Abstract
CD22 is an important immunotherapeutic target on B-cell malignancies, particularly hairy cell leukemia (HCL), but its soluble extracellular domain, sCD22, has not yet been reported in the blood. By immunoaffinity and enzyme-linked immunosorbent assay techniques using anti-CD22 monoclonal antibodies, we identified the 100-kDa extracellular domain of CD22 and an 80-kDa processed form in serum of patients with HCL. The median sCD22 level measured by enzyme-linked immunosorbent assay was 18 ng/mL for 93 patients with HCL. sCD22 levels varied from 2.1 to 163 ng/mL and were higher (P < .001) than 23 normal donors (median, 0.6 ng/mL). More than 95% of normal donors had sCD22 levels less than 1.9 ng/mL. sCD22 levels were proportional to concentrations of circulating HCL cells (P = .002), and HCL spleen size (P < .001). sCD22 levels normalized with complete but not partial response to treatment. sCD22 levels up to 300 ng/mL had less than a 2-fold effect on the cytotoxicity of the anti-CD22 recombinant immunotoxin BL22. sCD22 levels may be useful to follow in patients with HCL and may be more specific than sCD25 in patients with CD22+/CD25− disease. Trials are listed on www.cancer.gov as NCT00002765, NCT00021983, NCT00074048, NCT00085085, NCT00337311, and NCT00462189.
Introduction
Extracellular domains of tumor-associated antigens are often cleaved from the cell and may be quantified to follow tumor burden. A wide variety of soluble receptors and antigens have been reported associated with malignancies, including soluble granulocyte-monocyte colony-stimulating factor receptor in acute myelogenous leukemia (AML),1 soluble CD20 in non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), and Hodgkin disease (HD),2,3 soluble CD25 (soluble interleukin-2 receptor alpha or sTac) in CD25+ T- and B-cell malignancies, solid tumors and in autoimmune disorders,4-6 soluble CD27 in indolent NHL,7 soluble CD30 in autoimmune disorders8 and in HD,9,10 soluble CD40 in multiple myeloma (MM), CLL, mantle cell lymphoma (MCL), and AML,11 soluble CD44 in NHL and early CLL,12 soluble CD52 in CLL,13 soluble CD80 in CLL and MCL,14 soluble CD86 in MM and NHL,15,16 soluble CD137 in leukemias and lymphomas,3,17 soluble CD138 in MM18 and CLL,19 soluble CD307 (IRTA2, FcRH5) in hairy cell leukemia (HCL), MM, CLL, and MCL,20,21 beta2-microglobulin in HD, MM, and solid tumors,22-24 soluble tumor necrosis factor receptor in NHL,25 and soluble mesothelin in mesotheliomas.26 However, none of these markers is routinely used in the management of patients with B-cell malignancies.
One of the most commonly displayed antigens in hematologic malignancies is CD22, a 135-kDa phosphoglycoprotein adhesion molecule present on the surface of B cells, including human B-cell lymphomas and leukemias.27,28 CD22 is displayed in more than 90% of cases of CLL,29 60% to 70% of B-cell lymphomas,27 100% of HCL,28 and 96% to 100% of cases of pediatric acute lymphoblastic leukemia (ALL).30,31 Cell-surface CD22 can modulate B lymphocyte antigen receptor (BCR) signals, including BCR-induced cell death, and can regulate CD19 signal transduction.32,33 CD22 has been the target of a variety of antibody-based therapies, including the unlabeled monoclonal antibody (mAb) Epratuzumab (humanized LL2),34 anti-CD22 mAbs RFB4, and LL2 chemically conjugated to protein toxins derived from ricin35-37 and Pseudomonas exotoxin (PE),38,39 recombinant immunotoxins BL22 and HA22 containing RFB4-derived Fvs fused to truncated PE,40-42 recombinant immunotoxin DT2219 containing Fvs binding to CD22 and CD19 fused to truncated diphtheria toxin (DT),43 and LL2 conjugated to the ribonuclease protein onconase.44 However, CD22 has not yet been reported as a soluble protein that can be quantified in the plasma or serum.
To determine whether CD22 could serve as a useful tumor marker, we decided to attempt its detection in the serum of HCL patients and in the supernate of established HCL cells. We then quantified its concentration in the serum using an enzyme-linked immunosorbent assay (ELISA) assay. An additional goal was to determine whether the level of soluble CD22 (sCD22) could block the cytotoxicity of BL22, and if so, determine which concentrations of sCD22 would cause clinically important interference.
Methods
Patient samples
Blood samples were drawn as part of protocols approved by the National Cancer Institute (NCI) investigators review board. Serum samples from normal donors were purchased from Bioreclamation (East Meadow, NY). Serum samples were stored at −80°C until tested.
Consents used for obtaining samples used in this study were approved by the NCI Institutional Review Board and were obtained in accordance with the Declaration of Helsinki. A protocol to conduct research on stored human samples from terminated protocols was also approved by the NCI Institutional Review Board.
Assessment of tumor burden and CD22 density
Volume of disease was assessed by computed tomography and flow cytometry of the blood. Flow cytometry was performed as described.45,46 CD22 sites/cell were determined either by radiolabeled binding assay as described47 or by flow cytometry using the QuantiBRITE system for fluorescence quantitation (BD Biosciences, San Jose, CA) and anti-CD22 mAb conjugated 1:1 to phycoerythrin, according to the manufacturer's recommendations.
Preparation and purification of CD22 extracellular domain
The extracellular domain of CD22 protein was expressed as a fusion protein to human IgG Fc in transfected 293T cells, using plasmid pRB2k2, also referred to as pCDNA1.1–22-Fc, as described48 ; 293T cells were transfected either with calcium phosphate or with lipotransfectamine (Invitrogen, Carlsbad, CA). Culture supernatant was collected after 48-hour protein-free culture using Ultradoma (Lonza Walkersville, Walkersville, MD), concentrated, and the resulting protein sCD22-Fc was purified by sizing chromatography (TSK3000SW, 0.75 × 600 mm). Recombinant sCD22 alone was obtained from the same culture supernatant by concentrating the cleaved sCD22 in the flow-through of a protein A column, and then purifying by sizing chromatography. sCD22-Fc could also be produced from 293T cells in the presence of fetal bovine serum (FBS) by applying the supernatant to Protein A, washing with citrate buffer at pH 5 to remove bovine IgG, and then eluting the sCD22-Fc with glycine buffer at pH 2.5.
Cytotoxicity assay to determine competition of cytotoxicity
sCD22-Fc fusion protein was incubated with or without BL22 in 100-μL aliquots containing 104 Raji cells, RPMI 1640, and 10% FBS. After 24 hours at 37°C, cells were pulsed with [3H]-leucine 1 μCi/well for 4 to 6 hours, harvested, and counted as described.38
ELISA for measuring sCD22
Flat-bottomed (96-well) Immulon plates (Nalge Nunc International, Rochester, NY) were coated by the anti-CD22 mAb RFB4 (BioSource International, Camarillo, CA)49 in 100-μL aliquots of phosphate-buffered saline (PBS) containing 0.1 to 1 mg RFB4 for 2 hours at room temperature. After washing 2 to 3 times with 0.05% Tween-20 in PBS (TPBS), the plate was blocked with 1% bovine serum albumin (1% BSA-PBS) 300 μL/well for 1 hour at room temperature. After washing twice with TPBS, samples were added, either serum or plasma diluted 1:10 with PBS (final 10% serum), or purified protein diluted in 10% FBS. After incubating 15 to 20 hours at 4°C, plates were washed with TPBS 3 times, and treated with 100 μL aliquots of 50 ng mAb sHCL1 (BD Biosciences) biotinylated with sulfosuccinimidyl1–6-(biotinamide) hexanoate (Pierce Chemical, Rockford, IL). After incubating for 1 hour at room temperature, the plates were washed with TPBS 3 times and treated with 100-μL aliquots of 1:10 000 diluted avidin-horseradish peroxidase (HRP) solution (BioSource International) for 1 hour at room temperature. The plates were then washed 3 times with TPBS, treated with 100 μL/well TMB-H2O2 solution (Pierce Chemical), and incubated 5 to 15 minutes at room temperature. The reaction was stopped with 100 μL/well of 2N H2SO4 and the plates read at 450 nm.
Purification of sCD22 from cell culture medium or patient serum
RFB4 (10 mg) was bound to 100 mL cyanogen bromide-activated Sepharose in 1 M sodium borate, pH 8.0, by recirculation. Culture supernatant (500 mL), containing RPMI 1640 + 10% FBS, obtained from the HCL cell line Eskol (kindly provided by Dr M. Taylor, Indiana University, Indianapolis, IN) was loaded onto 25 mL RFB4-Sepharose. Diluted serum from a patient with HCL was used instead of culture supernatant. After washing with PBS, the protein was eluted with sodium dodecy sulfate-polyacrylamide gel electrophoresis sample buffer containing 5% 2-mercaptoethanol. After sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transfer to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA), the membrane was blocked with 0.5% BSA, treated with TPBS, and incubated with 3 μg/mL RFB4 solution for 1 hour. The washed membrane was incubated with HRP-labeled antimouse IgG and IgA polyclonal antibody (BioSource International) followed by chemiluminescence detection (enhanced chemiluminescence [ECL]; New England Nuclear, Boston, MA).
Statistical analysis
Standard curves from ELISA assays were analyzed using 4-parameter logistic regression, and unknown concentrations (X) determined using the equation:
X = X0*(((A/(Y − Y0)) − 1)^(1/B)), where Y is the OD450 and A, B, X0, and Y0 are the 4 parameters. To improve the utility of the standard curve for detecting low concentrations, later assays used a weighted regression analysis with the sum of the squares multiplied by 1/Y2.
When adding known concentrations of sCD22-Fc to human serum samples, accuracy and coefficient of variance (COV) were determined for each known concentration added and for each assay. Accuracy (%) was defined as 100(1 − {(x − e)/e}), where x and e are measured and expected concentrations, respectively. Expected concentrations for each of these spiked serum samples equaled the sum of the measured sCD22 concentration in the unspiked serum, plus the known added concentration of sCD22-Fc. The COV was defined as 100 × (SD/M), where SD is the standard deviation of the replicates tested for each assay and M is the mean of the measured concentrations.
In evaluating triplicates of simultaneous experiments with COV more than 10%, outliers were not removed unless more than 3 SD away from mean of the other 2 results.
sCD22 levels were compared between groups using 2-tailed t tests and the nonparametric (Wilcoxon) test. Pearson correlations were used to determine relationships between tumor burden and sCD22 level. Statistical P values were determined using SAS.
Results
Detection of sCD22
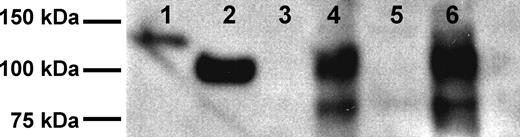
Although CD22 is an important marker on normal B cells and on B-cell malignancies, neither detection nor quantification of its extracellular domain in clinical samples has yet been reported. To determine whether the extracellular domain of CD22 (sCD22) could be detected in the blood, serum from a patient with HCL was applied to an affinity column containing anti-CD22 mAb, and the eluted protein analyzed by Western blot. As shown in Figure 1, an approximately 100-kDa band, corresponding to the extracellular domain of CD22, was observed, along with a smaller (∼ 80 kDa) fragment. The 100-kDa band comigrated with purified recombinant sCD22, and was lower than the recombinant sCD22-Fc fusion protein (130 kDa). sCD22 was also observed in culture supernatant of the HCL cell line Eskol, which is CD22+ (Figure 1). Neither normal serum nor culture medium alone contained sCD22. Thus, sCD22 is produced from HCL cells, which are strongly CD22+, and exists in the blood in association with CD22+ malignant cells.
Isolation of sCD22. Shown are Western blots of recombinant purified sCD22-Fc (lane 1), sCD22 (lane 2), serum from a healthy donor (lane 3), serum from an HCL patient (lane 4), culture medium (land 5), and culture medium from the HCL Eskol cell line (lane 6).
Isolation of sCD22. Shown are Western blots of recombinant purified sCD22-Fc (lane 1), sCD22 (lane 2), serum from a healthy donor (lane 3), serum from an HCL patient (lane 4), culture medium (land 5), and culture medium from the HCL Eskol cell line (lane 6).
ELISA to quantify sCD22
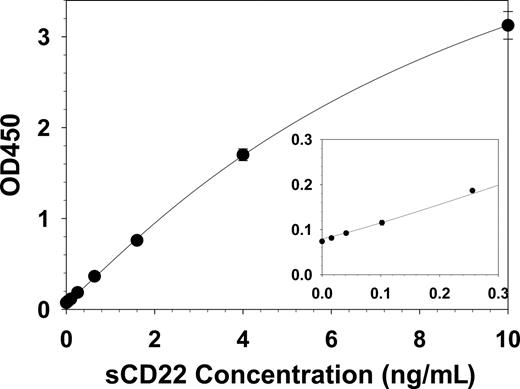
To quantify sCD22 in the serum or plasma, a sandwich ELISA assay was developed in which the anti-CD22 mAb RFB4 was bound to 96-well plates, diluted serum or standards were added, and the bound sCD22 detected with a biotinylated second anti-CD22 mAb sHCL1 followed by standard avidin-HRP treatment and development steps. A typical standard curve, using known concentrations of recombinant purified sCD22-Fc in FBS diluted 1:10 with PBS (10% FBS-PBS), is shown in Figure 2.
sCD22 standard curve. Known concentrations of purified sCD22-Fc were tested and the curve solved by 4-parameter logistic regression. Each point is the mean of 3 triplicate experiments, and SD values are shown when larger than the point markers. The inset curve magnifies the data at low concentrations.
sCD22 standard curve. Known concentrations of purified sCD22-Fc were tested and the curve solved by 4-parameter logistic regression. Each point is the mean of 3 triplicate experiments, and SD values are shown when larger than the point markers. The inset curve magnifies the data at low concentrations.
sCD22 levels in patients with HCL and normal controls
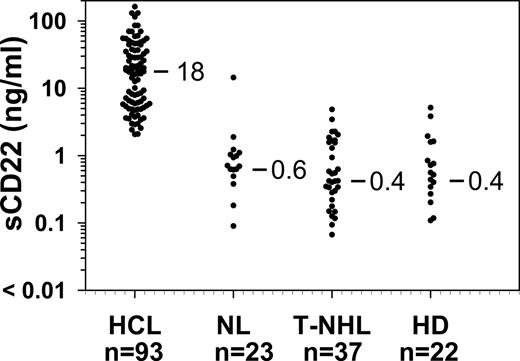
To determine whether sCD22 levels in patients with HCL would be elevated compared with normal, we performed the sCD22 ELISA assay using sera from 93 patients with HCL and 23 normal donors. All patients had evaluable disease. Each serum sample was diluted 1:10 with PBS, and the OD450 values compared with the standard curve made from purified sCD22-Fc diluted in PBS containing 10% FBS. Levels of sCD22 were determined using the equation of the weighted 4-parameter logistic regression of the standard curve. As shown in Figure 3, values of sCD22 in HCL varied from 2.1 to 163 ng/mL (median, 18 ng/mL). Of 23 normal controls, sCD22 ranged from less than 0.005 to 1.89 ng/mL, and one donor had a sCD22 level of 14.2 ng/mL. By rank order (Wilcoxon) analysis, the sCD22 in HCL was higher than in normal controls (P < .001). The status of the normal control with a sCD22 level of 14.2 ng/mL was unknown, including whether this donor had been screened for infectious, malignant, or autoimmune diseases. Thus, patients with HCL had sCD22 values more than 2 ng/mL, and 2 ng/mL was above 95th percentile of the normal controls. Therefore, the “normal range” of sCD22 is defined as 0 to 2 ng/mL.
sCD22 levels in HCL and CD22-negative malignancies. Serum samples were tested from the indicated number of patients with HCL, normal donors (NL), T-NHL, and HD. Median results of each set are shown.
sCD22 levels in HCL and CD22-negative malignancies. Serum samples were tested from the indicated number of patients with HCL, normal donors (NL), T-NHL, and HD. Median results of each set are shown.
sCD22 values in CD22-negative malignancies
To determine the specificity of sCD22 in patients with HCL, patients with T-cell lymphomas (T-NHL, n = 37) and HD (n = 22) were also tested. Of the 37 patients with T-cell lymphomas, 11 had adult T-cell leukemia, 20 had cutaneous T-cell lymphoma/mycosis fungoides, 4 had peripheral T-cell lymphoma, 1 had T-cell anaplastic large cell lymphoma, and 1 had T-cell CLL. As shown in Figure 3, patients with T-NHL and HD had median sCD22 levels no different from controls. Thus, elevated sCD22 levels in patients with HCL were probably the result of the CD22+ tumor burden in patients, rather than nonspecific causes associated with malignancy.
sCD22 assay accuracy and precision
To determine the accuracy and precision of the sCD22 ELISA assay, known quantities of sCD22-Fv were added to normal sera and the measured sCD22 levels were compared with expected levels. Triplicate experiments were performed for each assay and 2 or 3 independent assays were performed. For each known concentration of sCD22 added, percentage accuracy and COV were determined as described in “Statistical analysis.” Figure 4 shows the average accuracy and COV for each known concentration of sCD22 (0.041, 0.078, 0.156, 0.312, 0.625, 1.25, and 10 ng/mL) tested with each of 2 different serum samples. For the first normal human serum sample, accuracies ranged between 75% plus or minus 12% and 98% plus or minus 3% and COV ranged between 4% plus or minus 2% and 18% plus or minus 14%. For the second normal human sample, accuracy ranged between 72% plus or minus 14% and 98% plus or minus 1% and COV ranged between 2% plus or minus 1% and 20% plus or minus 19%. Thus, acceptable accuracy and precision were observed even at added sCD22 concentrations as low as 0.041 ng/mL. Unacceptable accuracy was observed at 0.0164 ng/mL (data not shown), indicating that 0.041 ng/mL was the lower limit of quantitation. Because each known sCD22 concentration was tested in the presence of human serum diluted 1:10, it may be concluded that as low as 0.41 ng/mL sCD22 in human serum could be detected with acceptable accuracy and precision. This value is approximately 20% of the upper limits of normal as defined in “sCD22 levels in patients with HCL and normal controls,” indicating that “abnormal” levels should be detectable with acceptable accuracy and precision.
Accuracy and precision. Serum samples from 2 normal donors (represented by gray and black), diluted 10-fold with PBS, contained the indicated known final concentrations of purified sCD22-Fc. ELISA-determined sCD22 concentrations in the samples were compared with known concentrations to determine accuracy (A) and precision (B). COV indicates coefficient of variance. Shown are the means of 2 or 3 independent experiments, and error bars represent SD.
Accuracy and precision. Serum samples from 2 normal donors (represented by gray and black), diluted 10-fold with PBS, contained the indicated known final concentrations of purified sCD22-Fc. ELISA-determined sCD22 concentrations in the samples were compared with known concentrations to determine accuracy (A) and precision (B). COV indicates coefficient of variance. Shown are the means of 2 or 3 independent experiments, and error bars represent SD.
Correlations between disease burden and sCD22 levels
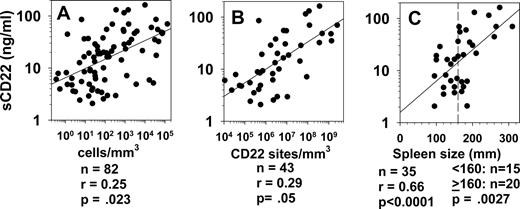
To determine whether sCD22 levels are proportional to HCL disease burden, concentrations of circulating HCL cells were obtained by flow cytometry in patients, and spleen size by caudocranial diameter (spleen height) was determined by computed tomography. As shown in Figure 5A, sCD22 was correlated with malignant cell counts in 82 patients (r = 0.25, P = .023). To correct for differences in CD22 density on the malignant cells, concentrations of circulating malignant cells were multiplied by CD22 sites/cell to obtain cell bound CD22 sites/mm3. sCD22 was proportional to sites/mm3 in the peripheral blood (Figure 5B, r = 0.29, P = .05). Rank order analysis showed that sCD22 levels were significantly higher with spleen size, accessed by caudocranial diameter, of at least 160 mm (Figure 5C, P = .003). sCD22 was also directly proportional to spleen size. Thus, sCD22 levels in patients with HCL are related to tumor burden as measured by malignant cells/mm3, cell-bound CD22 sites/mm3, and spleen size.
sCD22 vs tumor burden. sCD22 levels were obtained in 82 HCL patients with the indicated concentrations of circulating leukemic cells, determined by flow cytometry (A). The products of malignant cell counts and CD22 sites/cell are shown in panel B as cell-bound CD22 sites/mm3. sCD22 vs spleen height was assessed for 22 HCL patients with spleens present (C). The vertical dashed line indicates the spleen size (160 mm) used in comparing sCD22 levels in patients with large and small spleens by rank-order (Wilcoxon) analysis.
sCD22 vs tumor burden. sCD22 levels were obtained in 82 HCL patients with the indicated concentrations of circulating leukemic cells, determined by flow cytometry (A). The products of malignant cell counts and CD22 sites/cell are shown in panel B as cell-bound CD22 sites/mm3. sCD22 vs spleen height was assessed for 22 HCL patients with spleens present (C). The vertical dashed line indicates the spleen size (160 mm) used in comparing sCD22 levels in patients with large and small spleens by rank-order (Wilcoxon) analysis.
sCD22 in following disease status after treatment
To determine whether sCD22 levels could be used to follow patients after major response, patients with HCL were assayed before and after achieving partial response (PR) or complete response (CR) to recombinant immunotoxin BL22, which has a high response rate in HCL.41,42 As shown in Figure 6A and B, sCD22 decreased with both PR and CR, but the decrease was greater with CR. The log reduction in sCD22 was 0.11 to 1.6 (median, 0.5) for PR versus 0.75 to 2.4 (median, 1.4) for CR (P = .001). The decreases in sCD22 with response were similar to those measured by flow cytometry of the blood (Figures 6C,D). Moreover, the sCD22 levels at the time of response were predictive of PR versus CR, in that sCD22 levels were 2 to 26 ng/mL (median, 5.1 ng/mL) during PR, versus 0.18 to 1.7 ng/mL (median, 0.64 ng/mL) during CR (P < .001). Thus, sCD22 levels in HCL patients decreased to the normal range during CR but decreased to levels above the normal range with PR.
Tumor burden before and after response to BL22. Matched sCD22 levels before (PRE) and after achieving PR (A) or CR (B) in patients with HCL. HCL cell concentrations were determined by flow cytometry before (PRE) and after achieving PR (C) and CR (D) to BL22.
Tumor burden before and after response to BL22. Matched sCD22 levels before (PRE) and after achieving PR (A) or CR (B) in patients with HCL. HCL cell concentrations were determined by flow cytometry before (PRE) and after achieving PR (C) and CR (D) to BL22.
Competition of the cytotoxicity of BL22 by sCD22
To determine whether sCD22 levels would block the cytotoxic activity of anti-CD22 recombinant immunotoxin BL22, CD22+ Raji cells were incubated with different concentrations of BL22 in the presence and absence of different concentrations of sCD22. As shown in Figure 7, 30 ng/mL of sCD22 had essentially no effect on BL22 cytotoxicity. The IC50, the calculated concentration of BL22 necessary for 50% inhibition, was 0.35 ng/mL with or without 30 ng/mL sCD22; 300 ng/mL and 3000 ng/mL caused a dose-dependent competition in cytotoxicity (IC50 = 0.54 and 2.2 ng/mL) but even at 300 ng/mL sCD22 the effect on cytotoxicity was less than 2-fold. Thus, whereas the concentration of sCD22 may greatly exceed the concentration of BL22, the cytotoxicity of BL22 is preserved at clinically relevant sCD22 concentrations.
Competition of BL22 cytotoxicity by sCD22-Fc. Raji cells were incubated with the indicated concentrations of BL22 either alone (●) or combined with 30 (○), 300 (▾), or 3000 (▿) ng/mL of sCD22-Fc. Error bars as in Figure 2.
Competition of BL22 cytotoxicity by sCD22-Fc. Raji cells were incubated with the indicated concentrations of BL22 either alone (●) or combined with 30 (○), 300 (▾), or 3000 (▿) ng/mL of sCD22-Fc. Error bars as in Figure 2.
Discussion
Although many soluble receptors and antigens to follow disease burden have been examined in a wide variety of hematologic malignancies, none is in routine use for following patients with B-cell malignancies, which frequently express CD22. To determine whether the extracellular domain of CD22 could be detected and measured in the serum, HCL patient serum was studied by Western blot analysis and an ELISA was developed to detect sCD22. We found that sCD22 could be detected predominantly as a 100-kDa protein, that its level is 2.1 to 163 ng/mL in patients with HCL and less than 2 ng/mL in both normal donors and most patients tested with either T-cell malignancies or HD. sCD22 levels were proportional to disease burden in HCL, and its decrease after response to BL22 in HCL was greater with CR than with PR, suggesting that sCD22 levels could be useful in following these patients after treatment.
sCD22 compared with other markers for B-cell malignancies
Soluble CD25 is the most common assay performed for following leukemias and lymphomas, particularly adult T-cell leukemia and HCL where median levels of 69 000 and 48 000 units/mL (207 and 144 ng/mL) are observed.6 sCD25 is elevated at much lower levels in other lymphomas and leukemias, although it is also elevated in a wide variety of solid tumors. In most malignancies, the sCD25 is an “indirect” tumor marker, originating from activated normal T cells, rather than from the tumor. sCD22 would be considered a more specific marker, particularly for CD22+ and CD25− tumors, which are common among B-cell malignancies. An example might be the CD25− variant of HCL (HCLv). In 3 such patients who had CR to BL22,41 the log decrease in sCD22 compared with sCD25 was 2.4 versus 0.53, 1.2 versus 0.52, and 1.4 versus 0.28. Soluble IRTA2 (CD307) elevations were recently reported in CLL and MCL, with median levels approximately 5-fold and 2-fold higher than normal but with significant overlap with the normal range (< 30-600 ng/mL). The normal range of sCD22 (up to 2 ng/mL) is lower than those of sCD25 and sCD307, either because of more limited expression of CD22 or less rapid proteolysis or increased clearance of the extracellular domain of CD22 compared with those of the other markers. Finally, soluble (circulating) CD20 (cCD20) was reported to have a normal range of 124 to 547 nM (4-19 ng/mL). The median normal cCD20 level, 16 ng/mL, was only slightly less than the median cCD20 level of CLL patients (27 ng/mL), and both NHL and CLL patients with high cCD20 levels (up to 777 ng/mL) had poor prognoses.2,3 However, nearly half (40%) of NHL patients had normal (≤ 15 ng/mL) cCD20 levels.3 Even in patients with high cCD20, sCD22 may be more useful than cCD20 in following tumor burden in patients after rituximab therapy because the anti-CD20 mAb has a long half-life and would bind to cCD20.
Interaction between BL22 and sCD22
Unlike rituximab and other whole mAbs, BL22 is only 63 kDa, has a half-life of 2 to 3 hours, and should therefore not interfere with the sCD22 assay when used at least 1 to 2 days after BL22 therapy. As shown in Figure 7, even high levels of sCD22 only show modest competition for the cytotoxic activity of BL22. This is probably because BL22 binding to sCD22 is reversible, whereas BL22 binding to cell-associated CD22 is irreversible once internalization occurs. The pharmacokinetics of sCD22 are unknown. High levels of sCD22 are associated with biexponential rather than monoexponential disappearance of BL22 in the plasma, although this effect may in part be the result of the associated high levels of cell-associated CD22. Thus, sCD22 could be used in following response of HCL to chemotherapy, recombinant immunotoxins, and mAbs, such as rituximab or alemtuzumab. Its role in following patients with other B-cell malignancies is currently being tested.
Using sCD22 to follow HCL
To follow disease burden before and after tumor response, a tumor marker must be detectable after its concentration decreases by 1 to 2 or more logs in patients. We have recently published several methods to follow minimal residual disease in HCL, including flow cytometry, able to detect 1 HCL cell in 104 to 105 normal,45 and clone-specific real-time quantitative polymerase chain reaction, able to detect 1 HCL in 106 normal.50 As shown in Figure 6A and B, sCD22 decreased more than 1 log with CR and its decrease could discriminate between CR and PR. Whereas both CR and PR require improvement in normal blood counts, CR also requires a negative bone marrow biopsy. In most of these patients, CR could not otherwise be predicted without bone marrow biopsy or flow cytometry (Figure 6C,D) of the blood, tests that are much more invasive and/or expensive than a simple ELISA assay of serum. Thus, whereas sCD22 ELISA may not be appropriate for predicting very low levels of minimal residual disease, it might potentially be used in conjunction with blood counts for predicting when patients with HCL and possibly other B-cell malignancies achieve CR and may stop treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Waters at SAIC, Frederick for stocking and sending samples, Dr Milton Taylor at Indiana University for providing the HCL line Eskol, Dr Mitchell Ho and Richard Beers for providing some of the sCD22-Fc used as standard, nurses Karen Bergeron, Kelly Cahill, Rita Mincemoyer, Linda Ellison, and Elizabeth Maestri and patient care coordinator Sonya Duke for arranging samples from patients, Barbara Debrah for managing the data, Drs Wyndham Wilson, John Janik, John Morris, Jeff White, and Thomas Waldmann for referring patients with T-cell malignancies, and Drs Ira Pastan and Wyndham Wilson for helpful discussions regarding the project.
This work was supported by the intramural research program of the NCI, National Institutes of Health (NIH).
National Institutes of Health
Authorship
Contribution: R.J.K. and K.M. designed research and wrote the manuscript; K.M., I.M., and M.S.-S. performed research; R.J.K., K.M., and M.S.-S. analyzed data; and S.N. and M.O. contributed analytical tools.
Conflict-of-interest disclosure: R.J.K. and K.M. are coinvestors on the patent for sCD22, which is pending by the NIH. All other authors declare no competing financial interests.
Correspondence: Robert J. Kreitman, National Institutes of Health, Building 37, Room 5124b, 9000 Rockville Pike, Bethesda, MD 20854-4255; e-mail: kreitmar@mail.nih.gov.