The elusive mystery surrounding the intrinsic thermal instability of TAFI has finally been solved, thanks to the determination of the first crystal structure of TAFI, which uncovers a highly dynamic local structure responsible for its self-destruction.
As an important regulator in the blood coagulation system, linking the systems of coagulation and fibrinolysis,1,2 thrombin-activatable fibrinolysis inhibitor (TAFI) plays a significant physiological role, a role strictly controlled by a unique and mysterious autoregulatory mechanism. Since its discovery in the mid-1990s, a tremendous amount of effort has been invested in TAFI research, with more than 400 papers published in just 14 years (reviewed in Boffa and Koschinsky,3 Nesheim and Bajzar,4 and Bouma and Mosnier5 ).
Similar to many other procarboxypeptidases, the TAFI zymogen is activated through cleavage of an activation peptide to form activated TAFI (TAFIa), which is involved in the inhibition of plasmin-mediated clot lysis. However, once activated, TAFIa “destroys” itself in a matter of minutes, an act beautifully synchronized with the physiological process. Indeed, it has been long known that this “suicidal” action is achieved by the intrinsic structural instability of TAFIa.6,7 However, the molecular mechanism and structural basis underlying this “time bomb,” which leads to its self-destruction, have remained elusive. The striking difference in stability between TAFIa and other carboxypeptidases, despite high sequence homology (over 40% identity with human procarboxypeptidase B, for example), has left researchers completely puzzled until now.
The determination of the X-ray structure of TAFI by Marx and colleagues represents a major breakthrough in solving this mystery. At first glance, the overall structure, which is quite similar to those of other carboxypeptides, provides no surprises or immediate clues to the underlying basis for TAFI's intriguing dynamic instability. Indeed, this would explain why molecular modeling has not been informative.8 However, the “devil” is in the details. Through careful and systematic structural analyses, Marx et al have discovered a flexible “flap” that controls the fate of TAFI. The temperature factors (an indicator of thermal flexibility) in the “flap” segment are significantly higher than the average for the whole protein for each of the 3 crystallographically-independent molecules. This fact is further supported by the varying electron density levels for this region among the 3 molecules. These results, in conjunction with inhibitor complex and mutant structures, convincingly demonstrate that this “flap” is the “time bomb,” which is stabilized by interactions with the activation peptide. Removal of this peptide triggers a drastic increase in plasticity and, consequently, the complete unfolding of TAFIa. Thus, the dynamic “flap” is an inherent, flexible “hot spot” which lies dormant until destabilization is triggered by removal of the activation peptide, leading to the self-destruction of TAFIa through its complete unfolding.
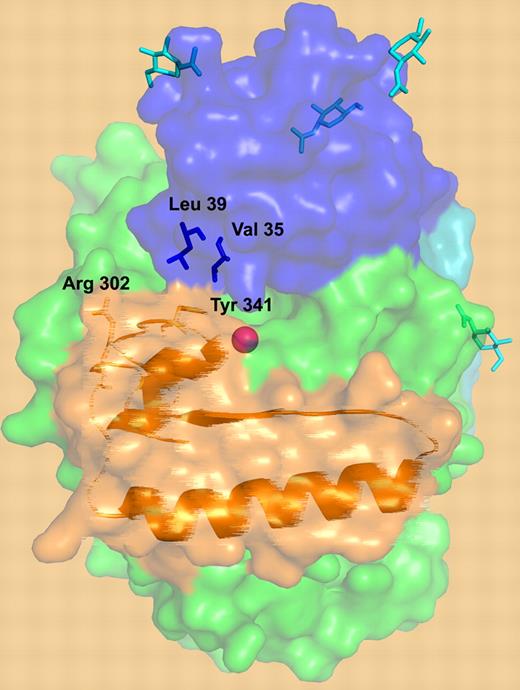
A surface representation of TAFI structure. The activation peptide is shown in blue, the catalytic domain is in green, and the dynamic “flap” residues are depicted in orange. Critical residues are depicted: residues involved in the interactions between the dynamic flap (Y341) and the activation peptide (V35 and L39), the cryptic thrombin cleavage site at Arg302, the N-linked glycans (cyan), and the catalytic zinc ion (magenta sphere). An artistic “blurring” rendition of the “flap” is utilized to depict its dynamic nature.
A surface representation of TAFI structure. The activation peptide is shown in blue, the catalytic domain is in green, and the dynamic “flap” residues are depicted in orange. Critical residues are depicted: residues involved in the interactions between the dynamic flap (Y341) and the activation peptide (V35 and L39), the cryptic thrombin cleavage site at Arg302, the N-linked glycans (cyan), and the catalytic zinc ion (magenta sphere). An artistic “blurring” rendition of the “flap” is utilized to depict its dynamic nature.
The newfound TAFI structure also explains much of the experimental data that has accumulated over the years. For example, it provides an elegant explanation for why mutations remotely located from the active site (IIYQ) have such a large impact on TAFI's stability. Moreover, it provides a structural rationalization for the biphasic decrease in tryptophan fluorescence during inactivation as well as for how the cryptic thrombin cleavage site at Arg302 becomes proteolytically accessible. Indeed, the discovery of this dynamic “flap” provides the long sought-after structural basis for many of the puzzles underlying TAFI autoregulation.
The “weak link” in this structural model of dynamic instability is the rather limited interaction of the activation peptide with the flexible “flap.” Only a single “flap” residue, Y341, provides a direct link to the activation peptide via hydrophobic interactions with V35 and L39. Yet this interaction putatively has a large influence on “flap” plasticity. Marx et al also suggest that the N-glycan on the activation peptide could play a role in stabilizing the “flap.” Are these interactions sufficient to stabilize the flap in its “dormant” state, or is this weak interaction a requirement to enable a quick trigger upon activation? Further experiments, such as mutagenesis, will be required to address this issue.
Determination of the structure of TAFI will now facilitate further structure-based optimization of inhibitory compounds. Additionally, if the contact between the activation peptide and the dynamic “flap” proves to be critical, one could attempt to weaken or interfere with this contact and design therapeutic TAFI activators.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal