In this issue of Blood, Hitchcock and colleagues provide novel insights into the molecular pathways regulating TPO-mediated c-Mpl trafficking, highlighting the essential role of AP2 in receptor internalization and lysosomal degradation as new pieces in the TPO/c-Mpl signaling puzzle.
Thrombopoietin (TPO) and its receptor c-Mpl are the primary regulators of megakaryocytopoiesis and play a critical role in hematopoietic stem cell biology. Upon TPO binding, c-Mpl facilitates tyrosine (Y) phosphorylation of cytoplasmic signaling proteins and activation of several pathways that control cellular proliferation, megakaryocyte development, and survival. Turning off the TPO signal is critical to prevent uncontrolled proliferation; however, the molecular processes governing this process are still not fully elucidated. Suppressors of cytokine signaling (SOCS), phosphatases, and other proteins, such as focal adhesion kinase and members of the Src family kinases (SFKs), have recently emerged as negative modulators of TPO signaling.1,2
Although it is still a matter of debate, soon after the discovery of TPO it was suggested that internalization of TPO/c-Mpl complexes by platelets and megakaryocytes is a primary means of regulating plasma TPO levels. A phosphotyrosine (Y112) and 2 dileucine motifs (L54L55 and I57L58) within the cytoplasmic domain of the receptor have been identified to promote TPO-mediated c-Mpl internalization and proliferative signaling. Furthermore, c-Mpl recycling from intracellular pools has also been demonstrated.3
Clathrin-mediated endocytosis constitutes the major route for selective receptor internalization in higher eukaryotes. Clathrin molecules are recruited to the cell surface where they interact with transmembrane proteins via adaptor proteins (AP) such as AP2 to form clathrin-coated pits. YXXφ and [DE]XXXL[IL] motifs in the cytoplasmic tails of transmembrane pro-teins have been identified as the binding sites for AP2.4
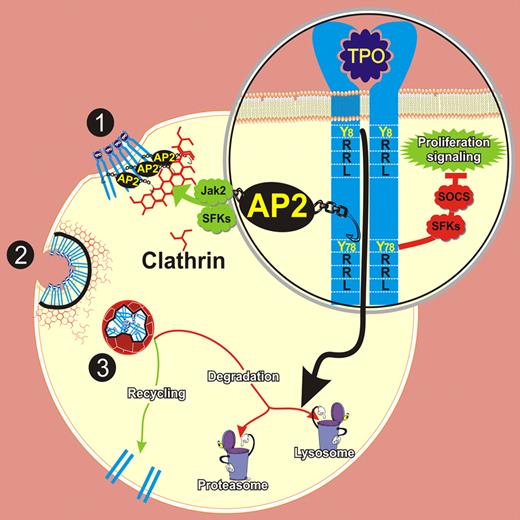
Hitchcock et al, in a series of elegant experiments, have demonstrated that AP2 has a key role in TPO-stimulated clathrin-mediated c-Mpl internalization in both Jak2 and SFKs activation-dependent pathways. Bearing in mind that c-Mpl contains 2 YXXφ motifs in its cytoplasmic domain at Tyr8 (Y8RRL) and Tyr78 (Y78RRL) and using BaF3-Mpl–expressing cells with point mutations at Y8 or Y78, the authors have identified Y78RRL as the binding motif for AP2 in the c-Mpl receptor. Interestingly, they also observed that cells expressing the Y78F mutation exhibited increased proliferation and prolonged activation of Jak2, STAT5, AKT, and ERK1/2 in response to TPO. The observation that knocking down AP2 did not have the same effects on signaling as the mutation led the authors to suggest that Y78 may be responsible not only for the internalization but also for the activation of other signaling pathways that restrain the TPO signal.
Although the clearance of c-Mpl was recently attributed only to the proteasome,5 this study unveiled that lysosomal degradation is another control mechanism of c-Mpl expression and also identifies Y8RRL as the lysosomal targeting motif. Accordingly, Y8F c-Mpl cells showed an increase in c-Mpl recycling to the cell surface (see figure).
Regulation of TPO-mediated c-Mpl receptor internalization and degradation. After TPO binding, clathrin-coated pits are formed through the interaction between the AP2 and Y78RRL motif in the cytoplasmic tail of c-Mpl. The internalization process is regulated by Jak2 as well as SFKs activation (1). Following invagination and budding, (2) clathrin-coated vesicles are routed to endocytic pathways that direct the c-Mpl receptor to recycling and/or degradation (3). While Y78RRL negatively regulates proliferation, Y8RRL targets c-Mpl lysosomal degradation.
Regulation of TPO-mediated c-Mpl receptor internalization and degradation. After TPO binding, clathrin-coated pits are formed through the interaction between the AP2 and Y78RRL motif in the cytoplasmic tail of c-Mpl. The internalization process is regulated by Jak2 as well as SFKs activation (1). Following invagination and budding, (2) clathrin-coated vesicles are routed to endocytic pathways that direct the c-Mpl receptor to recycling and/or degradation (3). While Y78RRL negatively regulates proliferation, Y8RRL targets c-Mpl lysosomal degradation.
This paper is the first report that describes the YXXφ motif for receptor internalization and lysosome targeting in the hematopoietic growth factor receptor family and raises many new questions that await an answer. What mechanisms control the balance between c-Mpl degradation/recycling? What contribution does c-Mpl internalization make to the TPO proliferative signal? What would be the biological impact of the c-Mpl Y78 and Y8 mutations in primary cells? To what extent are Y mutations linked to the pathogenesis of myeloproliferative diseases? Investigating these questions should help us to understand the interplay between the actors controlling the TPO/c-Mpl scenario.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal