Abstract
Red blood cell (RBC) transfusion is unique as a common large-scale intravenous introduction of foreign tissue and provides a valuable opportunity to study human immunologic response to intravenous foreign antigen. Patients receiving RBC transfusions are at risk of forming alloantibodies against donor RBC antigens, and valid estimates of alloimmunization risk are clinically important, but little is known about the factors governing this risk or, more generally, about determinants of human response to intravenous antigen. Here, we mine large electronic patient databases enabling us to model RBC alloimmunization as a stochastic process. We identify a subgroup of transfusion recipients that has a dramatically increased risk of alloimmunization that appears to be genetically determined because it is independent of common disease states, patient age, or the number of alloantibodies already formed, and only weakly dependent on transfusion count.
Introduction
Alloimmunization to red blood cell (RBC) antigens is of clinical importance in the practice of transfusion medicine and in the basic understanding of the human immune system. Because of the large number of polymorphic antigens and the large number of epitopes on each antigen, every RBC transfusion will introduce many foreign alloantigens.1 Physicians and blood banks expend significant resources on the limited number of patients who have mounted an immune response to RBC antigens. These patients often develop hemolysis; and if the hemolysis is symptomatic, the patients require an evaluation to diagnose the cause of the hemolysis and sometimes treatment to manage the effects of hemolysis and/or to correct the resulting anemia. Even if a patient does not develop symptomatic hemolysis, blood banks often need to determine the specificities of anti-RBC antibodies and locate RBC units lacking the corresponding antigens for transfusion. Once located, these RBC units usually require cross-match with the antihuman globulin technique to ensure compatibility of the RBC units.
An alternative approach for which there has been increasing interest would be to prospectively match patients and RBC units for multiple antigens. This approach is becoming feasible with the advent of DNA technology to determine extended RBC phenotypes of donor units and patients. Using this technology, laboratories may soon be able to type multiple RBC antigens simultaneously and inexpensively.2 However, even with the introduction of such technology, inventory limitations will prevent blood banks from providing extended matched RBC units for all patients. Hence, blood banks would need to prioritize the patients who prospectively receive extended matched RBC units.
If blood banks could predict who is most likely to make antibodies to RBC antigens, then they could prioritize extended matched RBC units for those patients. Although significant strides have been made in understanding the immune system, we cannot predict a priori who will respond immunologically to a transfusion containing foreign alloantigens. Progress in scientific understanding has been limited in part because most studies use animal models and in part because of the costs and ethical limitations associated with comparable human studies. One promising approach for studying the human immune system would involve analyzing variations in the responses between individuals in large populations who have been exposed to foreign antigens, for instance, during blood transfusion. People are frequently exposed to foreign antigens from blood transfusions, and there is significant variation in their immunologic response. However, transfusions are not controlled experiments in which one could control specific variables that may affect the immune responses to RBC transfusions. Each patient has a unique set of genes, has a specific disease state or states, and is transfused with a donor RBC unit with characteristics dependent on the genetics and physiologic state of the donor and the preparation and storage of the RBC unit. Nevertheless, if analysis were to demonstrate that certain groups of patients are more likely to respond immunologically to transfusions, then common characteristics among members of an immunologically responsive group would be hypothesized to be associated with, and perhaps to contribute to, the human immune response.
Several authors have speculated that there exists a subpopulation of patients (“responders”) who respond to RBC antigens to form antibodies at a much higher rate than the general transfused population.3-8 In other words, given the same number of exposures to RBC antigens, these responders will form many more antibodies than the general transfusion recipient population. Because each transfusion provides additional exposures, one expects greater numbers of transfusions to lead to greater numbers of alloantibodies. Although there is evidence for a generally increasing trend in alloantibodies with increasing numbers of transfusions,7,9,10 to our knowledge the incremental risk associated with each additional transfusion has not been measured.
Furthermore, we find no definitive demonstration of the existence of a subpopulation of responders in the published literature, despite frequent anecdotal speculation by transfusion medicine physicians that patients who form 2 or more alloantibodies have an intrinsically elevated risk. Efforts to implicate HLA variants have so far been unsuccessful, except in the case of a few isolated antigens.3,4 Some authors have proposed that sickle cell patients may be responders because they appear to form alloantibodies at a rate significantly higher than one would expect even after taking in to consideration the large number of transfusions they receive.5-7 It would be especially important to identify responders among sickle cell patients and others requiring frequent transfusion because alloantibodies significantly increase the difficulty of finding compatible units. Despite these frequent suggestions of higher response rates for sickle cell patients and those with other diseases,8 there are no definitive demonstrations of a substantially increased risk of alloimmunization for any particular subgroup of patients.
In this study, we provide estimates of alloimmunization risk by analyzing retrospective blood bank data for an adult and a pediatric patient population. We measure antibody frequencies in these 2 populations and conclude that alloimmunization in the transfused population depends very weakly on the number of transfusions a patient has received. We further find strong evidence for the existence of a subgroup of patients who have a significantly higher risk of alloimmunization. Indeed, we demonstrate that almost all patients who form any alloantibodies have a significantly higher risk of subsequent alloimmunization than the general transfused population and that their increased alloimmunization rate does not depend on the number of existing antibodies.
Methods
Patients
Institutional Review Board approvals for this study were obtained at Brigham and Women's Hospital and Children's Hospital Boston. We measured alloimmunization rates of all patients who were transfused over a 2-year period at a large tertiary care hospital and antibody frequencies in all patients who formed alloantibodies during a 15-year period (1991-2005) at 2 large tertiary care hospitals, one of which is a pediatric hospital. Both hospitals have active general surgery, cardiac surgery, oncology, trauma, and intensive care units whose patients frequently require transfusions. In addition, the adult hospital treats obstetric patients, including those at high risk, and the pediatric hospital has a thalassemia program. Except in the rare case of emergency, patients receive Rh(D)-compatible RBCs. There is an active sickle cell program at these hospitals in which these patients prospectively receive RBC units matched for C, E, and K antigens, and any antibodies for these patients were therefore excluded from the analysis. Patient records were accessed in accordance with research protocols approved by the Institutional Review Boards of Partners Healthcare and Children's Hospital Boston. We analyzed transfusion data for each patient at a single hospital. Some patients may have received transfusions at other hospitals or at times outside the scope of our analysis, but this single-hospital transfusion history provides a lower bound on the number of transfusions for each patient and serves as a reasonable estimate of the relative numbers of transfusions in each group.
Antibody detection
Antibody screens were valid for 3 days for recently transfused patients in both hospitals. Antibody screen methods for the pediatric population used standard tube techniques using low ionic strength saline in the beginning of this period and used polyethylene glycol enhancement for the latter portion of this period.11 Methods for the adult population studied from 1991 to 2005 include solid phase, gel, and polyethylene glycol.12,13 We defined “alloantibody” to include all antibodies reactive with RBC alloantigens. A small number (< 5%) of these antibodies were autoreactive as well.
Data acquisition
We generated a report of all antibodies detected in the blood bank of a large pediatric hospital for a 15-year period from the laboratory information system. Data for the adult population were identified using the Partners Healthcare System Research Patient Data Registry, which can identify patients with specific demographics, diagnoses, laboratory tests, medications, molecular medicine, health history, microbiology, procedures, providers, and/or transfusion services. We identified all patients at the hospital with a history of alloimmunization for a 15-year period from 1991 to 2005. These data were imported into Microsoft Access (Microsoft, Redmond, WA). Scripts were written in MATLAB (MathWorks, Natick, MA) for data analysis and statistical modeling.
Diagnostic definitions
“Pregnant” refers to patients with a coded diagnosis of current or past pregnancy in their electronic medical record.
“Sickle” includes patients with a diagnosis of sickle cell disease.
“Atherosclerosis” includes patients with a diagnosis of one of the following: atherosclerosis, angina, coronary disease, myocardial disease, or ischemic cardiac disease.
“Diabetes” includes patients with a diagnosis of diabetes mellitus.
“Hematologic malignancy” includes patients with a diagnosis of leukemia, lymphoma, or multiple myeloma.
“Orthopedic” patients are those with a diagnosis of fracture.
“Thalassemia” includes all patients who have a diagnosis of thalassemia.
“Inflammatory bowel disease” includes all patients with a diagnosis of Crohn disease or ulcerative colitis.
Results
The weak effect of transfusion count
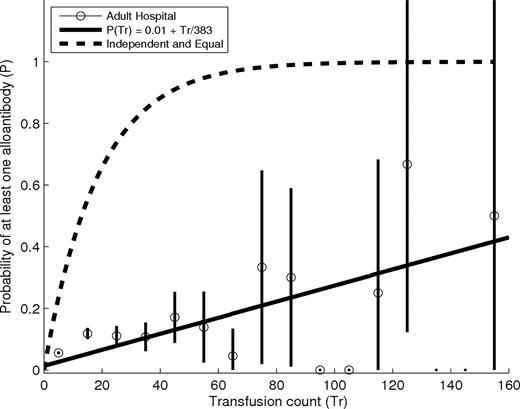
We first measured the overall prevalence of alloimmunization by determining the proportion of patients transfused in a 2-year period who formed new alloantibodies. A new alloantibody was any alloantibody for which there was serologic laboratory testing proving its absence before a transfusion as well as serologic evidence of its presence after a transfusion. Although anti-Rh(D) antibodies were included in this analysis, because patients generally receive Rh(D) compatible RBCs, our results may not apply to the immunology of this antigen, which may have very different properties.14 Our results treated all RBC transfusions equally and would not have detected differences that may be associated with massive transfusion occurring in a short period of time. Our results do not apply to immunization to non-RBC alloantigens, such as those on platelets or white blood cells (“Methods”). At our adult hospital, 13 255 patients received at least one RBC transfusion in 2003 or 2004, and 567 (∼4%) of these patients formed new alloantibodies as determined by antibody screens performed between 2003 and 2005. This 4% estimate of the prevalence of alloimmunization in the general transfusion recipient population is consistent with previous estimates,3,9 but experience suggests that some subgroups of patients have a much higher prevalence. An intuitive initial hypothesis is that transfusion count governs a patient's risk of alloimmunization. Under this model, differences in alloimmunization rate between patients simply reflect differences in transfusion counts. Although there is evidence for a generally increasing relationship between numbers of alloantibodies and transfusion count,7,9,10 prior studies have not precisely measured the incremental risk associated with each additional transfusion. Figure 1 shows how the prevalence of alloimmunization changes with average transfusion count at an adult study hospital during the study period.
Percentage of all transfused patients at the adult study hospital who are alloimmunized as a function of transfusion count. Circles represent the proportion of transfused patients who have developed at least one alloantibody binned by transfusion count. The first bin includes all patients transfused with 0 to 10 units, the second bin includes all patients transfused with 11 to 20 units, etc. The black lines are centered in each bin and extend up and down by 2 sample SDs from the mean. The solid line shows a least-squares linear fit to the data. This fit suggests that many (∼190) additional transfusions are needed before 50% of patients will form an alloantibody. The dashed line shows the relationship if each transfusion had an independent effect equal to that of the first, in which case approximately 17 transfusions would be required to cause 50% of patients to form alloantibodies.
Percentage of all transfused patients at the adult study hospital who are alloimmunized as a function of transfusion count. Circles represent the proportion of transfused patients who have developed at least one alloantibody binned by transfusion count. The first bin includes all patients transfused with 0 to 10 units, the second bin includes all patients transfused with 11 to 20 units, etc. The black lines are centered in each bin and extend up and down by 2 sample SDs from the mean. The solid line shows a least-squares linear fit to the data. This fit suggests that many (∼190) additional transfusions are needed before 50% of patients will form an alloantibody. The dashed line shows the relationship if each transfusion had an independent effect equal to that of the first, in which case approximately 17 transfusions would be required to cause 50% of patients to form alloantibodies.
Overall, the prevalence of alloimmunization among all transfused patients is weakly dependent on transfusion. If each additional transfusion had an independent effect equal to that of the first transfusion, which induces alloimmunization in approximately 4% of those with no alloantibodies, we would expect 50% of patients to be alloimmunized after approximately 17 transfusions, as shown by the dashed line in Figure 1. In contrast, data from our adult study hospital suggest that many more transfusions are required, perhaps as many as 190 or more, before 50% of patients will form alloantibodies. Differences in alloimmunization risk are therefore unlikely to be strongly determined by transfusion count.
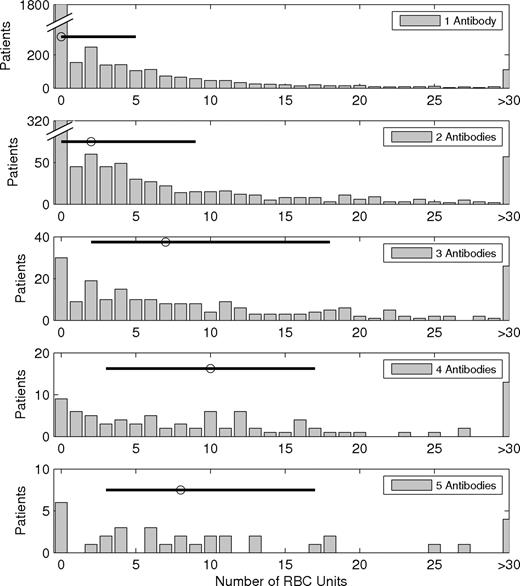
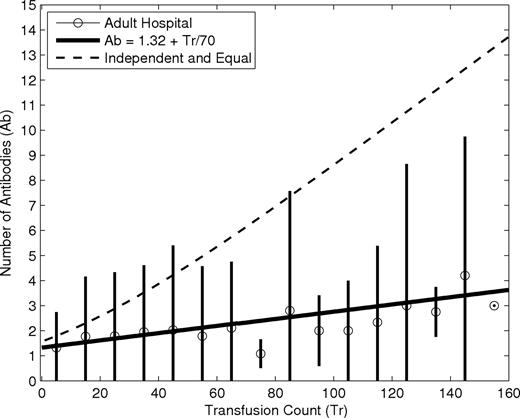
The large variation in transfusion count for patients with the same number of alloantibodies, as shown in Figure 2, also suggests that the effect of transfusion is weak. For example, more than 100 patients who formed just one antibody received more than 25 transfusions in the adult study hospital. It is almost certain that these highly transfused patients were exposed to many alloantigens to which their immune system failed to respond. Figure 3 demonstrates that, even among alloimmunized patients, the expected number of alloantibodies increases very slowly. There is a generally increasing trend in alloantibodies as the average number of transfusions increases, but the effect is very small, consistent with prior studies.5 A linear least-squares fit to the data suggests that approximately 70 transfusions beyond the first are required to increase the number of alloantibodies by one, whereas fewer than 20 transfusions would be required if each had an independent effect equal to that of the first transfusion. We calculated the “equal and independent” curve in Figure 3 by first measuring the probability distribution for the number of antibodies formed after a single transfusion. This distribution included all patients who had a negative antibody screen when they were transfused and who subsequently received an antibody screen. In 2003 and 2004, there were 4242 transfusions at our adult study hospital with associated antibody screens occurring at least 30 days later, allowing us to determine whether or not each of these transfusions led to the formation of alloantibodies. Although this retrospective analysis could be biased, because we cannot be certain that the subset of transfused patients who received an antibody screen 30 days later is a representative subset of all patients transfused, we have no reason to believe this sample is not representative. In addition, although we cannot separate the effects of multiple units transfused in the same time period, the estimate is valid for the purpose of qualitative comparison. Of these screened transfusions, 142 resulted in the formation of a single antibody, 50 resulted in the formation of 2 antibodies, 19 in 3, 11 in 4, 3 in 5, and 1 in 6. We then use this empirical probability distribution for the number of antibodies formed after a single transfusion to calculate the probability distribution for the number of antibodies formed after multiple transfusions. Under the assumption that the number of antibodies formed after each individual transfusion is independent and identically distributed, the distribution of the number of antibodies formed after multiple transfusions is equal to the repeated convolution of this single transfusion distribution.15 These results all suggest that transfusion count has a very weak effect on alloimmunization risk.
RBC transfusion counts for patients at an adult study hospital who formed different numbers of antibodies. The histogram shows the number of patients receiving a particular number of transfusions. The median number of transfusions is shown as a circle, and the horizontal line extends from the 25th percentile to the 75th percentile. The median for the single-antibody group is 0, meaning these patients responded to transfusions received outside our study hospital or study period, or had naturally occurring alloantibodies, or acquired their antibodies through some means other than transfusion, such as pregnancy.
RBC transfusion counts for patients at an adult study hospital who formed different numbers of antibodies. The histogram shows the number of patients receiving a particular number of transfusions. The median number of transfusions is shown as a circle, and the horizontal line extends from the 25th percentile to the 75th percentile. The median for the single-antibody group is 0, meaning these patients responded to transfusions received outside our study hospital or study period, or had naturally occurring alloantibodies, or acquired their antibodies through some means other than transfusion, such as pregnancy.
Number of alloantibodies as a function of transfusion count for patients with at least one alloantibody at an adult study hospital: 4452 patients, 6302 alloantibodies, and 28 106 transfusions. Circles represent the mean number of antibodies for patients binned by transfusion count. The black lines are centered in each bin and extend up and down by 2 sample SDs from the mean. There are 3 patients with more than 250 transfusions not shown. The line shows a least-squares linear fit to the data. This fit suggests that many (∼70) additional transfusions are needed for the acquisition of a single additional antibody. The dashed line shows the relationship if each transfusion had an independent effect equal to that of the first.
Number of alloantibodies as a function of transfusion count for patients with at least one alloantibody at an adult study hospital: 4452 patients, 6302 alloantibodies, and 28 106 transfusions. Circles represent the mean number of antibodies for patients binned by transfusion count. The black lines are centered in each bin and extend up and down by 2 sample SDs from the mean. There are 3 patients with more than 250 transfusions not shown. The line shows a least-squares linear fit to the data. This fit suggests that many (∼70) additional transfusions are needed for the acquisition of a single additional antibody. The dashed line shows the relationship if each transfusion had an independent effect equal to that of the first.
Evidence for a separate subgroup of responders
Alternatively, we hypothesize that there exists a separate small subgroup of patients who respond to RBC alloantigens at a significantly higher rate than the general population such that almost all alloimmunized patients are “responders.” We further hypothesize that alloimmunization for these responders is, as we have just shown, largely independent of the number of transfusions they receive and is also independent of the number of antibodies already formed. If almost all of the 4% of the general population who make alloantibodies are responders, we can evaluate their alloimmunization risk by restricting our analysis to those who are alloimmunized. In general, we do not expect the presence or absence of one alloantibody to affect the probability of forming additional alloantibodies, except when there is an association between the genes for the antigens in question. Thus, we can evaluate the risk of forming an additional antibody by comparing the number of individuals (N) with n antibodies (Nn) to the number with n + 1 antibodies (Nn + 1). We expect that this ratio (Nn/Nn + 1) will be constant for increasing values of n. We predict, then, that the process of alloantibody formation operates with no “memory” of the number of antibodies the patient has already formed as a result of current or prior transfusions. For example, under this responder hypothesis, we expect the probability that a patient with 2 antibodies will form a third antibody is the same as the probability that a patient with 8 antibodies will form a ninth. Such a stochastic process is called “memoryless”15 because it operates as if the immune system does not remember how many antibodies have already been formed.
Because antibody counts are discrete quantities, alloimmunization under this responder hypothesis can be modeled as a discrete stochastic process with the “memorylessness” property. The geometric probability distribution [P(n; p) = probability of forming n antibodies = (1 − p)pn] is the only discrete probability distribution with this property.15 The parameter p represents the probability of forming an additional antibody and does not vary with n. We therefore test the responder hypothesis by fitting a geometric probability distribution to alloantibody counts for patients at each of our 2 study hospitals as well as those from a study published previously.9 We note that, unlike many other biologic systems, the process of RBC alloimmunization is not a Poisson process because it is possible for multiple antibodies to be formed after the introduction of even very few foreign RBCs. The process of RBC alloimmunization therefore violates the law of rare events intrinsic to the Poisson process.16 Frequencies of alloimmunized patients forming different numbers of antibodies are shown in Figure 4.
Frequencies of patients with different numbers of antibodies. (Top panels) Data from a large adult hospital. (Middle panels) Data from a large pediatric hospital. (Bottom panels) Data from a previously published study.9 The left panels have a linear y-axis scale. The right panels show the same data with a logarithmic y-axis scale. Circles are actual data points. Error bars extend 2 sample SDs. Note that error bars are longer for larger numbers of antibodies first because the number of patients is small and second because the logarithmic scale amplifies length for smaller values. Lines are least-squares fits to a geometric probability distribution: (1 − p)pn. The distribution parameter p is the number of patients with n + 1 antibodies as a fraction of those with n; 95% confidence intervals for p are shown in the legends.
Frequencies of patients with different numbers of antibodies. (Top panels) Data from a large adult hospital. (Middle panels) Data from a large pediatric hospital. (Bottom panels) Data from a previously published study.9 The left panels have a linear y-axis scale. The right panels show the same data with a logarithmic y-axis scale. Circles are actual data points. Error bars extend 2 sample SDs. Note that error bars are longer for larger numbers of antibodies first because the number of patients is small and second because the logarithmic scale amplifies length for smaller values. Lines are least-squares fits to a geometric probability distribution: (1 − p)pn. The distribution parameter p is the number of patients with n + 1 antibodies as a fraction of those with n; 95% confidence intervals for p are shown in the legends.
Data from both our adult and our pediatric populations follow a geometric probability distribution with remarkable fidelity. The geometric distribution explains more than 99.97% of the variation in the data for both sets of data. This approximation is equally valid for data from another large population published previously9 and shown in Figure 4, with more than 99.99% of the variation explained by the geometric fit. Therefore, alloimmunization in patients who make antibodies appears to be a memoryless process in which additional antibodies are acquired randomly and independently of both the number of existing antibodies and any differences in the number of prior transfusions. The frequency of acquiring an additional antibody in this alloimmunized population is described very accurately by the distribution parameter p and is estimated to be approximately 30%. In other words, for every 100 patients with one alloantibody, there are approximately 30 with 2. For every 100 with 2 antibodies, there are 30 with 3, and for every 100 with 3 alloantibodies, there are 30 with 4.
It is important to note that the 30% proportion does not reflect a per-transfusion risk. Instead, it implies that, after a “typical” number of transfusions, there will be 30% as many patients with n + 1 antibodies as there will be patients with n antibodies. Because changes in transfusion number are not important, as shown in Figure 1, substantial deviations from this typical number of transfusions will not significantly change the rate at which patients form alloantibodies.
We can test this hypothesis by measuring the effect of a history of alloimmunization on the risk of future alloimmunization. Our responder hypothesis predicts that almost all alloimmunized individuals are responders and will therefore have a significantly higher frequency of future alloimmunization than patients who have not reacted to prior transfusions. We identified all transfusions during a 2-year period at our study hospital that were given to previously transfused patients and were followed by antibody screens at least 30 days later.17 We are then able to measure the per-transfusion risk of alloimmunization for all patients who have a history of transfusion and compare this risk between those who formed alloantibodies in response to their previous transfusions and those who did not. We find 84 such screened transfusions given to previously alloimmunized patients, 16 of which resulted in new antibodies, giving a per-transfusion alloimmunization risk of 19%. In contrast, 4158 such transfusions were given to previously transfused patients who had not formed alloantibodies, and 216 of them were followed by positive alloantibody screens, for a per-transfusion risk of 5%. The alloimmunization rates of 19% and 5% for these 2 groups are different with a P value less than 10−6. As the responder hypothesis predicts, among previously transfused patients, those with a history of alloimmunization are at a significantly increased risk of further alloimmunization, consistent with observations in prior studies.18,19
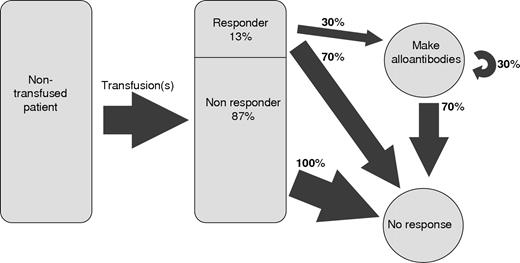
We use this model to estimate the proportion of responders among the general transfusion recipient population. Based on Figure 4, we infer that approximately 30% of responders are alloimmunized. In addition, because the observed curves in Figure 4 closely fit a geometric distribution with a 30% alloimmunization risk, the process of alloimmunization is probably homogeneous for all of these alloimmunized patients, meaning that virtually all patients in this population are responders. Hence, the 4% of the general population that is alloimmunized represents 30% of the responder population and the entire responder population must then make up approximately 0.04% to 0.30% to approximately 13% of the recipient population. Figure 5 is a schematic of this stochastic model of alloimmunization.
Schematic of the responder hypothesis. A transfusion recipient is represented by the rectangle on the left; 13% of these patients are responders and have a 30% chance of forming additional antibodies. The other patients form no antibodies.
Schematic of the responder hypothesis. A transfusion recipient is represented by the rectangle on the left; 13% of these patients are responders and have a 30% chance of forming additional antibodies. The other patients form no antibodies.
The responder phenotype is independent of age and several disease states
It would be clinically useful to identify responders before they form their first alloantibody, perhaps on the basis of an unknown but recognizable trait that is sufficient for the responder phenotype. Ideally, every patient expressing this trait would be among the 13% of the transfusion recipient population comprising the entire responder population, and its prevalence will increase almost 8-fold (100%/13%) between the general transfused population and the alloimmunized population. We compared prevalences of common inflammatory and noninflammatory diseases among transfused and alloimmunized patients at our adult study hospital in Table 1 and did not find any significant changes. Prior studies have reported different alloimmunization rates in some patient subgroups, but these changes are generally quite small or involved small numbers of patients and are therefore consistent with our findings.3,8,20
Changes in prevalence of common diagnoses between transfused and alloimmunized populations
| Trait . | Prevalence in transfused, % . | Prevalence in alloimmunized, % . | Fold change . |
|---|---|---|---|
| Pregnancy | 2.5 | 3.0 | 1.23 |
| Diabetes | 24.5 | 28.0 | 1.14 |
| Atherosclerosis | 45 | 52 | 1.16 |
| Orthopedic fracture | 11 | 15 | 1.36 |
| Hematologic malignancy | 12 | 15 | 1.25 |
| Splenectomy | 3.5 | 4.5 | 1.28 |
| Trait . | Prevalence in transfused, % . | Prevalence in alloimmunized, % . | Fold change . |
|---|---|---|---|
| Pregnancy | 2.5 | 3.0 | 1.23 |
| Diabetes | 24.5 | 28.0 | 1.14 |
| Atherosclerosis | 45 | 52 | 1.16 |
| Orthopedic fracture | 11 | 15 | 1.36 |
| Hematologic malignancy | 12 | 15 | 1.25 |
| Splenectomy | 3.5 | 4.5 | 1.28 |
There is a 1.32-fold overall increase in the prevalence of all diagnoses in the alloimmunized population, but there are no large increases beyond this background increase and therefore nothing with an 8-fold increase in prevalence. The similar prevalences of the traits in both the transfused and alloimmunized populations, ie, P(diagnosis|alloimmunized)/P(diagnosis)∼1, imply that the prevalence of alloimmunization in each diagnostic group is similar to the overall prevalence of alloimmunization, consistent with prior observation8 :
We can learn more about the distribution of responders among these subgroups by fitting geometric distributions. Figure 6 shows that a current or past diagnosis of pregnancy is associated with a lower frequency of additional alloantibodies. These patients acquire alloantibodies less often (19%) than other responders (∼30%), suggesting that the process of alloimmunization during pregnancy may be fundamentally different from that occurring during transfusion. Patients who form alloantibodies during pregnancy may not be at increased risk of subsequent alloimmunization and perhaps should not be considered responders.
Frequencies of patients with different numbers of antibodies in diagnostic subgroups at an adult study hospital. The y-axis has a logarithmic scale. Circles represent actual data points. Error bars extend 2 sample SDs from the sample mean. Note that error bars are longer for larger numbers of antibodies first because the number of patients is small and second because the logarithmic scale amplifies length for smaller values. The lines represent least-squares fits to a geometric probability distribution: (1 − p)pn. The distribution parameter p is the risk of forming an (n + 1)th antibody after having formed an nth antibody; 95% confidence intervals for estimates of this parameter are shown in the legend of each panel. Smaller values of p lead to a steeper slope because the slope of this fit on a logarithmic scale is log(p), as can be seen in the top panel for the “pregnancy” patients, where the frequency falls to 10−5 faster than in the other panels.
Frequencies of patients with different numbers of antibodies in diagnostic subgroups at an adult study hospital. The y-axis has a logarithmic scale. Circles represent actual data points. Error bars extend 2 sample SDs from the sample mean. Note that error bars are longer for larger numbers of antibodies first because the number of patients is small and second because the logarithmic scale amplifies length for smaller values. The lines represent least-squares fits to a geometric probability distribution: (1 − p)pn. The distribution parameter p is the risk of forming an (n + 1)th antibody after having formed an nth antibody; 95% confidence intervals for estimates of this parameter are shown in the legend of each panel. Smaller values of p lead to a steeper slope because the slope of this fit on a logarithmic scale is log(p), as can be seen in the top panel for the “pregnancy” patients, where the frequency falls to 10−5 faster than in the other panels.
Because the estimates of the parameter p for diagnoses other than pregnancy in Figure 6 are so similar to the overall estimate of p and because alloimmunization prevalence in these diagnostic groups is similar to the overall prevalence, if we extrapolate to zero alloantibodies, we can conclude that the hypothetical responders comprise about the same fraction of patients in these diagnostic subgroups as they do in the overall alloimmunized population. In other words, Figure 6 also supports the surprising conclusion that the estimated proportion of responders (∼13%) may be robust across all of these diagnostic groups with the exception of pregnancy. Further, because our adult and pediatric patient populations appear to have similar alloimmunization prevalences as shown in Figure 4, it is unlikely that the responder phenotype is necessarily linked with age or with age-related diseases, such as atherosclerosis and diabetes. Indeed, there is no evidence that the responder phenotype is strongly correlated with any preexisting diagnostic or demographic group.
Discussion
The demonstration of the existence of a distinct population of responders will facilitate future studies to determine the factor(s) that cause people to be responders. Our results suggest that such factor(s) are not likely to be dependent on the disease state or on the age of the patient. Whereas some have proposed that inflammatory stimuli are randomly and inadvertently introduced into transfused units and lead to alloimmunization,21 our results are very consistent between different hospitals at different times, and these hospital blood banks used different RBC preservative solutions and had different leukoreduction methods and policies. Hence, although a characteristic of transfused RBCs may contribute to the immune response, it is not likely to be sufficient to induce an immune response. If it were sufficient to induce an immune response in approximately 4% of transfusions, then nearly all patients who have been transfused with at least 25 units should be alloimmunized. Our findings are entirely consistent with genetic factor(s) causing the responder phenotype; however, further studies would be required to prove this hypothesis.
Previous authors have speculated that RBC alloimmunization results from the unusual shunting of donor RBCs from less immunogenic compartments to more immunogenic compartments.21 We can speculate that non-HLA genetic variants might govern the immunologic handling of RBCs, determining whether they are shunted to immunogenic compartments and thus controlling the expression of the responder phenotype. Studies in mice have suggested that some sort of a danger signal is necessary in combination with antigen to cause alloimmunization.3 Although our analysis of a small number of diagnostic subgroups did not find any disease process associated with such a danger signal, the possible role for a danger signal warrants future investigation. Indeed, it is possible that, although all responders are genetically capable of mounting an immunologic response, the risk of doing so is only approximately 30% because the presence of a danger signal is also required to mount such a response.
Because almost all nonpregnant patients who have made at least one antibody are responders, blood banks may choose to provide RBC units that are matched at multiple antigens for all future transfusions for alloimmunized patients. An analysis of the data at our adult hospital estimates that potentially 14% (115/867) of all alloantibodies are formed by patients with prior positive antibody screens and therefore can possibly be prevented by extended phenotype matching. These preventable antibodies are more probable to be problematic than other alloantibodies exactly because they occur in patients with existing antibodies. The process of locating crossmatch-compatible blood products for these multiply alloimmunized patients can be extremely challenging, especially with the limited time available in emergencies. Future best clinical practice could involve identifying these patients as responders at the time of initial alloantibody identification and locating antigen-matched units when feasible. Currently, this policy would not be practical at most blood banks because it would require significantly increased testing of both patients and donor units. However, even using current technologies, some blood banks may be able to provide extended matched RBC units for those responders at increased risk of complications secondary to alloantibodies. Examples may include patients with sickle cell disease and females of childbearing potential.
In this study, we mined a large electronic patient database and developed a stochastic model enabling us to discover 2 distinct human populations with different immunologic responses to transfusions. Identification of these populations will facilitate investigations of factors conferring a responder phenotype, and the statistical model implies epidemiologic characteristics of such factors, further narrowing the search. This or similar statistical approaches could potentially be used to study other quantifiable medical and biologic conditions using large electronic patient databases. Even before the initiation of such studies, transfusion medicine specialists should view every patient who has made any alloantibody as a responder and should consider prospectively matching products for these patients if such measures are feasible.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Shawn Murphy, Henry Chueh, and the Partners Health Care Research Patient Data Registry group for facilitating use of their database, and Alfred Mass for assistance in acquiring data.
Authorship
Contribution: J.M.H. and S.R.S. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: S.R.S. has served on the scientific advisory board of Bioarray Solutions. The authors declare no other competing financial interests.
Correspondence: Steven R. Sloan, Blood Bank, Bader 406, Children's Hospital Boston, 300 Longwood Avenue, Boston, MA 02115; e-mail: steven.sloan@childrens.harvard.edu.


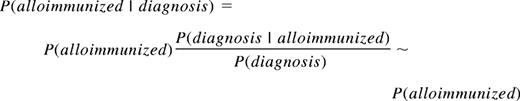

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal