In this issue of Blood, Bellon and Nicot describe an elegant mechanism used by HTLV-I to up-regulate telomerase gene expression and function in the absence of the viral tax oncoprotein.
Adult T-cell leukemia was first reported as an entity in Blood in 1977.1 Three years later, Gallo et al identified the human T-cell leukemia/lymphoma virus-I (HTLV-I), a deltaretrovirus, as the causative agent for this debilitating disease.2 Currently, the estimated number of people infected with HTLV-I worldwide is between 15 and 25 million. However, due to insidious characteristics of this virus, most HTLV-1–infected individuals remain asymptomatic for at least 20 to 30 years before developing HTLV-related clinical disorders. Besides causing adult T-cell leukemia and lymphoma (ATLL), HTLV-I has been associated with other human diseases, including a neurologic disorder known as HLTV-I–associated myelopathy/tropical spastic paraparesis, and perhaps other hematologic and nonhematologic disorders.
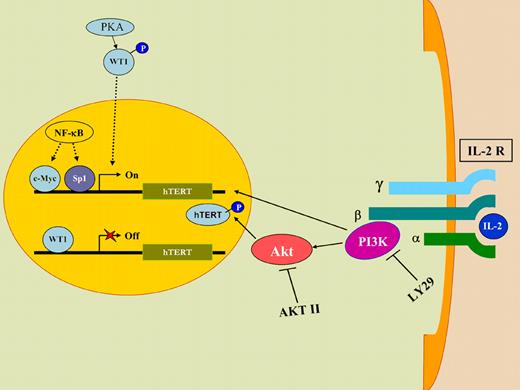
HTLV-I has been intensely studied, primarily because it has the capacity to transform primary human T cells in vitro and in vivo.3 To convert a normal T cell to a leukemic cell, the HTLV-I tax oncoprotein must participate in several cellular pathways in order to overcome innate cellular barriers to transformation.4 To achieve clonal expansion in the early phase of viral infection, HTLV-I–infected cells also acquire an increased capacity to replicate DNA. The complete replication of linear chromosomal DNA relies on the enzymatic activity of a specialized cellular RNA-dependent DNA polymerase (telomerase) that synthesizes telomeric sequences at the 3′ ends of linear chromosomes.5 The tax protein of HTLV-I has been shown to up-regulate expression of the catalytic protein subunit of telomerase (hTERT) in infected cells, via the nuclear factor κB (NF-κB) signaling pathway.6 Clonal expansion of HTLV-I–infected cells is also dependent on the presence of interleukin-2 (IL-2), as this cytokine is required for the differentiation and long-term proliferation of T cells.7 However, as noted by Bellon and Nicot, expression of tax protein in IL-2–dependent cells and in primary tissues of ATLL patients is almost undetectable, yet these cells retain high levels of telomerase enzymatic activity. These observations suggest an alternative mechanism for telomerase activation, independent of the viral tax protein. To establish a link between IL-2 and telomerase activation, the authors withdraw this growth factor in cultures of immortalized HTLV-I–infected cells and show that telomerase activity is reduced. By using known pharmacological inhibitors of PI3K, a downstream target of the IL-2 receptor complex, and of its immediate downstream target Akt, the authors show that these inhibitors can also reduce telomerase activity, suggesting that the PI3K/Akt pathway is involved in activating telomerase in virus-infected cells. Perhaps more importantly, the authors provide data to show that PI3K regulates telomerase gene expression at a transcriptional level independent of Akt, which increases telomerase activity by phosphorylating hTERT posttranscriptionally, and that IL-2 results in sequestration of a known transcriptional repressor of the hTERT gene, WT1, in the cytoplasm, thereby increasing telomerase gene expression and function.
A model of how HTLV-I can up-regulate telomerase gene expression and function in the absence of the viral tax oncoprotein. Upon IL-2 stimulation, PI3K transcriptionally up-regulates telomerase (hTERT) gene expression, whereas Akt increases telomerase function by phosphorylating hTERT protein. LY29 and AKTII, known pharmacological inhibitors of PI3K and Akt, respectively, reduce telomerase activity, confirming the involvement of the PI3K/Akt pathway in activating telomerase function. IL2 stimulation also results in sequestration of WT1, a known transcriptional repressor of hTERT gene, in the cytoplasm of the cell, thereby increasing telomerase gene expression and function.
A model of how HTLV-I can up-regulate telomerase gene expression and function in the absence of the viral tax oncoprotein. Upon IL-2 stimulation, PI3K transcriptionally up-regulates telomerase (hTERT) gene expression, whereas Akt increases telomerase function by phosphorylating hTERT protein. LY29 and AKTII, known pharmacological inhibitors of PI3K and Akt, respectively, reduce telomerase activity, confirming the involvement of the PI3K/Akt pathway in activating telomerase function. IL2 stimulation also results in sequestration of WT1, a known transcriptional repressor of hTERT gene, in the cytoplasm of the cell, thereby increasing telomerase gene expression and function.
One potential problem with this approach is that the 2 small molecule inhibitors of PI3K/Akt activity used in the study may have other targets in the cell that are not specific to the PI3K/Akt pathway. It would next be important to investigate whether the same effects are seen in cells deficient for components of the PI3K/Akt pathway (eg, knockout or knockdown cells) or in cells expressing dominant negative mutants of some components in this signaling pathway. In addition, as the authors have correctly pointed out, it is not known if PI3K is directly responsible for WT1 cytoplasmic retention or whether additional IL-2R signaling pathways are activated and/or repressed in order to increase telomerase functions in ATLL patients.
In summary, Bellon and Nicot provide experimental evidence to explain a seemingly paradoxical phenomenon, in which even in the absence of the HTLV-I tax protein, a strong inducer of telomerase gene expression, telomerase activity remains elevated in IL-2–dependent cells and ATLL patient samples. This is consistent with the fact that after HTLV-I–infected cells become transformed, they no longer require tax protein or IL-2 stimulation. For the first time, these authors show that the PI3K/Akt signaling pathway is invoked by HTLV-I infection to effect telomerase activation and long-term proliferation of infected cells. This study underscores the importance of this signaling pathway in regulating telomerase function in cancer cells, as both telomerase and PI3K/Akt functions are commonly found to be activated in cancer cells.8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal