Abstract
The humoral immune system senses microbes via recognition of specific microbial molecular motifs by Toll-like receptors (TLRs). These encounters promote plasma cell differentiation and antibody production. Recent studies have demonstrated the importance of the TLR system in enhancing antibody-mediated defense against infections and maintaining memory B cells. These results have led the way to the design of vaccines that target B cells by engaging TLRs. In hematologic malignancies, cells often retain B cell–specific receptors and associated functions. Among these, TLRs are currently exploited to target different subclasses of B-cell leukemia, and TLR agonists are currently being evaluated in clinical trials. However, accumulating evidence suggests that endogenous TLR ligands or chronic infections promote tumor growth, thus providing a need for further investigations to decipher the exact function of TLRs in the B-cell lineage and in neoplastic B cells. The aim of this review is to present and discuss the latest advances with regard to the expression and function of TLRs in both healthy and malignant B cells. Special attention will be focused on the growth-promoting effects of TLR ligands on leukemic B cells and their potential clinical impact.
Introduction
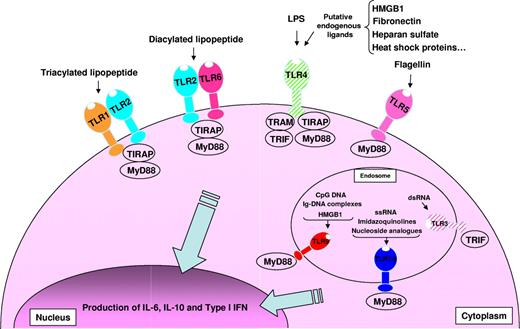
The human immune system is continuously challenged by commensal microflora as well as invasive infectious agents. The decision to mount a rapid and protective immune response to a pathogen is a consequence of the activation of the innate immune system via pattern recognition receptors, such as Toll-like receptors (TLRs), that sense microbial products.1 TLR polymorphisms have been implicated in increased severity and predisposition to infection and septic shock2 in both mice and humans. TLRs recognize highly conserved structures of viral (TLR3, 7, 8, and 9) and bacterial (TLR1, 2, 4, 5, 6, 7, 8, and 9) origin, known as pathogen-associated molecular patterns (PAMPs; Figure 1). TLR2 heterodimerization with TLR1 or TLR6 is triggered by bacterial lipopeptides, whereas TLR3 is activated by double-stranded RNA, TLR4 is activated by lipopolysaccharide (LPS), TLR5 is activated by flagellin, TLR7 and TLR8 are activated by single-stranded RNA (ssRNA), and TLR9 is activated by unmethylated CpG DNA motifs. Moreover, endogenous ligands released during cellular stress or matrix degradation (eg, heat-shock proteins, fibronectin, heparan sulfates) are thought to activate TLRs.3 Numerous reports have described how TLRs orchestrate the immune response to pathogens in dendritic cells (DCs) and macrophages.4 Much less is known about the effects of TLR-mediated B-cell activation, although the design of vaccines could benefit from a more detailed understanding of this process.
Overview of TLRs-TLR ligands and their signaling complexes in normal and malignant human B cells. TLR2 associates with TLR6 or TLR1 to form receptors that recognize diacylated and triacylated lipopeptides, respectively. TLR3 recognizes virally derived dsRNA. TLR4 recognizes LPS from Gram-negative bacteria, and several putative endogenous ligands. TLR5 recognizes bacterial flagellin. TLR7 and TLR8 recognize ssRNA from viruses, imidazoquinolines, and nucleoside analogs. TLR9 recognizes CpG DNA from bacteria and viruses, immunoglobulin-DNA complexes, and HMGB1. Unlike TLR1, 2, 5, 6, 7/8, and 9, which are expressed by normal and malignant human B cells, TLR3 and TLR4 are expressed solely by malignant B cells and are shown hatched. A total of 4 signaling adaptors are involved in TLR signaling: MyD88, TRIF, TRAM, and TIRAP. TLR signaling pathways result in the production of IL-6, IL-10, and type I IFN.
Overview of TLRs-TLR ligands and their signaling complexes in normal and malignant human B cells. TLR2 associates with TLR6 or TLR1 to form receptors that recognize diacylated and triacylated lipopeptides, respectively. TLR3 recognizes virally derived dsRNA. TLR4 recognizes LPS from Gram-negative bacteria, and several putative endogenous ligands. TLR5 recognizes bacterial flagellin. TLR7 and TLR8 recognize ssRNA from viruses, imidazoquinolines, and nucleoside analogs. TLR9 recognizes CpG DNA from bacteria and viruses, immunoglobulin-DNA complexes, and HMGB1. Unlike TLR1, 2, 5, 6, 7/8, and 9, which are expressed by normal and malignant human B cells, TLR3 and TLR4 are expressed solely by malignant B cells and are shown hatched. A total of 4 signaling adaptors are involved in TLR signaling: MyD88, TRIF, TRAM, and TIRAP. TLR signaling pathways result in the production of IL-6, IL-10, and type I IFN.
Leukemic B cells often retain the expression of markers specific for their cellular origin (eg, CD5, CD10, CD138). Furthermore, several reports have demonstrated TLR expression and function in neoplastic B cells. Because DCs can be activated and matured upon triggering of TLRs, immunotherapeutic protocols in leukemia have recently included TLR agonists to improve tumor antigen presentation and subsequent T-cell activation.5 However, recent reports have indicated that leukemic cells could hijack the TLR machinery to their own benefit. A better understanding of the effects of TLR ligands on normal B cells and their leukemic counterparts could therefore help avoid adverse vaccination effects. In this review, we will discuss the role of TLRs in generating the humoral immune response and their dual effects on different leukemic cell types.
TLR expression in normal and neoplastic B cells
Normal B-cell subsets and modulation of expression
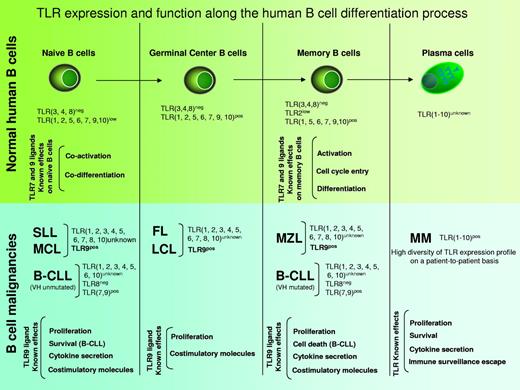
The TLR expression pattern is specific for each cell type and is summarized in Figure 2 for human B cells. TLRs expression in human B cells is characterized by high expression of TLR1, 6, 7, 9, and 10.6-8 Low expression of TLR2 allows for the formation of the functional heterodimers TLR1/2 and TLR2/6, which are required to respond to diacylated and triacylated lipoproteins. The inability to be activated by LPS is a hallmark of human B cells because they lack TLR4, in contrast to mouse B cells. However, human B cells are well equipped to recognize nucleic acids given their expression of TLR7 and TLR9. This profile allows them to act synergistically as part of the innate immune system with plasmacytoid dendritic cells (pDCs) that bear the same TLR expression pattern. This cellular duo can be further stimulated with immune complexes (Ab-RNA/DNA, Ab-viruses), and therefore represents a crucial player in the initiation and amplification of antimicrobial responses as well as the propagation of autoimmune diseases such as systemic lupus erythematosus.9-11 More detailed studies on human B-cell populations have shown that peripheral blood–derived naive and memory B cells express distinct levels of TLR6, 7, and 9. Indeed, TLR6, 7, 9, and 10 are barely expressed by circulating naive B cells,7 whereas memory B cells display a higher sensitivity to TLR activation with a concomitant higher capacity for differentiation into plasma cells. Naive B cells are thus poorly responsive to circulating PAMP and require additional signals to mature into plasma cells.
TLR expression and function during normal B-cell differentiation and in B-cell malignancies. MCL, B-cell SLL, B-CLL, FL, diffuse LCL, MZL, and MM.
TLR expression and function during normal B-cell differentiation and in B-cell malignancies. MCL, B-cell SLL, B-CLL, FL, diffuse LCL, MZL, and MM.
Human B-cell localization seems to influence their TLR expression profile, as similarly high expression levels of TLR are found in naive, germinal center (GC), and memory B cells in the tonsils, while TLR9 levels are lower in circulating blood B cells.12 Interestingly, TLR2 expression is restricted to a fraction of circulating B cells (IgG−, CD27−) that are characterized by intermediate expression levels of CD19 and CD69, whereas most tonsillar B cells express TLR2.12,13 Increased TLR expression in tonsillar B cells may thus result from local infections.14 Indeed, activation via the B-cell receptor (BCR) and/or CD40L, or by CpG oligodesoxynucleotides (ODNs), temporarily increases expression of TLR7, 9, and 10 in resting B cells.7,8 Furthermore, type I interferon (IFN), which is highly produced by pDCs during infections, induces TLR7 and MyD88 expression in naive peripheral blood B cells.15 However, TLR levels in B cells of infected and uninfected hyperplastic tonsils were found to be similar.12 TLR expression regulation may therefore also differ depending on the local environment.
TLR expression studies in different subsets of murine B cells reveal a pattern slightly different from that found in human B cells, as murine naive B cells express high levels of TLRs. While TLR1, 2, 6, 7, and 9 are expressed to various degrees, TLR3 expression is primarily restricted to marginal zone B cells. Of note, TLR4 is conserved independently of the B-cell subtype. As in humans, expression of TLR5 and TLR8 is absent or negligible.16,17
Human B cells and DCs share the expression of several TLRs. In both cell types, intracellular signal transduction initiated by TLR activation depends on the key signaling molecule MyD88 (except for TLR3) and results in nuclear translocation of NFκB, activation of PKB/Akt, Erk, and JNK mitogen-activated protein (MAP) kinases, and phosphorylation of interferon regulatory factor (IRF) transcription factor family members18 (Figure 1). These pathways induce similar cytokine secretion patterns (IL-6 and IL-10) and up-regulation of activation markers (ie, CD80, CD86, MHC II) in both cell types.19
Notably, TLR3 and TLR4, which both signal via TRIF, an adapter molecule that promotes MyD88-independent signaling, are not expressed in human B cells. To date, it is unclear whether TRIF plays a role in TLR-mediated B-cell activation. Similarly, a role for the TLR2/4-associated adapter proteins Mal/TIRAP and TRAM has thus far not been described in B cells. Another discrepancy exists in the recognition of LPS between B cells and DCs. Indeed, CD180 (RP105) structurally resembles TLRs but lacks a Toll/IL-1 receptor (TIR) domain and promotes LPS-driven B-cell responses in mice. In contrast, CD180 acts as a negative regulator of TLR4 signaling in monocytes and DCs.20 Furthermore, human B cells are much less responsive than DCs to bacterial cell wall components due to their low or absent expression of surface TLRs. Thus, recognition of microbes is restricted to endosomal activation of nucleic acid–sensing TLRs in pDCs and B cells. This stands in marked contrast to human myeloid DCs, which are characterized by the absence of TLR7 and TLR9 but are fully equipped to react quickly to LPS and lipopeptides.
Heterogeneous TLR expression in B-cell neoplasms
Information regarding TLR expression in B-cell chronic lymphocytic leukemia (B-CLL) is restricted to TLR7, 8, and 9, and mirrors expression in normal B cells (Figure 2). Although variations exist among patients and cell lines, TLR7 and TLR9 have been detected in most samples,21-25 whereas TLR8 was found to be absent.25 Molecules involved in TLR signaling (IRAK1, IRAK4, TRAF6, and MyD88) are also expressed with high heterogeneity.22 B-CLL cells express TLR9 levels similar to those of peripheral blood B cells. TLR7 expression is frequently lower than in human peripheral blood mononuclear cells, where it is expressed by pDCs and memory B cells.22
Precursor B acute lymphoblastic leukemia (pre–B-ALL) cells express TLR1, 2, 3, 4, 5, 6, 7, and 9; however, the question remains as to whether the aberrant expression of TLR3, 4, and 5 is a consequence of malignant differentiation or just a reflection of the normal B-cell precursor phenotype.
In multiple myeloma (MM), most primary tumors and cell lines express TLR1, 2, 3, 4, 7, 8, and 9. Among those, TLR1, 4, 7, and 9 are most frequently expressed.26,27 This expression pattern does not correlate with that of B cells or normal plasma cells (G.J. et al, unpublished data, August 2006), as TLR10 is lost, while TLR3, 4, and 8 are acquired by MM cells. A high level of heterogeneity exists among patients and cell lines; in some cases, this can be associated with disease activity markers. Indeed, DNA microarray analysis of a large panel of patients with MM has identified that TLR4 is overexpressed in patients bearing activated c-MAF and MAFB proto-oncogenes, a condition that represents approximately 6% of patients and is associated with a poor outcome (Zhan et al28 and G.J., unpublished data, December 2006). Furthermore, a recent report presented at the 11th International Myeloma Workshop suggested that high levels of TLR and MyD88 expression could be acquired during the transition from monoclonal gammopathy of undetermined significance (MGUS) to overt MM (Munshi et al, meeting report). Thus, TLR expression may be associated with disease progression or even with poor outcome. Monitoring TLR and MyD88 expression during long-term follow-up of individuals with MGUS is necessary to confirm this observation.
Physiologic responses to TLR binding in normal B cells
The first encounter of naive B cells with a pathogen induces secretion of both antigen-specific and -unspecific IgM. Accumulating evidence suggests that TLR engagement in B cells could be instrumental in the initiation and amplification of this response. Bacterial cell walls contain various molecules that activate surface TLRs (TLR1/2, TLR2/6, or TLR4). However, expression of these TLRs is low or even absent in naive human B cells. In marked contrast, endosomally located nucleic acid–sensing TLR7 and TLR9 are present in naive human B cells and are thought to be major players in B-cell stimulation by microbes. Due to their endosomal localization, TLR7 and TLR9 stimulation must be preceded by endocytosis of microbial nucleic acids, which may occur upon bacterial disintegration or cellular infection via cell-surface receptor–mediated uptake. This leads to spatial and temporal segregation of TLR ligand availability, which translates into different levels of B-cell activation. Indeed, activation of human B cells by Staphylococcus aureus cell walls or TLR2 ligands depends on the presence of a costimulus, such as BCR ligation with anti–human Ig, or virulence factors, such as staphylococcal surface protein A (SpA). Only dual stimulation by SpA- and TLR2-active lipopeptides induces cell cycling, and additional IL-2 is required to fully differentiate a small number of Ab-secreting cells.29 On the contrary, CpG ODN and synthetic TLR7 ligands are more potent stimuli that are sufficient to induce Ig synthesis. These findings suggest that safety mechanisms have evolved to avoid premature responses to ubiquitous TLR2 ligand–bearing commensal bacteria while allowing potent B-cell activation in the context of proliferation-induced microbial disintegration.
Another level of TLR-mediated B-cell activation control resides in the nature of naive B cells themselves. Indeed, in contrast to memory B cells, activation of naive B cells is difficult,30 and TLR stimulation alone only induces limited activation.15,31 Full activation requires a combination of 3 signals32 : BCR triggering, assistance by T cells, and TLR stimulation. The absence of the latter prevents strong activation and plasma cell differentiation. This triple requirement suggests that full responsiveness of human naive B cells to TLR-activating PAMPs occurs only after specific antigenic engagement of the BCR.
Adjacent immune cells can regulate B-cell sensitivity to TLR ligands and provide costimulatory signals, such as BAFF and APRIL, that enhance TLR signaling (I.B.-D., unpublished data, April 2007).15 It has also been shown that DCs and particularly pDCs can control the humoral memory response to viruses through TLR expression and subsequent secretion of type I IFN.33,34 Thus, TLR activation can regulate the humoral response at several checkpoints.
In many aspects, the murine immune system is more sensitive to stimulation than that of humans. This is true for TLR function, as both naive and memory murine B cells respond equally to TLR agonists in vitro despite the absence of BCR activation.16,17 Furthermore, the murine B-cell subsets that are involved in T-independent responses (marginal zone B cells, B-1a and B-1b cells) respond to various TLR agonists (with the exception of flagellin and polyI:C) with strong proliferation and differentiation into plasma cells in vitro. This permits polyclonal secretion of antibodies during infections and thus increases the innate response. However, the role of TLRs in T-dependent immune responses has been explored with controversial results. One in vivo study showed that TLR4 and MyD88 in B cells were necessary for optimal GC formation and plasma cell differentiation.35 In marked contrast, 2 other studies showed either no difference in Ab responses as compared with wild-type mice36 or only a role restricted to response amplification.37 The reasons for this discrepancy are still a matter of intense debate that will not be discussed in this review.38
Ig class-switch recombination (CSR) permits the transition from IgM to the IgG, IgE, and IgA isotypes that have higher affinity to the target antigen and can enhance opsonization of microbes via Fc receptors. Studies in mouse models have shown that TLR can initiate CSR through up-regulation of the activation-induced cytidine deaminase (AID).39 Additional signals from BCR, CD40L, BAFF, APRIL, IL-4, and/or IL-10 are needed, however, to fully produce the Ig CSR. Furthermore, active CSR has been observed during murine B-cell bone marrow development after BCR or TLR engagement.40,41 In humans, CSR has been demonstrated upon TLR9 stimulation in combination with IL-10, BAFF, CD40L, or APRIL, the latter having been shown to induce CSR to IgA.
Formation of long-term humoral immunity is obtained through a persistent pool of memory B cells and long-lived plasma cells. Indeed, Ag-specific memory B cells must survive in the absence of cognate Ag, a situation that can last for months or even years. Their reactivation permits replenishment of their pool and is a source for new plasma cells. Recent reports suggest that TLRs play a role in this particular aspect of long-term memory. Because TLR triggering induces memory B-cell proliferation in the absence of BCR triggering, polyclonal activation by TLR ligands can occur during any kind of infection. In the absence of infection, minute amounts of TLR ligands may be sufficient to sustain homeostasis by allowing self-perpetuation of the system. Furthermore, this process represents a very efficient means for generating plasma cells. Indeed, vaccine boosts are accompanied by increased numbers of circulating plasma cells that are specific for vaccine antigens in addition to plasma cells specific for other recall antigens.42 However, the origin of the circulating polyclonal plasma cells has been challenged by studies suggesting that these cells represent resident bone marrow plasma cells that are reconducted to the blood by the newly formed antigen-specific plasma cells.43,44 This mechanism is thought to generate space in the bone marrow niches, which have a finite capacity. However, because MyD88 is necessary to maintain long-lived mouse plasma cells,45 and we have detected TLRs in normal plasma cells generated in vitro (G.J. et al, unpublished data, August 2006), we hypothesize that direct activation of plasma cells by TLR ligands could contribute to plasma cell–derived Ig secretion in addition to migration and survival.
B-cell neoplasms' response to TLR ligands
Proliferation
The effects of TLR activation in leukemic cells frequently mirror those observed in normal B cells. Upon CpG ODN stimulation, increased proliferation was observed in different lymphomas (mantle cell, B-cell small lymphocytic, follicular, and diffuse large B cell).21 Despite this common increase in proliferation, a highly heterogeneous level of response was observed when comparing different lymphomas and even on a patient-to-patient basis. Together with the variability in TLR expression, the variability in the response of malignant B cells to TLR binding is representative of the heterogeneity of B-cell neoplasias.
The highest proliferation induction was seen in marginal zone lymphomas (MZLs), possibly because MZL is derived from memory B cells. Memory B cells are very sensitive to TLR9 activation when compared with naive and GC B cells. However, a broader panel of patients in each category is necessary to validate these data and fully explore the cause of the variability because the number of patients was limited.
In contrast to data on B-cell lymphomas, several reports have shown a frequent increase of proliferation in B-CLLs. Stimulating B-CLLs with CpG ODNs induces cell-cycle entry and increases thymidine incorporation.21,46,47 Synergy with IL-2– or CD40L-induced proliferation of B-CLLs has also been observed.46,48 The latter effect could be explained by up-regulation of CD40 expression in B-CLLs upon CpG ODN stimulation.21 On the contrary, heterogeneous and mostly weak proliferation of B-CLL cells has been observed upon TLR7 stimulation.49,50 TLR9-induced proliferation is mainly observed in samples from patients with progressive diseases or bearing unmutated immunoglobulin variable heavy chain genes (VH), which correlate with bad prognoses.23 Contrarily, CpG ODNs frequently induce apoptosis in patients with stable disease, VH mutations,23 or low serum thymidine kinase activity.51 Interestingly, the differences observed between patients in these 2 B-CLL subgroups do not correlate with TLR9 expression levels23,52 but rather with prolonged activation of signaling pathways, including Akt, MAP kinase p38, and NFκB.
Unlike findings obtained in other B-cell leukemic cell types, no change in proliferation was observed for pre–B-ALL cells in the presence of ligands for TLR2, 7, and 9 despite changes in cell-surface marker expression.53,54
Similar to B-CLLs, MMs frequently respond with strongly increased proliferation when challenged with ligands for TLR2, 3, 4, 5, 7, or 9. Cell viability and absolute cell numbers for cell lines and primary MM cells increase over the time in culture.26 In several MM cell lines, TLR-induced proliferation and survival is mediated by autocrine secretion of IL-6, which is the major MM growth factor. IL-6–independent increases in proliferation have also been observed for some MM cell lines (G.J. et al, unpublished observation, June 2007). The TLR responsiveness of MM cells raises the question of whether this phenomenon can be considered a hallmark of the plasma cell differentiation that is conserved throughout the oncogenic process or rather a characteristic of malignant transformation. However, evidence of a central role for MyD88 in bone marrow plasma cell formation supports the former.45
Immunomodulatory effects of TLR on neoplastic B cells
Because triggering TLRs induces DC maturation and increases their stimulatory properties, several studies have focused on the use of TLR ligands to improve the immunostimulatory potential of leukemic B cells as a therapeutic tool.
B-CLL cells display poor antigen-presenting capacity and costimulatory activity when cocultured with autologous or allogeneic T cells. This is partially due to low expression of costimulatory molecules.55 Interestingly, ligands for TLR7 and TLR9 but not ligands for TLR2 and TLR3 can induce changes in costimulatory molecule expression in B-CLLs. CpG ODNs and imidazoquinolines equal or even surpass CD40L in their ability to up-regulate CD40, CD54, CD80, CD86, and MHC class I and II.21,22,48
Lymphoma cells similarly respond to TLR9 triggering by increasing their expression of these costimulatory molecules. However, differences exist because mantle cell lymphoma (MCL) cells show no significant up-regulation of CD80, CD86 or MHC class I, and large cell lymphoma (LCL) cells do not show increased MHC class I and II expression.
Recent questions have addressed whether these phenotypic changes are sufficient to trigger T-cell responses against tumor antigens or whether they actually contribute to tumor evasion of the immune response. It should be noted that partial or inappropriate T-cell activation can induce anergy and regulatory T cells.56 This may be particularly true for ALLs because the effect of CpG ODNs on the costimulatory capacity of pre-ALL cells is limited; CD40 is the only costimulatory molecule that is up-regulated. Furthermore, ALL cells treated with CpG ODNs are unable to induce proliferative allogenic T-cell responses, and their cytokine profile is shifted toward IFNγ and IL-10 secretion without IL-5 production, a phenotype that closely corresponds to that of Tr1 cells. This cytokine secretion profile is further accompanied by high levels of IL-6 and IL-10, 2 cytokines with known immunosuppressive function in the absence of IL-12p70.53 This cytokine profile is reminiscent of normal B cells upon CpG ODN stimulation (I.B.-D. et al, unpublished data, September 2007). Similarly, stimulation of B-CLL cells with CpG ODNs or imidazoquinoline induces little or no T-cell proliferation,46,50 but generates high levels of IL-6 and IL-10 synthesis. Upon restimulation with imidazoquinoline, this cytokine pattern is either sustained or slightly enhanced, while TNFα secretion is strongly inhibited.24 Given that TNFα is a DC maturation factor, we hypothesize that repeated stimulation may shift the immune response toward tolerization. Interestingly, increased IL-10 levels have been described in patients with B-CLL with progressive disease.50
Moreover, MM cell stimulation via activation of TLR2, 4, or 9 inhibits cytotoxic T lymphocyte (CTL) generation by inducing B7-H1 expression.57 Furthermore, our unpublished data (June 2006) suggest that the resistance of MM cells to these effectors is increased. Altogether, there are abundant data that support a direct role for TLR ligands in the shaping of the immune response via activation of leukemic B cells.
Therapeutic approaches to improve the immunostimulatory potential of leukemic cells must be combined with a therapeutic tool that will abrogate protumoral machinery while preserving or even enhancing the expression of costimulatory molecules. In B-CLLs, exogenous IL-2 strongly increases CD80 and CD86 expression and allogeneic T-cell stimulation when combined with CpG ODNs or imidazoquinoline.46,50 However, recent in vitro and in vivo studies have demonstrated that IL-2 contributes to regulatory T-cell development,58,59 thus bringing the potential benefit of this combination into question. More recently, protocols including concomitant administration of a protein kinase C (PKC) agonist with a TLR7 ligand have attracted attention. Initial studies have observed inhibition of tumor cell proliferation and reduced IL-10 secretion, in addition to increased T-cell proliferation.50
Drug sensitivity in the context of TLR stimulation
Targeting TLRs on malignant B cells results in phenotypic and functional changes that affect cellular sensitivity to pharmacologic substances. These alterations can induce resistance to chemotherapeutic agents but can also be exploited for combined therapeutic approaches. For example, CD20 expression is increased in many different types of malignant B cells after CpG ODN stimulation (small lymphocytic lymphoma [SLL], MZL, and CLL strongly; MCL, LCL, and follicular lymphoma [FL] weakly).21 Because increased CD20 expression could improve the efficacy of the anti-CD20 monoclonal antibody rituximab, clinical trials are under way to investigate the efficacy of this combination. Moreover, the sensitivity of B-CLL cells to fludarabine alone or in combination with mafosfamide can be strongly enhanced by a TLR7 ligand (loxoribine).60 It has also become clear that the time of TLR ligand administration is critical to properly enhance the effect of cytotoxic drugs. For example, a single treatment of B-CLL cells with a TLR7 ligand protected the cells against high-dose vincristine toxicity, whereas repeated exposure sensitized the cells.24 In MM, however, CD20 expression has not been observed after stimulation with TLR ligands (Jahrsdorfer et al21 and G.J., unpublished data, November 2007), and TLR7 and TLR9 ligands protect MM cells from dexamethasone-induced apoptosis.26 Therefore, MM cells may form a separate entity with different characteristics.
Putative involvement of endogenous TLR ligands in leukemic cell growth
Apart from microbial molecular patterns, a broad array of molecules released in the context of tissue damage, cellular stress, or cell death may also be recognized by TLRs. These danger-associated molecular patterns (DAMPs) represent host-derived TLR ligands. Recently, a broad variety of molecules has been described as endogenous TLR ligands and mainly target TLR2 and TLR4. Among them, fibronectin, fibrinogen, heparan sulfate, and heat-shock proteins comprise the most prominent examples.3,61 Interestingly, DAMPs can also be generated by cell death during chemotherapy; one of the molecules that accumulates in this setting is HMGB1, which is described as a TLR4 or TLR9 agonist.62,63 However, care must be taken regarding the ability of endogeneous molecules to bind TLRs; difficulty in chemically separating contaminating TLR2-active lipopeptides and LPS from candidate molecules results in a very heterogeneous panel of identified autologous TLR ligands. Only very few of these ligand will be validated over time.64 Despite this drawback, accumulating evidence suggests that endogenous TLR ligands are involved in solid tumor progression,65,66 although evidence for leukemia-promoting effects has not yet been found. Nevertheless, HMGB1 is released by osteoclasts67 and could stimulate adjacent MM cells; in addition, heparan sulfate proteoglycans expressed on MM cells such as CD138 could bind to TLR4 and act as autocrine survival factors. Despite the findings that soluble CD138 and heparanase levels as well as TLR4 expression all correlate with poor prognoses and degradation of the MM cellular matrix could facilitate the interaction of endogenous TLR ligands with tumor cells, further investigations are required to validate this association.
TLR activation of both immune and neoplastic cells: how to shift the balance?
Synthetic TLR ligands improve DC function and break immune tolerance in vitro and in mouse models of cancer. These findings prompted investigators to start clinical trials using TLR ligands as adjuvants. Although there is evidence for immune cell activation, the clinical benefits have been limited. This could be due to tumor-derived immunosuppressive substances that are released into the tumor environment. Importantly, this release of immunosuppressory mediators could be enhanced via direct activation of the tumor cells through TLRs.
Moreover, the number of regulatory T (Treg) cells and DCs that secrete high levels of immunosuppressive cytokines are often increased in patients with lymphoma and MM.68-70 In MM, the monocyte-derived DCs generated by the patients are functionally defective; this was found to be due to cytokines released by MM cells.71 TLR ligands may amplify these effects and inhibit DC maturation. Although recent reports show that Treg-cell function can be inhibited by directly targeting TLR8,72 studies using other TLR ligands have observed enhanced Treg-cell induction and improved Treg-cell function.73 In particular, CpG ODNs and LPS promote Treg-cell induction through IL-10 production by DCs.74,75
Conversely, the efficacy of anthracycline-based therapy may be partly mediated by HMGB1 released from dying tumor cells. This process would permit optimal cross-presentation by DCs and thus may provide an explanation for the faster relapse of patients with breast cancer carrying loss-of-function TLR4 mutations.
Taken together, these findings suggest that targeting TLRs in B-cell neoplasia can result in both positive and negative outcomes. Therefore, more refined strategies designed to selectively neutralize the tumor-promoting effects may represent a decisive step in the improvement of immunotherapy. In support of this strategic approach, neutralization of IL-10 secreted by DCs in response to TLR ligands has been shown to improve the efficacy of CpG ODNs in mouse models of cancers.75,76
Conclusions
Elucidating mechanisms that lead to robust and long-lasting humoral immunity is critical for developing new vaccination strategies. The success of one of the most effective vaccines available, the yellow fever vaccine, is thought to be due to the combined triggering of several TLRs on DCs.77 In addition, myeloid DCs or pDCs promote plasma cell differentiation upon stimulation with whole viruses or synthetic ligands for TLR7 and TLR9,34 but it remains unclear whether vaccine efficacy could partially arise from direct B-cell activation. Many studies summarized in this review support direct B-cell activation because TLR triggering is mandatory for full activation of naive human B cells and strong amplification of T-dependent responses and because polyclonal stimulation of memory B cells by TLR ligands appears to be involved in maintaining the human memory B-cell pool. Therefore, selected TLR ligands are promising candidates for novel adjuvants that could improve the efficacy and duration of vaccine-induced immunity.
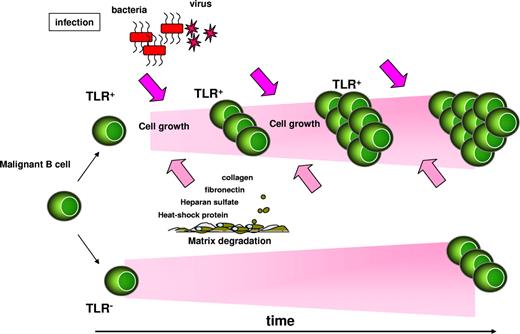
Although the humoral immune system is activated to protect organisms against infections, it can also give rise to different types of leukemic cells. The normal differentiation process involves multiple steps in which oncogenic events can occur. Fortunately, these events are rare and generally not stable over time because fatal mutations in the clone, elimination by the immune system, and DNA repair mechanisms efficiently prevent the development of malignancies. However, the capacity of TLR ligands to promote B-cell cycling and the secretion of cytokines with immunosuppressive capacity could favor the persistence of malignant clones. In this context, conserved TLR expression or acquisition of new TLR expression patterns could be critical for promoting leukemic ontogenesis. We therefore suggest a model (Figure 3) in which repeated polyclonal activation of leukemic B cells by microbial molecules during natural infection or inflammation is the initial step in the oncogenic process that lowers the threshold for outgrowth of malignant cell clones.
Model of cell growth–promoting effects of TLR ligands on malignant B cells. Recurrent bacterial or viral infections, or endogenous TLR ligands released by cellular stress (eg, collagen, fibronectin, heparin sulfate, heat-shock proteins) could favor the expansion of TLR-expressing malignant B-cell clones over those that have not acquired or retained TLR expression. TLR ligands would then promote cell growth and/or escape from immune surveillance.
Model of cell growth–promoting effects of TLR ligands on malignant B cells. Recurrent bacterial or viral infections, or endogenous TLR ligands released by cellular stress (eg, collagen, fibronectin, heparin sulfate, heat-shock proteins) could favor the expansion of TLR-expressing malignant B-cell clones over those that have not acquired or retained TLR expression. TLR ligands would then promote cell growth and/or escape from immune surveillance.
Current knowledge of MM and B-CLL pathophysiology favors this hypothesis. In addition, concomitant B-cell deficiency predisposes patients with MM and B-CLL to infection. Recent epidemiologic studies have shown that several episodes of pneumonia prior to MM or B-CLL diagnosis increase the risk of developing MM.78,79 Although additional epidemiologic studies are necessary to validate these observations, it is striking that these malignancies are often newly diagnosed in the context of infections and that recurrent infections remain a major cause of morbidity in patients.
Due to the adverse effects of TLR ligands on neoplastic B cells, their therapeutic use needs to be addressed with care. This is also complicated because a broad heterogeneity of TLR responsiveness exists among B-cell neoplasias. Among B-CLL subtypes, opposite responses to TLR ligation have been described depending on VH mutation status. Furthermore, only protumoral effects have been observed in vitro for ligands for TLR7 and TLR9 in MM cells. Therefore, TLR-based therapy should only be performed in carefully selected patients. Future clinical trials will have to account for cellular heterogeneity and evaluate possible predictors of in vivo response. Soon, the first results will be available from several early-stage clinical trials that have already examined B-CLLs using TLR agonists as a single agent80 ; however, future protocols should include combined therapeutic approaches to prevent undesirable effects due to direct activation of tumor TLRs.
As TLRs are broadly expressed and display functional activity in normal and neoplasic B cells (Figure 2), we conclude that both antimicrobial vaccine design and immunotherapy in B-cell leukemia will benefit from studies that decipher TLR function in the human B-cell lineage.
Acknowledgments
We thank Dr Martine Amiot and Dr Thierry Defrance for their critical review of the manuscript.
This study was supported by grants from La Ligue Contre le Cancer (équipe labélisée 2008).
Authorship
Contribution: G.J. provided the concept and designed and wrote the review; D.C. and I.B.-D. contributed to the writing and some of the original work described in this review; and C.P.D. and R.B. contributed to the writing of the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gaëtan Jego, Inserm U892, 9 quai Moncousu, 44093 Nantes Cedex 01, France; e-mail: gaetanjego@yahoo.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal