Abstract
Expression of the fusion gene FIP1-like 1/platelet-derived growth factor receptor alpha (FIP1L1/PDGFRα, F/P) and dysregulated c-kit tyrosine kinase activity are associated with systemic mastocytosis (SM) and chronic eosinophilic leukemia (CEL)/hypereosinophilic syndrome (HES). We analyzed SM development and pathogenesis in a murine CEL model induced by F/P in hematopoietic stem cells and progenitors (HSCs/Ps) and T-cell overexpression of IL-5 (F/P-positive CEL mice). These mice had more mast cell (MC) infiltration in the bone marrow (BM), spleen, skin, and small intestine than control mice that received a transplant of IL-5 transgenic HSCs/Ps. Moreover, intestinal MC infiltration induced by F/P expression was severely diminished, but not abolished, in mice injected with neutralizing anti–c-kit antibody, suggesting that endogenous stem cell factor (SCF)/c-kit interaction synergizes with F/P expression to induce SM. F/P-expressing BM HSCs/Ps showed proliferation and MC differentiation in vitro in the absence of cytokines. SCF stimulated greater migration of F/P-expressing MCs than mock vector–transduced MCs. F/P-expressing bone marrow–derived mast cells (BMMCs) survived longer than mock vector control BMMCs in cytokine-deprived conditions. The increased proliferation and survival correlated with increased SCF-induced Akt activation. In summary, F/P synergistically promotes MC development, activation, and survival in vivo and in vitro in response to SCF.
Introduction
Systemic mastocytosis (SM) is characterized by the accumulation of neoplastic mast cells (MCs) in multiple organs and can exhibit either an indolent or an aggressive clinical course.1,2 The organs most frequently affected are bone marrow (BM), skin, liver, spleen, and the gastrointestinal tract.2,3 A subset of patients with SM has been shown to have a clonal MC and eosinophil expansion due to an interstitial deletion in chromosome 4q12, which results in the generation of a fusion gene, FIP1L1/PDGFRA (F/P).4,5 The F/P fusion gene product acts as a constitutively active tyrosine kinase.6 Eosinophilia (in BM and/or peripheral blood) commonly accompanies SM (20%-40% of cases, termed SM-Eo or SM-CEL) and up to one half of SM-Eo patients carry the F/P fusion gene.7 Expression of the F/P fusion gene has been detected in MCs as well as eosinophils because the F/P mutation occurs in multipotential hematopoietic progenitor cells capable of giving rise to multiple lineages.8,9 SM induced by constitutively active c-kit mutations and SM induced by FIP1L1/PDGFRα appear to have distinct clinical characteristics.10 The basis for the preferential expansion of eosinophils and MCs in the patients with the F/P fusion gene remains unclear. The role of the c-kit receptor in FIP1L1/PDGFRα-induced SM has not yet been explored.
Recently, we have established a murine F/P-positive CEL-like model by retroviral introduction of the F/P fusion gene into hematopoietic stem cells and progenitors (HSCs/Ps) in the presence of IL-5 transgene overexpression.11 The mice demonstrated severe eosinophilia with leukocytosis and tissue infiltrations of a large number of eosinophils in most organs. In these mice, as in patients with F/P-positive CEL, early hematopoietic progenitors are responsible for the F/P induction of the hypereosinophilic syndrome, implying that the F/P fusion gene may also be expressed in MCs in this murine model of F/P-positive CEL disease.12
Until now, only one murine myeloproliferative SM model has been reported, based on the c-kit receptor mutation D816V that transforms c-kit to a constitutively active tyrosine kinase.3,13 We postulated that a murine model of F/P-induced SM might represent an opportunity to clarify the mechanisms of tyrosine kinase–dependent SM and may be useful for developing alternative therapies in SM-CEL. In this study, we aimed to examine the impact of F/P expression on MC levels and MC distribution, activation, and signaling in response to the MC growth factor c-kit ligand (stem cell factor [SCF]).
Methods
Mice
Age- and sex-matched wild-type and CD2-IL-5 transgenic (Tg) BALB/c mice11,14 were used as BM donors. BALB/c (wild-type) female mice (7-12 weeks old) were obtained from Taconic Farms (Germantown, NY). All mice were maintained under specific pathogen-free conditions in Cincinnati Children's Hospital Animal Facility. Animal protocols were approved by the Animal Care Committee of Cincinnati Children's Hospital Medical Center.
Retroviral constructs and viral supernatants
Retroviral constructs were murine stem cell virus (MSCV)–based15 bicistronic vectors denominated MSCV-F/P-IRES-EGFP and MSCV-IRES-EGFP (mock vector) (kindly provided by Drs Gary Gilliland and Jan Cools, Harvard Medical School). Efficient expression of F/P by this retroviral vector has been previously shown.16,17 Retrovirus supernatant was generated in the Phoenix-gp cells17 as previously described.18
Retroviral transduction into hematopoietic progenitor cells
Mice were treated with 150 mg/kg 5-fluorouracil (5-FU) administered intraperitoneally beginning 6 days before BM harvest. Femora, tibiae, and iliac crests were harvested and their BM content was isolated. Low-density BM (LDBM) cells were separated by density gradient fractionation according to the manufacturer's instructions (Histopaque 1083; Sigma-Aldrich, St Louis, MO), prestimulated in the presence of recombinant mouse IL-3 (rmIL-3, 6 ng/mL; PeproTech, Rocky Hill, NJ), recombinant rat stem cell factor (rrSCF, 10 ng/mL; Amgen, Thousand Oaks, CA), and rmIL-6 (10 ng/mL; PeproTech), and transduced for 2 days in a protocol that included 2 rounds of spinoculation (1800g for 90 minutes) with retroviral supernatants separated by 24 hours of incubation, and followed by 6 additional hours of incubation at 37°C, 5% CO2. Transduction efficiency of all experiments was approximately 10% without any significant difference among the different groups as previously described.11
HSC/P transplantation
Retrovirally transduced cells were transplanted 6 hours after the second round of spinoculation/transduction. A total of 2.5 to 6.5 × 106 cells/mouse were injected into the lateral tail vein of previously lethally irradiated (4.5 Gy × 2 doses, 3 hours apart, 137Cs source, dose rate: 60-65 cGy/min) recipient mice (BALB/c, wild-type).11
Antibody administration
To deplete MCs, mice were administered 5 doses (first dose intravenously, then intraperitoneally) of 1 mg anti–c-kit antibody (Ab: ACK2) or a control Ab (J1.2) starting 3 weeks after transplantation as previously described.19 All mice were killed 2 days after the last injection.
Histopathology
For tissue histology, relevant organs were fixed in 10% buffered formalin and embedded in paraffin. The tissue sections were stained for chloroacetate esterase (CAE) activity and lightly counterstained with methyl green as previously described.19,20 At least 3 random sections per mouse were analyzed. Quantification of stained cells per square millimeter of intestinal lamina propria and epidermis and dermis was performed by blind morphometric analysis using ImagePro Plus version 4.1 (Media Cybernetics, Silver Spring, MD).11,19 Masson trichrome staining was performed as previously described21 where blue deposits denote abundant collagen deposition.
MC content analysis by flow cytometry
BM and spleen cell suspensions were obtained after mechanical treatment as previously described.22 Intestinal cell suspensions were obtained from the ileum. After washing in phosphate-buffered saline (PBS), the tissue was minced, treated with collagenase (0.12 mg/mL, Liberase CL; Roche, Indianapolis, IN) and DNAse (0.25 mg/mL; Sigma-Aldrich) for 30 minutes at 37°C, and then squeezed through 40-μm mesh filters. Cells were resuspended in RPMI-1640 with 10% fetal calf serum (FCS).23 Single-cell suspensions were stained with the following mAbs: PECy7-conjugated anti-CD45 (clone 30F-11; BD-Pharmingen, San Jose, CA), APC-conjugated anti–c-kit (clone 2B8; BD-Pharmingen), and PE-conjugated anti-Fc∈RIα (clone MAR-1; eBiosciences, San Diego, CA). Dead cells were excluded from analysis by staining with 7-aminoactinomycin D (7-AAD; Invitrogen, Carlsbad, CA). Multicolor flow cytometric analysis was performed with a FACScalibur or a FACSCanto flow cytometer, and the data were analyzed using CellQuest or FACSDiVa software (Becton Dickinson, San Jose, CA).
Plasma mouse MC protease-1 concentration analysis
The plasma level of plasma mouse MC protease-1 (MMCP-1) was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Mouse MMCP-1 OptEIA ELISA set; BD Biosciences Pharmingen).19
In vitro MC development
Retrovirally transduced EGFP+HSCs/Ps (empty vector and F/P expressing) were sorted (Fluorescence-Activated Cell Sorting [FACS] Vantage SE DiVa; Becton Dickinson) 2 days after the last transduction round (day 0 of culture). Sorted cells were cultured at a starting density of 1 to 2 × 105 cells/mL in IMDM supplemented with l-glutamine, penicillin, streptomycin, 10% FCS (Invitrogen), and either no cytokines or supplemental cytokines (100 ng/mL rrSCF and/or 100 ng/mL rmIL-3 [Peprotech]). The cytokines were added every 2 to 3 days, and the medium was renewed every week. Migration and survival experiments were performed in cultures containing more than 90% MCs after a minimum of 4 weeks of culture.24,25
Bone marrow–derived MC chemotaxis assay
Migration assays in response to SCF-induced chemotactic gradient were performed by seeding 500 000 bone marrow–derived mast cells (BMMCs) on 8-μm pore size, polycarbonate membrane transwells (Corning, Lowell, MA), in triplicate, on a gradient formed by 0, 1, 10, or 100 ng/mL SCF for 4 hours at 37°C, 5% CO2.25 The migrated cells in the bottom chamber were collected, resuspended, and divided in aliquots for cell enumeration.
Survival assay of BMMCs
BMMCs were starved in IMDM supplemented with 10% FCS in absence of cytokines for 48 hours and stained with annexin V–APC (BD-Pharmingen) and 7-AAD according to the manufacturer's instructions. Survival was analyzed as the percentage of annexin V−/7-AAD− events.25
Immunoblotting
After starvation for 24 hours in IMDM containing 1% BSA, BMMCs were lysed in a buffer containing 10 mM TrisHCl, pH 7.4, 130 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, and protease and phosphatase inhibitors (5 mM EDTA, 5 mM EGTA, 10 mM NaF, 10 mM β-glycerophosphate, 1 mM Na3VO4) and Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN). The lysates were incubated on ice for 20 minutes, cleared by centrifugation at 12 000g for 15 minutes, and separated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel. For Western blot analysis, proteins were transferred onto a PVDF membrane, blocked with 5% BSA in TBS-0.1% Tween 20, and probed with antibodies specific for phospho-Erk (p42/p44 MAPK, Thr202/Tyr204; 1:1000 dilution), phospho-Akt (Ser473; 1:1000), phospho-STAT5 (Tyr694; 1:1000), and phospho-c-kit (Tyr719; 1:1000) (all from Cell Signaling Technology, Danvers, MA); phospho-Tyr (4G10, 1:1000; Upstate, Billerica, MA); or β-actin (AC-15, 1:5000; Sigma-Aldrich). Blots were then incubated with HRP-conjugated secondary antibodies (Cell Signaling Technology) and developed with LumiGLO reagents (Cell Signaling Technology).25
Statistical analysis
Data were expressed as means plus or minus standard error of the mean except when otherwise stated. Statistical analysis of data was performed by Student t test comparing 2 groups and 1-way or 2-way analysis of variance (ANOVA) followed by multiple comparisons for 3 or more groups. When nonparametric analyses were required, statistical comparisons were performed with Mann-Whitney U test for 2 groups and Kruskal-Wallis test for 3 or more groups. P values less than .05 were considered significant.
Results
Multiorgan infiltration of F/P-expressing MCs in F/P-positive CEL mice
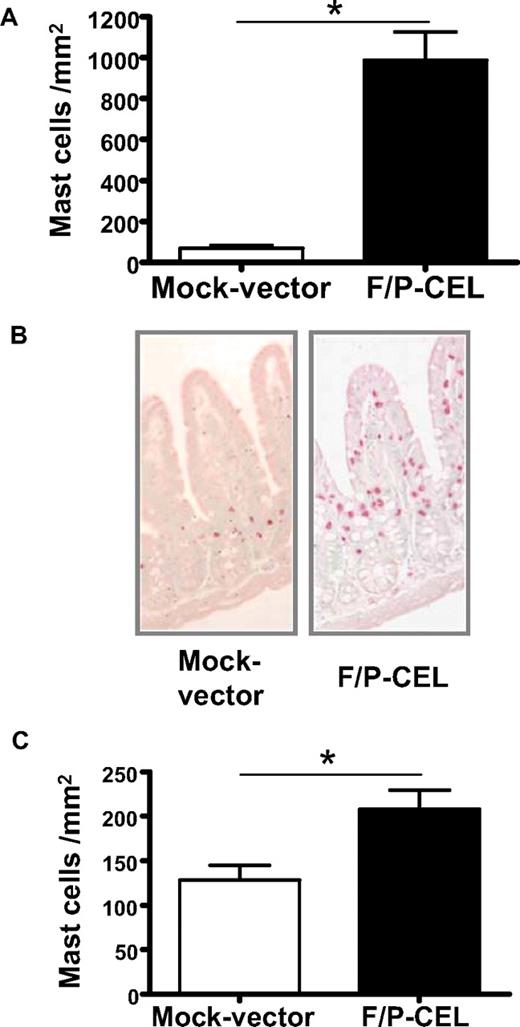
We initially investigated whether F/P-positive CEL mice demonstrate tissue MC infiltration. The MC content of organs from mice with overt CEL11 was analyzed at 4 to 5 weeks after transplantation by CAE staining in tissue sections and flow cytometry of cell suspensions. The F/P-positive CEL mice showed approximately 10-fold higher levels of MC infiltration in the small intestine compared with mice that received a transplant of donor-matched, mock vector–transduced HSCs/Ps (Figure 1A,B). Interestingly, the intestinal MC infiltration of F/P-positive CEL mice was primarily associated with MCs residing in the lamina propria and intraepithelial locations associated with villi, whereas MCs in control mice were primarily in the crypt areas, similar to what has been described in human intestinal mastocytosis.20,26 These data suggested that MCs in F/P-positive CEL mice might possess higher migration activity to epithelium in vivo.
F/P-positive CEL mice have MC infiltration in relevant nonhematopoietic organs. (A) Levels of mast cells in the small intestine. Levels of intestinal mast cells in F/P-positive CEL and CD2-IL-5Tg/mock vector–transduced recipient mice were assessed after development of disease (at 4-5 weeks after transplantation) by morphometric analysis of chloroacetate esterase (CAE)–stained cells. Results are shown as mean (± SEM) from 4 mice per group of one representative experiment (n = 3 experiments). *P < .001. (B) Representative small intestine section from F/P-introduced F/P-positive CEL and control mice with CAE staining (original magnification, ×125), indicating a preferential intraepithelial localization of MCs in F/P-positive CEL mice. Images taken with Leica DMI6000B microscope (Wetzlar, Germany) 10× PH1 acquired with color Leica camera and Open Lab software version 5.50 (Improvision, Waltham, MA). (C) Levels of cutaneous mast cells in F/P-induced, F/P-positive CEL and donor-matched control mice were assessed as in panel A. Results are shown as means (± SEM) from 4 mice per group of one representative experiment. Three independent experiments were conducted with similar results; *P < .05.
F/P-positive CEL mice have MC infiltration in relevant nonhematopoietic organs. (A) Levels of mast cells in the small intestine. Levels of intestinal mast cells in F/P-positive CEL and CD2-IL-5Tg/mock vector–transduced recipient mice were assessed after development of disease (at 4-5 weeks after transplantation) by morphometric analysis of chloroacetate esterase (CAE)–stained cells. Results are shown as mean (± SEM) from 4 mice per group of one representative experiment (n = 3 experiments). *P < .001. (B) Representative small intestine section from F/P-introduced F/P-positive CEL and control mice with CAE staining (original magnification, ×125), indicating a preferential intraepithelial localization of MCs in F/P-positive CEL mice. Images taken with Leica DMI6000B microscope (Wetzlar, Germany) 10× PH1 acquired with color Leica camera and Open Lab software version 5.50 (Improvision, Waltham, MA). (C) Levels of cutaneous mast cells in F/P-induced, F/P-positive CEL and donor-matched control mice were assessed as in panel A. Results are shown as means (± SEM) from 4 mice per group of one representative experiment. Three independent experiments were conducted with similar results; *P < .05.
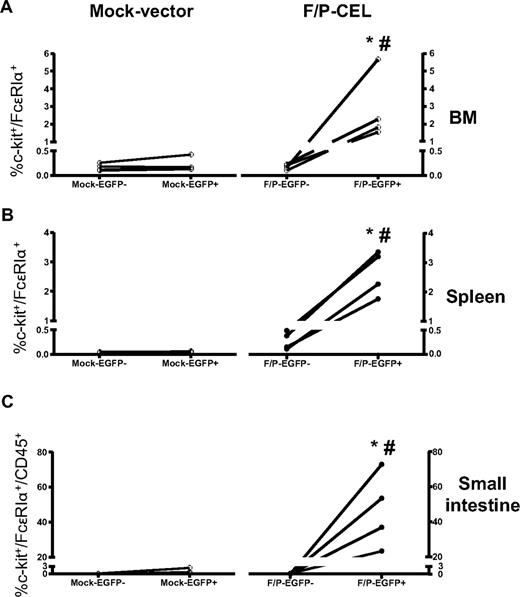
The skin is one of the most common organs involved in patients with mastocytosis and HES. Cutaneous MCs were detected in the dermis in both F/P-positive CEL mice and control mice; however, the cutaneous MC level was significantly increased in F/P-positive CEL mice compared with vector control mice (Figure 1C). To determine whether the MC infiltration was affecting multiple organs, we analyzed the content of F/P-expressing cells based on the expression of EGFP. The content of EGFP+ and EGFP− MCs (c-kit+/Fc∈RIα+ cells) in single-cell suspensions of BM and spleen (Figure 2A,B) and small intestine (CD45+/c-kit+/Fc∈RIα+ cells; Figure 2C) was analyzed by flow cytometry. The vast majority of MCs infiltrating BM, spleen, and intestine of F/P-positive CEL mice expressed EGFP, although there was no expansion of the EGFP-negative MC population compared with the mock vector control, demonstrating that SM induced in these mice was due to expression of F/P.
MC intrinsic expression of F/P induces SM. Frequency of viable MCs was analyzed by flow cytometry of c-kit+/Fc∈RIα+ cells on gated EGFP+ or EGFP-negative cells of BM (A) and spleen (B), and of viable CD45+/c-kit+/Fc∈RIα+ cells on gated EGFP+ or EGFP− cells of small intestine (C). *P < .05 compared with EGFP-negative MC content. #P < .05 compared with mock vector–transduced (EGFP+) MC content. Results are shown as means (± SEM) from 4 mice per group pooled from 2 independent experiments.
MC intrinsic expression of F/P induces SM. Frequency of viable MCs was analyzed by flow cytometry of c-kit+/Fc∈RIα+ cells on gated EGFP+ or EGFP-negative cells of BM (A) and spleen (B), and of viable CD45+/c-kit+/Fc∈RIα+ cells on gated EGFP+ or EGFP− cells of small intestine (C). *P < .05 compared with EGFP-negative MC content. #P < .05 compared with mock vector–transduced (EGFP+) MC content. Results are shown as means (± SEM) from 4 mice per group pooled from 2 independent experiments.
BM sections of F/P-positive CEL mice demonstrated the presence of increased collagen deposits compared with control mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), a finding frequently associated with myeloproliferative disorders.27 MCs in F/P-positive CEL mice were dysmorphic, with irregular shape and frequent cytoplasm extensions (examples of intestinal MCs are shown in Figure S2A,B), compared with the relatively round shape of MCs in control mice. This irregular shape is reminiscent of “spindle shape” found in clinical systemic mastocytosis.
To determine whether IL-5 overexpression had any significant role in the induction of mastocytosis in these mice, we analyzed MC tissue infiltration in mice that received a transplant of non–IL-5Tg, mock-transduced and non–IL-5Tg, F/P-expressing BM cells. IL-5 overexpression did not significantly modify the MC tissue content in mice engrafted with mock-transduced HSCs/Ps. However, the intestinal MC infiltration in mice that received a transplant of F/P-transduced HSCs/Ps was approximately 2-fold higher when IL-5 was overexpressed (Figure S3A). IL-5 overexpression did not significantly change the MC infiltration of the skin of mice receiving either mock- or F/P-transduced HSC/P transplants (Figure S3B).
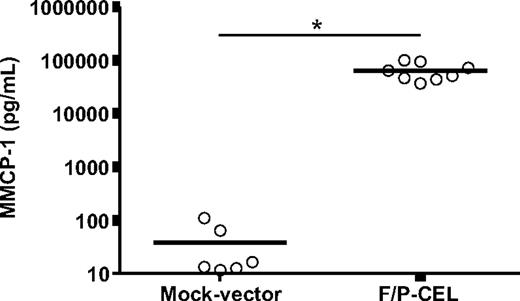
Similar to serum tryptase determination in SM patients, a systemic assay of MC content and degranulation in the mouse is the determination of circulating mouse MC protease 1 (MMCP-1) levels. The levels of plasma MMCP-1 were extremely elevated in F/P-positive CEL mice (averaging 1684-fold higher than in control mice, Figure 3), indicating the presence of a large MC burden in these mice.
Mice with F/P-induced SM have elevated serum levels of MMCP-1. Levels of MMCP-1 in CEL mice and mock vector–transduced recipient mice were assessed after development of disease (at 4-5 weeks after transplantation) by ELISA. Each dot represents results from 1 mouse, and the horizontal bars show mean values (n = 6-8, *P < .001). The results are pooled from 2 independent experiments.
Mice with F/P-induced SM have elevated serum levels of MMCP-1. Levels of MMCP-1 in CEL mice and mock vector–transduced recipient mice were assessed after development of disease (at 4-5 weeks after transplantation) by ELISA. Each dot represents results from 1 mouse, and the horizontal bars show mean values (n = 6-8, *P < .001). The results are pooled from 2 independent experiments.
Similar to the observation of intestinal MC infiltration, IL-5 overexpression did not significantly modify the MMCP-1 serum levels of mice receiving mock-transduced HSC/P transplants but it increased the MMCP-1 serum levels of mice that received a transplant of F/P-transduced HSCs/Ps by approximately 2-fold (Figure S3C).
SCF synergizes with F/P expression to induce MC infiltration in F/P-positive CEL mice
Because c-kit receptor–dependent signaling is crucial for normal MC development and function, we analyzed whether F/P-induced SM was still c-kit dependent. To reduce or eliminate c-kit signaling, we used parenteral administration of an anti–c-kit blocking antibody (ACK.2) that specifically neutralizes the c-kit receptor28 and has been shown to completely abolish MC infiltration in models of allergen-induced mastocytosis.19 Because ACK2 antibody may interfere with HSC/P function when administered during the engraftment process,29 we administered this antibody starting on day +21 after transplantation when the engraftment of HSCs/Ps is considered largely complete. To verify this point, we analyzed the white blood cell count of mice 2 days after the administration of the last dose of antibodies. Administration of ACK2 antibody did not significantly change the total white blood cell count of mice compared with an isotype control antibody after transplantation of IL-5Tg, F/P-expressing HSCs/Ps (8.8 ± 4.4 and 5.5 ± 1.4 × 103/mm3, respectively, P = NS).
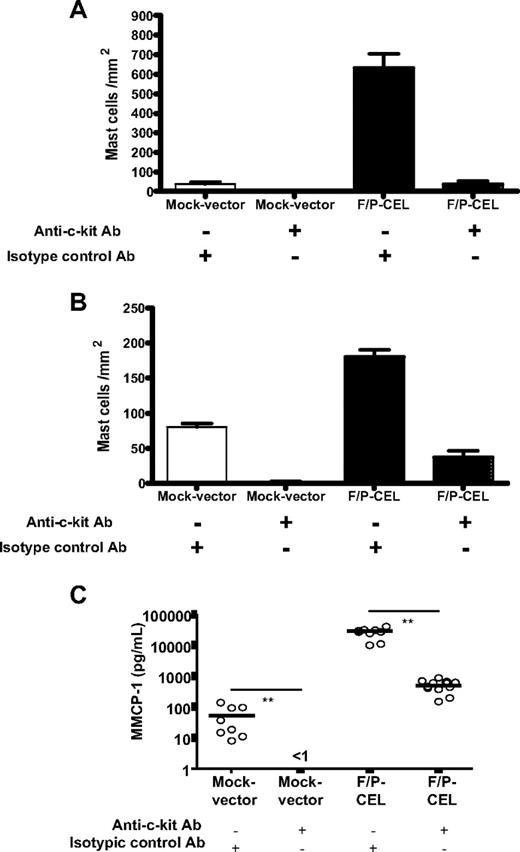
Anti–c-kit antibody administration significantly diminished SM (Figure 4A,B), suggesting that c-kit–mediated signaling is partly responsible for the SM of F/P-positive CEL mice. Likewise, flow cytometry analysis of single-cell suspensions of BM, spleen, and small intestine showed that the administration of anti–c-kit antibody significantly decreased EGFP+ MC infiltration in spleen and small intestine (Table 1), although not in the BM. The systemic burden of MCs (as determined by elevation of MMCP-1) was diminished by the anti–c-kit antibody treatment (Figure 4C). These data suggest that tissue infiltrations of F/P+ MCs are associated with SCF/c-kit signaling.
Anti–c-kit treatment dramatically reduces the tissue MC infiltration and serum level of MMCP-1. After hematopoietic engraftment, on day +21 after transplantation, mice were treated with an anti–c-kit neutralizing antibody (ACK2) or an isotype-matched control antibody (J1.2). The effect of ACK2 treatment on the levels of intestinal (A) and skin (B) mast cells in F/P-positive CEL and mock vector–transduced recipient mice was assessed by morphometric analysis of chloroacetate esterase (CAE)–stained cells. Results are shown as means (± SEM) from 4 to 6 mice per group of 1 representative experiment of 2 independent experiments. *P < .05; #P < .001, compared with isotype control–treated mice. (C) The effect of ACK2 treatment on levels of MMCP-1 in the same groups of mice was assessed by ELISA. Each dot represents results from each individual mouse, and horizontal bars show mean values (n = 7-11 pooled from 2 independent experiments; **P < .001).
Anti–c-kit treatment dramatically reduces the tissue MC infiltration and serum level of MMCP-1. After hematopoietic engraftment, on day +21 after transplantation, mice were treated with an anti–c-kit neutralizing antibody (ACK2) or an isotype-matched control antibody (J1.2). The effect of ACK2 treatment on the levels of intestinal (A) and skin (B) mast cells in F/P-positive CEL and mock vector–transduced recipient mice was assessed by morphometric analysis of chloroacetate esterase (CAE)–stained cells. Results are shown as means (± SEM) from 4 to 6 mice per group of 1 representative experiment of 2 independent experiments. *P < .05; #P < .001, compared with isotype control–treated mice. (C) The effect of ACK2 treatment on levels of MMCP-1 in the same groups of mice was assessed by ELISA. Each dot represents results from each individual mouse, and horizontal bars show mean values (n = 7-11 pooled from 2 independent experiments; **P < .001).
Frequency of tissue EGFP+ MCs found after treatment with anti–c-kit antibody
| . | Mock vector isotype control . | Mock vector anti–c-kit . | F/P-CEL isotype control . | F/P-CEL anti–c-kit . |
|---|---|---|---|---|
| Spleen | 0.1 ± 0 | 0.035 ± 0.017* | 1.3 ± 0.36 | 0.74 ± 0.38* |
| BM | 0.33 ± 0.058 | 0.015 ± 0.024* | 0.98 ± 0.47 | 2.4 ± 1.21 |
| Small intestine | 0.36 ± 0.17 | 0.45 ± 0.31 | 30 ± 25.04 | 5.6 ± 4.68* |
| . | Mock vector isotype control . | Mock vector anti–c-kit . | F/P-CEL isotype control . | F/P-CEL anti–c-kit . |
|---|---|---|---|---|
| Spleen | 0.1 ± 0 | 0.035 ± 0.017* | 1.3 ± 0.36 | 0.74 ± 0.38* |
| BM | 0.33 ± 0.058 | 0.015 ± 0.024* | 0.98 ± 0.47 | 2.4 ± 1.21 |
| Small intestine | 0.36 ± 0.17 | 0.45 ± 0.31 | 30 ± 25.04 | 5.6 ± 4.68* |
Data are shown as mean percentages plus or minus standard deviation.
P < .05 compared with isotype-matched control-treated mice in each group.
F/P fusion protein induces increased survival of BMMCs and specifically synergizes with SCF to induce ex vivo MC output
To determine the role of F/P on preventing BMMC apoptosis, their survival after cytokine deprivation was analyzed by flow cytometry in an annexin V binding assay. Expression of the F/P fusion gene was associated with significantly reduced apoptosis of BMMCs in cytokine-deprived conditions (Figure 5A). To examine whether F/P expression favors BMMC expansion in culture, F/P fusion– or empty vector–transduced wild-type BM HSCs/Ps were cultured in the absence or presence of SCF and/or IL-3 (followed for up to 4 weeks; Figure 5B-D). In these conditions, F/P-expressing BM HSCs/Ps expanded modestly into a differentiated MC population (Figure 5). Expansion of F/P-expressing MCs was significantly higher in the presence of SCF (Figure 5C, ∼ 70-fold higher than the culture without cytokines on week 4 of culture). In contrast, mock vector–transduced BM-derived HSCs/Ps in the absence of cytokines were not able to differentiate into MCs, and SCF alone induced negligible MC output from mock vector–transduced BM HSCs/Ps (Figure 5B,C). Although IL-3 induced a striking expansion of MCs from mock vector–transduced HSCs/Ps as previously shown by others,24,25 it had only a marginal effect on MC output from F/P+ HSCs/Ps beyond the level observed in non–cytokine-containing cultures (Figure 5D). Taken together, these data show the expansion of MC output is enhanced by F/P in a synergistic fashion with SCF but not with IL-3.
F/P fusion promotes differentiation and proliferation of BMMCs and increases survival after cytokine depletion. (A) F/P-expressing or mock vector–transduced BMMCs were cultured in the absence of cytokines for 48 hours, and then the survival of BMMCs was assessed by annexin V and 7-AAD using flow cytometry. Data represent means (± SEM) of BMMCs that were annexin V and 7-AAD double negative. *P < .05 (n = 3, from 1 representative experiment of 3). (B-D) BMMC output associated with FIP1L1/PDGFRα expression. F/P-transduced (■) or mock vector–transduced (▲) 5-FU–treated BM HSC/Ps were cultured in the absence (B) or presence of recombinant stem cell factor (rSCF, 100 ng/mL; C) or rIL-3 (100 ng/mL; D). MC content of cell cultures from panels B through D was assessed by flow cytometry of EGFP+/c-kit+/Fc∈RIα+ cells. The data are depicted as fold expansion (MC [EGFP+/c-kit+/Fc∈RIα+cell] numbers divided by seeded HSC/P numbers in indicated periods). *P < .05; **P < .01; and ***P < .001, compared with mock vector (EGFP+) MC cells (n = 3 from 1 experiment representative of 2 or 3 experiments).
F/P fusion promotes differentiation and proliferation of BMMCs and increases survival after cytokine depletion. (A) F/P-expressing or mock vector–transduced BMMCs were cultured in the absence of cytokines for 48 hours, and then the survival of BMMCs was assessed by annexin V and 7-AAD using flow cytometry. Data represent means (± SEM) of BMMCs that were annexin V and 7-AAD double negative. *P < .05 (n = 3, from 1 representative experiment of 3). (B-D) BMMC output associated with FIP1L1/PDGFRα expression. F/P-transduced (■) or mock vector–transduced (▲) 5-FU–treated BM HSC/Ps were cultured in the absence (B) or presence of recombinant stem cell factor (rSCF, 100 ng/mL; C) or rIL-3 (100 ng/mL; D). MC content of cell cultures from panels B through D was assessed by flow cytometry of EGFP+/c-kit+/Fc∈RIα+ cells. The data are depicted as fold expansion (MC [EGFP+/c-kit+/Fc∈RIα+cell] numbers divided by seeded HSC/P numbers in indicated periods). *P < .05; **P < .01; and ***P < .001, compared with mock vector (EGFP+) MC cells (n = 3 from 1 experiment representative of 2 or 3 experiments).
F/P expression synergizes with SCF to induce Akt activation
To evaluate the signaling pathways activated by F/P and the observed synergism in MC growth, we examined downstream signaling pathways implicated in SCF/c-kit ligand/receptor interactions in MCs. Because Akt is a known downstream effector of c-kit receptor signaling and has also been implicated in the survival, proliferation, and migration of several cell types,30 including F/P-transduced human CD34+cells,31 we analyzed Akt activation after SCF stimulation. As seen in Figure 6A (top panel) and Figure S4, SCF induced an approximately 6.5-fold increased Akt activation in F/P-expressing BMMCs compared with mock vector–transduced cells. This activation was prevented by preincubation with AktVIII, an Akt activation inhibitor, which did not modify SCF-dependent Erk or STAT5 activation levels (Figure S5). Interestingly, there were significant differences neither in the level of c-kit activation after SCF stimulation between mock-transduced and F/P-expressing MCs nor in the levels of phosphorylated F/P when F/P-expressing MCs were stimulated with SCF (Figure 6A second and third panels). In addition, there was no synergistic effect of c-kit activation and F/P on STAT5 or Erk activation (Figure 6A fourth and fifth panels), suggesting a specific synergistic stimulation of Akt signaling pathway by F/P and SCF, downstream of both c-kit and F/P.
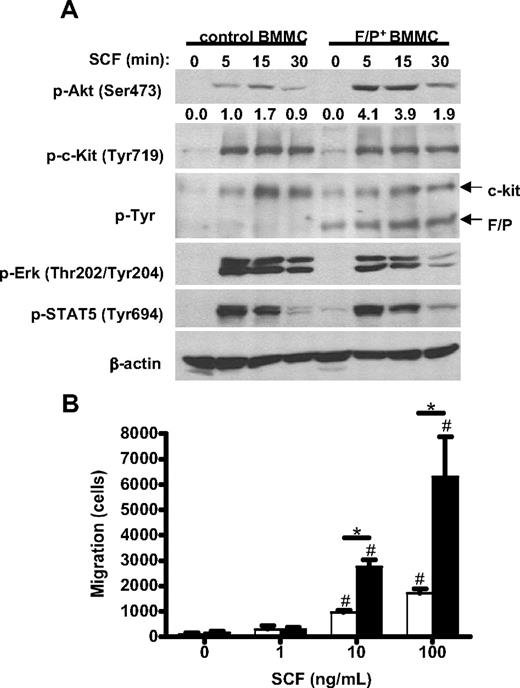
Role of SCF in migration and signaling activation in F/P-expressing BMMCs. (A) Time course of SCF-induced activation of Akt (p-Akt), Erk (p-Erk), STAT5 (p-STAT5), c-kit (p-c-Kit), and overall tyrosine phosphorylation (p-Tyr) in mock vector–transduced and F/P-expressing MCs. β-Actin is used as loading control. The numbers represent the densitometric quantification of p-AKT relative to β-actin in this experiment. One representative experiment (of 3-4 completely independent experiments) is shown. (B) Migration of F/P-expressing BMMCs toward stem cell factor (SCF). F/P-expressing (■) or mock vector–transduced (□) BMMCs were allowed to migrate in response to a gradient of different concentrations of SCF. Data represent means (± SEM) of BMMCs that migrated into lower chambers. *P < .05, compared with mock vector–transduced in each concentration of SCF; #P < .05, compared with medium control in each group (n = 3, from 1 representative experiment of 3 independent experiments with similar results).
Role of SCF in migration and signaling activation in F/P-expressing BMMCs. (A) Time course of SCF-induced activation of Akt (p-Akt), Erk (p-Erk), STAT5 (p-STAT5), c-kit (p-c-Kit), and overall tyrosine phosphorylation (p-Tyr) in mock vector–transduced and F/P-expressing MCs. β-Actin is used as loading control. The numbers represent the densitometric quantification of p-AKT relative to β-actin in this experiment. One representative experiment (of 3-4 completely independent experiments) is shown. (B) Migration of F/P-expressing BMMCs toward stem cell factor (SCF). F/P-expressing (■) or mock vector–transduced (□) BMMCs were allowed to migrate in response to a gradient of different concentrations of SCF. Data represent means (± SEM) of BMMCs that migrated into lower chambers. *P < .05, compared with mock vector–transduced in each concentration of SCF; #P < .05, compared with medium control in each group (n = 3, from 1 representative experiment of 3 independent experiments with similar results).
F/P+ BMMCs show enhanced migration in response to SCF
Finally, because we observed increased numbers of MCs infiltrating different tissues and a synergism with SCF in the expansion of BMMCs and Akt activation and because SCF is a well-described chemotactic factor for MCs, we next determined whether the expression of F/P affected the motility of MCs in response to SCF. BMMCs expressing either mock vector or F/P were generated in cultures containing both IL-3 and SCF and tested in an SCF-dependent transwell migration assay. MC migration through an SCF gradient was significantly higher (∼ 3-fold increase for 10 and 100 ng/mL SCF gradients) for F/P-expressing BMMCs than those of mock vector (Figure 6B). These data are consistent with the systemic tissue infiltration of MCs found in vivo in F/P-positive CEL mice.
Discussion
F/P-induced disease is characterized by increased cell numbers and activation of the MC and eosinophil lineages. F/P expression leads to clinical SM and CEL (SM-CEL) with infiltration of hematopoietic tissues and nonhematopoietic tissues. Among the nonhematopoietic tissues, the skin and intestine are commonly involved.
There has been a significant degree of controversy on the role of FIP1L1/PDGFRα in SM.5,10,32-35 Mutation of the PDGFRA gene has been found in eosinophils and MCs in SM-F/P–positive CEL or gastrointestinal stromal tumors, which do not have KIT mutations,33,36 pointing out the involvement of common downstream signaling pathways common to c-kit and PDGFRA in the development of different diseases. This suggests the potential influence of cell type–specific activation pathways and the possible role of stromal expression of SCF in the pathogenesis of SM. This controversy has been due, at least partly, to the absence of satisfactory pathogenetic models for SM-F/P–positive CEL, which would allow the study of the intrinsic role of F/P-expressing MCs in vivo and in vitro.
Here we show that a murine model of F/P-positive CEL11 also demonstrates biologic features comparable with those of SM. This model of SM is a direct result of the expression of F/P in hematopoietic progenitors and MCs because despite hypereosinophilia, the vast majority of tissue-infiltrating MCs express F/P, although there is no significant expansion of F/P-negative MCs. This model of SM-F/P–positive CEL demonstrates increased levels of F/P-expressing MCs in the skin, small intestine, BM, and spleen. This model, similar to some models of parasite-induced mastocytosis, demonstrates MC infiltration within the intestinal epithelium of F/P-positive CEL mice.20,26 SCF-expressing mesenchymal cells surrounding the intestinal epithelium are thought to nurture the intestinal stem cell niche composed of intestinal stem cells and progenitors.37 These mesenchymal cells generate a gradient of SCF that may attract large numbers of proliferating MCs in SM, leading to their presence in the intestinal epithelium. An increased migration response to SCF by F/P-expressing MCs would explain their preferential localization in the epithelial layer within the intestine.
We hypothesized that preferential MC development associated with F/P fusion in vitro and in vivo would be dependent on synergistic effects of SCF with the F/P fusion protein. Indeed, MC intestinal and spleen infiltrations and the elevated levels of MMCP-1 induced by F/P expression were significantly diminished but not abolished by administration of an anti–c-kit antibody, suggesting that SCF/c-kit interaction acts synergistically with F/P expression to induce mastocytosis. Interestingly, BMMC infiltration was not decreased after administration of anti–c-kit antibody. Because ACK2 appears to adequately target c-kit–expressing BM cells,29 these data suggest that BMMCs are less dependent on SCF signaling to survive in vivo. Although F/P+ BMMC differentiation into MCs in vitro occurred even in the absence of cytokines, such expansion was augmented in the presence of SCF alone. Although SCF is a key cytokine in the development, activation, and survival of MCs, SCF alone is not sufficient for the development of MCs from BM cells as this process requires cofactors such as IL-3 and IL-4.38,39 Interestingly, SCF does not require any MC-extrinsic cofactors to develop F/P+ MCs from F/P+ HSCs/Ps, suggesting that F/P fusion may alternatively work as a cofactor. Interestingly, F/P not only induces an increased response to SCF, but also synergizes with SCF to induce increased MC proliferation and survival. We found cooperation between SCF- and F/P-dependent signaling to specifically activate Akt that correlated with increased proliferation and migration. However, there was not a consistent activation of Erk or STAT5 by F/P6,31,40 or cooperation with SCF in hyperactivation of Erk or STAT5, suggesting that Erk and STAT5 activation do not have major roles in F/P-induced mastocytosis. Interestingly, the absence of an effect by IL-3 on proliferation of F/P-expressing MCs suggests that IL-3 does not modify the level of tyrosine kinase dependency of the MCs in this murine model. Thus, SM induced by F/P in this murine model could be “tyrosine kinase addicted.”41 These findings support the importance of SCF in F/P-associated MC development, survival, and activation, and the cooperative signaling of multiple tyrosine kinases in neoplasia.42
In summary, our results show that the F/P fusion gene induces mastocytosis characterized by increased levels of MCs in hematopoietic organs, skin, and small intestine, and elevated serum levels of MMCP-1, along with prolonged survival and higher migration activity of MCs in response to SCF. It is likely that these F/P+ MCs directly contribute to the disease pathogenesis, as patients with this subset of disease have increased tissue damage including fibrosis.4 The finding that 2 tyrosine kinases (PDGFRA and c-kit) cooperate in the pathogenesis of SM-F/P–positive CEL highlights the potential utility of blocking single or multiple kinases in these patients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by American Heart Association Ohio Valley Affiliate Postdoctoral Fellowship (Columbus, OH; Y.Y.), University of Cincinnati Cancer Center Grant (Cincinnati, OH; J.A.C), Campaign Urging Research for Eosinophilic Disease (CURED, Buffalo Grove, IL; M.E.R), and the Buckeye Foundation (Chicago, IL; M.E.R.).
Authorship
Contribution: Y.Y., A.S.-A., E.B.B., M.M., N.J.H.A.-M., and J.A.C. performed experiments; F.D.F. analyzed results and provided anti–c-kit and isotype control antibodies for in vivo neutralization; and Y.Y., D.A.W., J.A.C., and M.E.R. designed the research, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc E. Rothenberg, Division of Allergy and Immunology, Cincinnati Children's Hospital Medical Center, MLC 7028, 3333 Burnet Avenue, Cincinnati, OH 45229-3039; e-mail: rothenberg@cchmc.org.
References
Author notes
*J.A.C. and M.E.R. contributed equally to this work.

![Figure 5. F/P fusion promotes differentiation and proliferation of BMMCs and increases survival after cytokine depletion. (A) F/P-expressing or mock vector–transduced BMMCs were cultured in the absence of cytokines for 48 hours, and then the survival of BMMCs was assessed by annexin V and 7-AAD using flow cytometry. Data represent means (± SEM) of BMMCs that were annexin V and 7-AAD double negative. *P < .05 (n = 3, from 1 representative experiment of 3). (B-D) BMMC output associated with FIP1L1/PDGFRα expression. F/P-transduced (■) or mock vector–transduced (▲) 5-FU–treated BM HSC/Ps were cultured in the absence (B) or presence of recombinant stem cell factor (rSCF, 100 ng/mL; C) or rIL-3 (100 ng/mL; D). MC content of cell cultures from panels B through D was assessed by flow cytometry of EGFP+/c-kit+/Fc∈RIα+ cells. The data are depicted as fold expansion (MC [EGFP+/c-kit+/Fc∈RIα+cell] numbers divided by seeded HSC/P numbers in indicated periods). *P < .05; **P < .01; and ***P < .001, compared with mock vector (EGFP+) MC cells (n = 3 from 1 experiment representative of 2 or 3 experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2007-11-126268/7/m_zh80170822790005.jpeg?Expires=1767712111&Signature=PooE3~qzNlRiu0u0LQA1OppWN3FSQ6aJU9gfZfuTHs42bZI2MfFXaQGftqstmdBkh-~znx3GmrveHESfzbTKVQOeQIT2UBREtR3LWhmxyl6W7VQOUUR8UFUA9jE8uZnOchCyIPCAZ5aE9E-tM6NRyWGix0rVwVCGjJjA342uRNNBRQ6Z0g7RXnYpkX3pUXkMKLQt1qHFypC14M81rmscJUYi~UF9N8ZSrt8e1vLmOKOFKFic6kwrq8ONux1kKFUhyPU2ZjICdlxoWAwrmondpfahoGPdSY01QpSX2Ao-M1H~ilsidix0pdzL0KPv1kbvuXMiepwE4IrwmK03RuueyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal