therapy with either TBI + cyclophosphamide or BEAM/BEAC has led to identical PFS,5,6 and the projected long-term PFS of our BEAM/BEAC-treated cohort speaks against any superiority of TBI in MCL. Addition of rituximab to the myeloablative regimen appears promising, being it purely chemotherapy-19 or TBI-based.27 In the present study, the high-dose therapy and stem cell support were given shortly after the in vivo purging with 2 rituximab infusions, securing therapeutic circulating antibody levels at the time of stem cell infusion, which may be important to catch up with minimal tumor-cell contamination.
The MCL-2 regimen was well tolerated, and the nonrelapse mortality of 5%, only one case of myelodysplasia, and an admission rate during the induction phase of 15% is comparable with those reported by others.5,6,19-21 Indeed, only 5 of 154 responders (3%) did not proceed to the high-dose regimen because of toxicity or refusal, much less than the reported 29% who did not complete the planned 6 or 8 hyperCVAD/MTX/ cytarabine cycles.21 The MCL-2 regimen, however, cannot be intensified further. Serious side effects, including cardiotoxicity, were more frequent in MCL-2 patients who received extra immunochemotherapy cycles for logistic reasons before the high-dose therapy. After stem cell harvest, the patients should proceed directly to the high-dose regimen.
The lymphoma cell proliferation rate in MCL as assessed by Ki-67 expression parallels that assessed by proliferation-gene expression.28-31 Recently proposed new cutoffs for the Ki-67 expression for MCL patients treated with immunochemotherapy32 also gave the strongest discrimination in our cohort and was the single independent prognostic factor for EFS, whereas for PFS, it had only borderline significance, because the 6 nonresponders who did not qualify for assessment of PFS had high Ki-67 values. Of note, even patients with high values of Ki-67 expression, blastoid/ pleomorphic cytology, diffuse growth pattern, or high IPI, achieved EFS plateaus of approximately 50%, indicating that also high-risk subgroups benefit from this treatment. The cell-cycle active drug cytarabine may be particularly important in patients with rapidly proliferating MCL. In the MCL-1 trial, the only independent predictor of EFS was the achievement of CR before transplantation, but this had no independent significance in the present trial, possibly because the final, stem cell mobilizing course of high-dose cytarabine after the restaging may have induced CR in some partial responders.
There are only few reports on allogeneic stem cell transplantation as first-line treatment (reviewed by Dreger and Laport33 ). Because of a rather high nonrelapse mortality allogeneic stem cell transplantation after both standard and reduced intensity conditioning, has not improved the outcome of MCL and is not indicated as first-line treatment of MCL. Beyond first remission, however, reduced intensity conditioning followed by allogeneic stem cell transplantation has been reported to lead to 57% PFS at 42 months, with a suggested survival plateau and a 2-year nonrelapse mortality of 20%,34 and may become the modality of choice for younger, fit patients beyond first remission.
Whereas the achievement of molecular remission may be a prerequisite for cure of MCL, we confirm that it does not per se predict long-term remission and that early molecular relapse is a harbinger of clinical relapse.17,35,36 The significance of tumor cells in the stem cell product is unknown and the efficacy of ex vivo purging of MCL is questionable.6,35 In the MCL-1 trial6 where only 27% had a CR and only 38% a molecular remission, the number of tumor cells in the stem cell product had no prognostic importance despite the reinfusion of up to 80 million tumor cells, still much less than the at least 109 tumor cells by definition harbored endogenously in a patient not in CR. In contrast, in the MCL-2 trial with the majority of patients in clinical as well as molecular CR, the reinfusion of 80 million tumor cells might indeed contribute to relapse. After the in vivo purging, tumor cells were detectable in only 14% of stem cell products, in accordance with previous reports.19 A recent meta-analysis of available data indicates that rituximab improves remission induction, PFS, and OS in MCL.37 In addition, ít greatly diminishes the concern about tumor cell reinfusion in autologous transplantation of mantle cell lymphoma.
Can this approach be curable? The emerging PFS plateau after 5 years could raise the hope that a proportion of younger MCL may be cured, but this will take longer follow-up to demonstrate than our median follow-up of only 3.4 years. Accordingly, cohorts like the present should be followed and duly reported for a long time. Long-term follow-up with rigorous restaging including CT scans, however, poses certain problems, partly ethical, partly concerning radiation doses. We found it difficult to impose rigorous restaging for a longer period than 5 years, well aware that smoldering subclinical relapse might go undetected for a while. We estimate, however, that the majority of MCL relapses will soon be symptomatic or clinically detectable at the regular visits on physical and laboratory examination.
In conclusion, we report here a significantly improved outcome of MCL in younger patients, compared with other reports to date, including our own MCL-1 trial, presumably because of the intensification of the induction therapy to include high-dose cytarabine and rituximab. Our results indicate a shift of the paradigm of MCL as one of the subtypes of malignant lymphoma with the most dismal prognosis. The projected 6-year EFS on intent-to-treat of 56% and PFS of 66% respectively, now suggest that a substantial proportion of younger patients with MCL may achieve long-term freedom from lymphoma and perhaps cure.
The online version of this article contains a data supplement.
Presented in part at the 49th Annual Meeting of the American Society of Hematology, December 11, 2007, Atlanta, GA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the medical and nursing staffs of all contributing departments and blood banks for their contribution and the patients for their willingness to participate.
This work was supported by grants from the Danish Cancer Society, the Nordic Cancer Union, the John and Birthe Meyer Foundation, and the NovoNordisk Foundation.
Authorship
Contribution: All authors have taken part in the creation of this protocol during NLG plenary meetings and have contributed to data collection and reporting; C.H.G., P.B., and N.S.A. analyzed the data; C.H.G., A.L., E.E., A.K., and M. Eriksson wrote the manuscript; C.H.G. made the figures; and all coauthors subsequently collaborated on finalizing the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian H. Geisler, Department of Hematology L4042, Rigshospitalet, DK 2100 Copenhagen, Denmark; e-mail: christian.geisler@rh.regionh.dk.