Abstract
Emerging data suggest a critical role for bone marrow angiogenesis in hematologic malignancies. The angiopoietin/Tie ligand-receptor system is an essential regulator of this process. We evaluated whether circulating angiopoietin-2 (Ang-2) is a predictor for the probability of disease-free survival (DFS) in allogeneic hematopoietic stem cell transplantation (allo-HSCT) for high-risk acute myeloid leukemia or myelodysplastic syndrome. Ang-2 was measured by enzyme-linked immunosorbent assay in serum from 20 healthy controls and 90 patients with acute myeloid leukemia or myelodysplastic syndrome before conditioning for HSCT. Circulating Ang-2 was elevated in patients (median, 2.21 ng/mL; range, 0.18-48.84 ng/mL) compared with controls (median, 0.87 ng/mL; range, 0.27-4.51 ng/mL; P < .001). Multivariate analyses confirmed the independent prognostic impact of Ang-2 (hazard ratio [HR] = 2.46; 95% confidence interval [CI], 1.27-4.76, P = .005), percentage of bone marrow infiltration (HR = 1.14; 95% CI, 1.01-1.29, P = .033), and chemotherapy cycles before HSCT (HR = 1.38; 95% CI, 1.01-1.08, P = .048). Regression tree analysis detected optimal cutoff values for Ang-2 and recursively identified bone marrow blasts and Ang-2 as the best predictors for DFS. Because few predictors for DFS exist in the setting of allo-HSCT, Ang-2 may be used as a readily available powerful biomarker to pre-estimate DFS and may open new perspectives for risk-adapted treatment of high-risk myeloid malignancies.
Introduction
Despite improvements in therapy during the last 25 years, the majority of patients with high-risk myeloid malignancies, including acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) frequently require allogeneic hematopoietic stem cell transplantation (HSCT) in addition to standard chemotherapy protocols.1 High-risk disease is characterized by delayed response to chemotherapy,2 unfavorable karyotype,3-5 a history of preceding neoplasia and/or chemotherapy,6,7 and the occurrence of relapse.8
Although several clinical and biologic features of myeloid malignancies have been identified as prognostic factors, including loss or overexpression of tumor suppressor genes or oncogenes, expression of drug-resistant proteins, elevated levels of circulating cytokines,9-12 response to induction therapy, and duration of first remission,13 the estimation of disease recurrence after allogeneic HSCT remains difficult. This is particularly true for patients with high-risk myeloid malignancies. Thus, a readily available marker that could predict the risk of relapse after allogeneic HSCT in advance is needed.14
The Angiopoietin/Tie ligand-receptor system is the second class of vascular-specific receptor tyrosine kinases (the first being the vascular endothelial growth factor (VEGF)/VEGF-receptor system) to be linked to overall survival in patients with de novo AML.15,16 Angiopoietin-2 (Ang-2) tightly controls the endothelial phenotype during angiogenesis in a unique and nonredundant fashion.17,18 Specifically, Ang-2 facilitates angiogenesis via negative interference with constitutive Ang-1/Tie2 signaling, thus altering the integrity of interendothelial junctional complexes and the cell-surface expression of different growth factor receptors, including receptors for inflammatory and angiogenic cytokines.18,19
Although virtually absent in quiescent endothelium, Ang-2 expression is only observed at sites of active vascular remodeling and neoangiogenesis.20,21 Recently, endothelial Weibel-Palade bodies have been identified as the primary source of Ang-2, which is dramatically up-regulated on endothelial-cell activation and proliferation.22 Thus, Ang-2 constitutes a dynamic regulator of endothelial activation, which facilitates inflammation, vessel remodeling, and neo-angiogenesis.18,22
The importance of angiogenesis for the progressive growth and viability of solid tumors is well established.23,24 Emerging data indicate a crucial involvement of angiogenesis in the pathophysiology of hematologic malignancies as well. Studies comparing the extent of bone marrow angiogenesis in normal and neoplastic bone marrow have demonstrated significantly increased angiogenesis in patients with AML.25,26 The increased density of microvessels in the neoplastic bone marrow results from several angiogenic molecules (eg, Ang-2, VEGF, and basic fibroblast growth factor) that are induced on interplay between bone marrow endothelial cells and leukemic blasts.27-29 Vice versa, the expanded endothelium supports leukemic hematopoiesis through paracrine release of hematopoietic growth factors.30,31
Recently, it has been demonstrated that, among several investigated circulating angiogenic molecules (Ang-2, Tie2, VEGF, and basic fibroblast growth factor), only Ang-2 had prognostic impact on survival in de novo AML.16,32 Accordingly, high levels of Ang-2 at the time of diagnosis represents an unfavorable prognostic factor, whereas low Ang-2 level is a favorable prognostic factor for overall survival in de novo AML.15,16
The aim of this study was to analyze whether circulating Ang-2 might serve as a prognostic marker in HSCT for high-risk AML or MDS. Therefore, we determined the serum expression of Ang-2 by enzyme-linked immune assay in 90 adult patients before HSCT for high-risk myeloid malignancies. Ang-2 levels were correlated with clinicopathologic features and disease-free survival.
Methods
Patients' eligibility criteria
Patients were included if they fulfilled at least one of the following criteria defining high-risk AML: (1) primary induction failure (PIF) after 2 or more cycles of chemotherapy, (2) first early relapse after a remission duration of less than 6 months, (3) relapse refractory to salvage combination chemotherapy containing high-dose AraC, (4) second or subsequent relapse,33 and (5) cytogenetics. Patients with MDS were included if their disease was classified as high risk (IPSS int-2 and high).3 Further inclusion criteria were age between 18 and 70 years and the availability of a family or unrelated stem cell donor with 8 of 8 or, when done, 10 of 10 HLA match. One major mismatch (HLA-A, -B, -C, or -DRB1 mismatch) was accepted. Exclusion criteria were creatinine clearance less than 50 mL/min, bilirubin or transaminases greater than 3 times the upper limit of normal, cardiac shortening fraction less than 30%, and pregnancy.
For cytoreduction, patients received fludarabine 30 mg/m2, high-dose cytarabine 2 g/m2, and amsacrine 100 mg/m2 from days −12 to −9 (FLAMSA regimen33 ). After 3 days of rest, reduced-intensity conditioning consisted of 4 Gy total body irradiation on day −5, cyclophosphamide (40 mg/kg for HLA-identical sibling, 60 mg/kg for unrelated or mismatched donors) on days −4 and −3, and rabbit antithymocyte globulin (10 mg/kg for HLA-identical sibling, 20 mg/kg for unrelated or mismatched donors) from day −4 to day −2. For transplantation, granulocyte colony-stimulating factor mobilized peripheral blood stem cells were preferred; bone marrow was accepted at the donor's preference. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine from day −1, and mycophenolate mofetil (15 mg/kg), starting from day 0. In the absence of GVHD, mycophenolate mofetil was discontinued by day 50 and cyclosporine was tapered from days 60 to 90. Standard infection prophylaxis was used. Patients received prophylactic donor lymphocyte infusions if they were in complete remission (CR) without signs of GVHD at day 120 or 30 days after discontinuation of immunosuppression. In the absence of GVHD, prophylactic donor lymphocyte infusion was repeated up to 3 times, using escalating cell doses.33
All patients were treated at the Department of Hematology, Hemostasis, Oncology and Stem-Cell Transplantation at Hannover Medical School between 2004 and 2007. The study was done in accordance with the Declaration of Helsinki and approved by the institutional review board. Informed consent was obtained from all patients and controls in accordance with the Declaration of Helsinki. Twenty healthy body mass index– and age-matched volunteers of the Hannover Medical School staff served as controls (Table 1).
Patient characteristics
| Characteristic . | Value . |
|---|---|
| No. of patients | 90 |
| Median age, y (range) | 54 (19.1-71.2) |
| Sex | |
| Male | 45 |
| Female | 45 |
| Diagnosis | |
| De novo AML | 39 |
| AML secondary to MDS | 30 |
| AML secondary to other malignancies | 10 |
| MDS | 8 |
| MDS secondary to other malignancies | 3 |
| Risk stratification according to cytogenetics | |
| Good | 3 |
| High | 49 |
| Standard | 35 |
| Unknown | 3 |
| Stage at transplantation | |
| Untreated | 16 |
| Primary induction failure | 28 |
| First CR | 13 |
| Second CR | 4 |
| First relapse after CR | 24 |
| Second relapse after CR | 3 |
| Third relapse after CR | 2 |
| Donor type | |
| MRD | 24 |
| MMRD | 1 |
| MUD | 45 |
| MMUD | 20 |
| CMV status | |
| Patient−/donor− | 25 |
| Patient+/donor− | 18 |
| Patient−/donor+ | 8 |
| Patient+/donor+ | 36 |
| NE | 3 |
| Stem cell source | |
| PBSCs | 81/1* |
| Bone marrow | 8/1* |
| GVHD | |
| Acute | 39 |
| Chronic | 19 |
| Chemotherapy cycles, no. (range) | 1 (0-4) |
| Median marrow blasts, % (range) | 10 (0-90) |
| Median peripheral blasts, per μL (range) | 0 (0-34.560) |
| Median white blood count, per μL (range) | 2.85 (200-35.000) |
| Median follow-up, d (range) | 327 (10-1161) |
| Characteristic . | Value . |
|---|---|
| No. of patients | 90 |
| Median age, y (range) | 54 (19.1-71.2) |
| Sex | |
| Male | 45 |
| Female | 45 |
| Diagnosis | |
| De novo AML | 39 |
| AML secondary to MDS | 30 |
| AML secondary to other malignancies | 10 |
| MDS | 8 |
| MDS secondary to other malignancies | 3 |
| Risk stratification according to cytogenetics | |
| Good | 3 |
| High | 49 |
| Standard | 35 |
| Unknown | 3 |
| Stage at transplantation | |
| Untreated | 16 |
| Primary induction failure | 28 |
| First CR | 13 |
| Second CR | 4 |
| First relapse after CR | 24 |
| Second relapse after CR | 3 |
| Third relapse after CR | 2 |
| Donor type | |
| MRD | 24 |
| MMRD | 1 |
| MUD | 45 |
| MMUD | 20 |
| CMV status | |
| Patient−/donor− | 25 |
| Patient+/donor− | 18 |
| Patient−/donor+ | 8 |
| Patient+/donor+ | 36 |
| NE | 3 |
| Stem cell source | |
| PBSCs | 81/1* |
| Bone marrow | 8/1* |
| GVHD | |
| Acute | 39 |
| Chronic | 19 |
| Chemotherapy cycles, no. (range) | 1 (0-4) |
| Median marrow blasts, % (range) | 10 (0-90) |
| Median peripheral blasts, per μL (range) | 0 (0-34.560) |
| Median white blood count, per μL (range) | 2.85 (200-35.000) |
| Median follow-up, d (range) | 327 (10-1161) |
Data are numbers unless otherwise defined.
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome; CR, complete remission; MRD, matched related donor; MMRD, mismatched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; CMV, cytomegalovirus; NE, PBSCs, peripheral blood stem cells; and GVHD, graft-versus-host disease.
One patient received stem cells from both PBSCs and bone marrow.
Serum levels of Ang-2 were measured in peripheral blood samples in 90 patients before allogeneic HSCT. Samples were taken within one week before beginning cytoreductive/conditioning therapy, immediately centrifuged, and stored at −80°C.
Cytogenetic analysis and risk stratification
Pretreatment samples from all patients were studied centrally by G-banding and fluorescence R-banding analysis and fluorescence in situ hybridization.34,35 Conventional cytogenetic studies were performed using standard techniques, and chromosomal abnormalities were described according to the International System for Human Cytogenetic Nomenclature.36 All specimens were also analyzed by fluorescence in situ hybridization using a comprehensive DNA probe set allowing for the detection of the most relevant AML-associated genomic aberrations.37 In addition, diagnostic samples from all patients with AML were analyzed for mutations in FLT3 (internal tandem duplications and activation loop mutations at D835).38 Risk stratification of cytogenetics (favorable, intermediate, and unfavorable karyotype) was done in accordance with Southwest Oncology Group/Eastern Cooperative Oncology Group Study5 criteria. FLT3 mutations were considered as unfavorable cytogenetics.39,40
Ang-2 enzyme-linked immunosorbent assay
We developed a sandwich enzyme-linked immunosorbent assay (ELISA) assay for the detection of Ang-2 using the DuoSet methodology purchased from R&D Systems (Minneapolis, MN).41,42
Recombinant human Ang-2 (95% purity, NSO derived) was also purchased from R&D Systems. ELISA plates (NUNC A/S, Roskilde, Denmark) were coated overnight at 4°C with 2 μg/mL monoclonal Ang-2 antibody (AB) in 0.1 M Na-carbonate buffer (pH 9.5) and then washed 3 times with 300 μL phosphate-buffered saline with Tween-20 (PBST). Serum samples (50 μL) were then diluted 1:1 with assay buffer-1 (30 g/L bovine serum albumin, 10 g/L bovine IgG, 1% goat serum, 0.1% NaN3, 1 M NaCl, 40 mM Na-phosphate buffer, pH 7.4), added to the tubes, and incubated for 2 hours at room temperature on an orbital shaker. After removal of the serum samples, the tubes were washed 3 times with PBST; 100 μL assay buffer-2 (0.5% bovine serum albumin, 1% mouse serum, 0.15 M NaCl, 40 mM Na-phosphate buffer, 0.1% Thimerosal, pH 7.4) containing 1 μg/mL biotinylated anti–Ang-2 AB were added to each tube and incubated for 4 hours at room temperature. After 3 washing steps, 100 μL of streptavidin in assay buffer-2 was added to each tube and incubated for 20 minutes at room temperature. After 3 final washing steps with PBST, the 100 μL substrate solution (1 mL of tetramethylbenzidine, 10 mL 0.1 M citrate buffer, pH 5, 4 μL H2O2) were added to each tube and incubated for 15 minutes. After incubation, the assay was stopped by sulfuric acid (1 M H2SO4) stop-solution. Absorbance was measured at 450 nm using a microplate reader (Spectra Mini; Tecan, Crailsheim, Germany). All measurements were performed in duplicate. In each experiment, a standard curve of Ang-2 was used to calculate Ang-2 concentrations in individual samples. The average value intra-assay coefficient of variation for Ang-2 was 3.8%, and the average value interassay coefficient of variation was 4.24%. The sensitivity threshold was 0.2 ng/mL.
Statistics
Differences in Ang-2 levels and healthy controls were analyzed using the Mann-Whitney rank sum test. The difference between disease groups according to diagnosis was analyzed by the Kruskal-Wallis 1-way analysis of variance. In addition, t test and analysis of variance (ANOVA) were performed after logarithmic transformation of Ang-2 values. Data are presented as medians and ranges, unless otherwise stated.
Correlations between variables were assessed by the Spearman rank correlation coefficient (Ang-2) or by the Pearson correlation coefficient (logAng-2). The distribution of the time-to-event variables were estimated using the Kaplan-Meier method with log-rank testing.
Disease-free survival was the primary outcome studied and was calculated from the day of HSC infusion to relapse or death in complete remission. Overall survival was calculated from the day of HSC infusion to death from any cause. Time to relapse was calculated from the day of transplantation to day of relapse. Patients who did not suffer from any event within the follow-up were censored at the date of last contact.
Parameters independently associated with disease-free survival were identified by univariate and multivariate Cox proportional hazards models. Variables found to be statistically significant at a 10% level in the univariate analysis were included into the multivariate model. Different models were established, incorporating Ang-2 as either a continuous variable or in a dichotomous fashion using the median value as the cutoff. Two-sided P values less than .05 were considered statistically significant. All of the afore-described statistical analyses were performed with the SPSS package (SPSS, Chicago, IL).
In an explorative approach, data were analyzed using recursive partitioning (decision tree analysis) to identify optimal cutoff values for Ang-2 with regard to disease-free survival. In addition, all continuous variables incorporated in the multivariate Cox model were concurrently subjected to regression tree analysis to recursively identify best predictors and patient subgroups with different prognosis for disease-free and overall survival.43,44 The validity of this risk stratification model was controlled by minimizing the 10-fold cross validated relative error. The rpart software (R package version 3.1-24, www.stats.ox.ac.uk/pub/MASS3/Winlibs/) was used for regression tree analysis.
Results
Serum levels of Ang-2 in HSCT patients and healthy controls
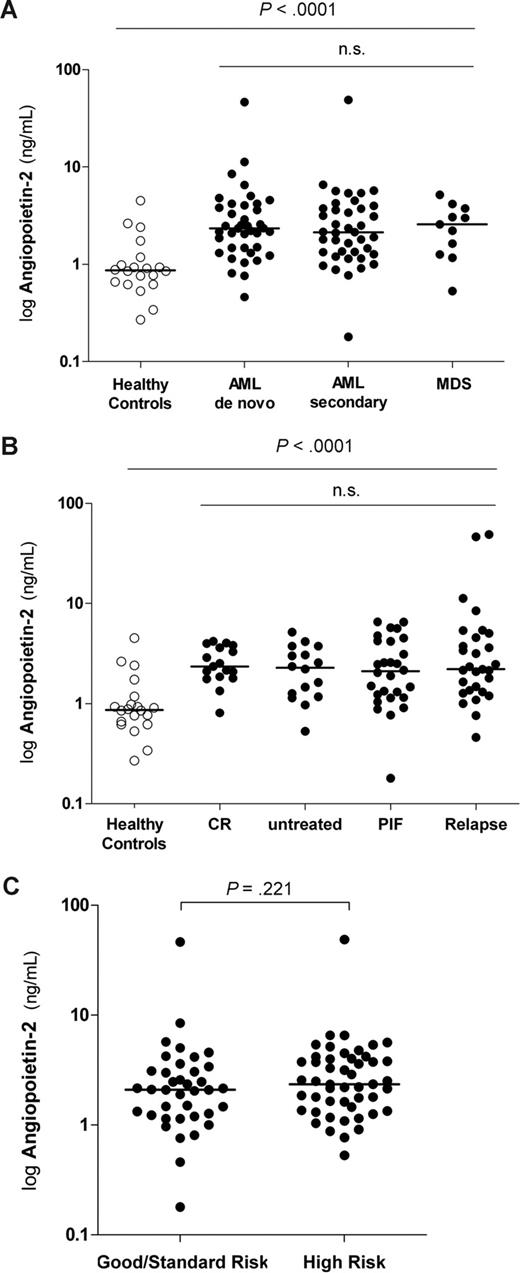
Serum levels of Ang-2 were analyzed by ELISA in 90 patients who underwent allogeneic HSCT for high-risk AML or MDS within 1 week before conditioning therapy. Circulating Ang-2 serum levels were significantly elevated in patients compared with healthy controls (P < .001; Figure 1A). In contrast, Ang-2 levels did not significantly differ between disease groups according to diagnosis (P = .579; Table 2; Figure 1A). The same results were obtained when logAng-2 was compared by ANOVA (P = .543).
Pre-HSCT serum levels of Ang-2 lack association with disease, response to prior treatment, and cytogenetic risk groups. (A) Pre-HSCT serum levels of Ang-2 are elevated in patients compared with healthy controls. Levels of Ang-2 in healthy controls (n = 20; median, 0.87 ng/mL; range, 0.27-4.51 ng/mL) were significantly lower than pre-HSCT Ang-2 levels in patients with myeloid malignancies (median, 2.21 ng/mL; range, 0.18-48.84 ng/mL; P < .001). Ang-2 levels did not differ between patients with de novo AML (n = 39; median, 2.34 ng/mL; range, 0.36-4.03 ng/mL), secondary AML (n = 40; median, 2.13 ng/mL; range, 1.18-48.84 ng/mL), or MDS (n = 11; median, 2.57 ng/mL; range, 0.53-5.17 ng/mL), respectively (P = .579). Horizontal bars indicate median values. (B) Pre-HSCT serum levels of Ang-2 are not associated with response to prior treatment. Levels of Ang-2 in healthy controls (n = 20; median, 0.87 ng/mL; range, 0.27-4.51 ng/mL) were significantly lower than pre-HSCT Ang-2 levels in patients who achieved first or second complete remission (CR, n = 17: median, 2.35 ng/mL; range, 0.81-4.19 ng/mL), patients who were untreated (n = 16: median, 2.29 ng/mL; range, 0.53-5.17 ng/mL), patients with primary induction failure (PIF, n = 28: median, 2.11 ng/mL; range, 0.18-6.56 ng/mL), and patients who had a relapse (n = 29: median, 2.21 ng/mL; range, 0.46-48.84 ng/mL). Ang-2 levels did not differ between patients with different disease stages at transplantation (P = .798). Horizontal bars indicate median values. (C) Pre-HSCT Ang-2 levels are not different according to distinct cytogenetic risk groups. Pre-HSCT Ang-2 levels were not different between patients with good or standard risk cytogenetics compared with high risk (according to Southwest Oncology Group/Eastern Cooperative Oncology Group Study5 criteria and FLT3 mutations63,64 ) good risk/standard risk (n = 38: median, 2.10 ng/mL; range, 0.18-46.42 ng/mL) and high risk (n = 49: median, 2.35 ng/mL; range, 0.53-48.84 ng/mL; P = .221). Three patients with cytogenetics of unknown significance could not be considered. Horizontal bars indicate median values.
Pre-HSCT serum levels of Ang-2 lack association with disease, response to prior treatment, and cytogenetic risk groups. (A) Pre-HSCT serum levels of Ang-2 are elevated in patients compared with healthy controls. Levels of Ang-2 in healthy controls (n = 20; median, 0.87 ng/mL; range, 0.27-4.51 ng/mL) were significantly lower than pre-HSCT Ang-2 levels in patients with myeloid malignancies (median, 2.21 ng/mL; range, 0.18-48.84 ng/mL; P < .001). Ang-2 levels did not differ between patients with de novo AML (n = 39; median, 2.34 ng/mL; range, 0.36-4.03 ng/mL), secondary AML (n = 40; median, 2.13 ng/mL; range, 1.18-48.84 ng/mL), or MDS (n = 11; median, 2.57 ng/mL; range, 0.53-5.17 ng/mL), respectively (P = .579). Horizontal bars indicate median values. (B) Pre-HSCT serum levels of Ang-2 are not associated with response to prior treatment. Levels of Ang-2 in healthy controls (n = 20; median, 0.87 ng/mL; range, 0.27-4.51 ng/mL) were significantly lower than pre-HSCT Ang-2 levels in patients who achieved first or second complete remission (CR, n = 17: median, 2.35 ng/mL; range, 0.81-4.19 ng/mL), patients who were untreated (n = 16: median, 2.29 ng/mL; range, 0.53-5.17 ng/mL), patients with primary induction failure (PIF, n = 28: median, 2.11 ng/mL; range, 0.18-6.56 ng/mL), and patients who had a relapse (n = 29: median, 2.21 ng/mL; range, 0.46-48.84 ng/mL). Ang-2 levels did not differ between patients with different disease stages at transplantation (P = .798). Horizontal bars indicate median values. (C) Pre-HSCT Ang-2 levels are not different according to distinct cytogenetic risk groups. Pre-HSCT Ang-2 levels were not different between patients with good or standard risk cytogenetics compared with high risk (according to Southwest Oncology Group/Eastern Cooperative Oncology Group Study5 criteria and FLT3 mutations63,64 ) good risk/standard risk (n = 38: median, 2.10 ng/mL; range, 0.18-46.42 ng/mL) and high risk (n = 49: median, 2.35 ng/mL; range, 0.53-48.84 ng/mL; P = .221). Three patients with cytogenetics of unknown significance could not be considered. Horizontal bars indicate median values.
Pre-HSCT serum angiopoietin-2 levels in patients and healthy controls, ng/mL
| Diagnosis . | No. . | Mean . | SD . | Median . | Range . |
|---|---|---|---|---|---|
| De novo AML | 39 | 4.00 | 7.30 | 2.34 | 0.46-46.42 |
| AML secondary to MDS | 30 | 2.48 | 1.57 | 2.01 | 0.77-6.56 |
| AML secondary to other malignancies | 10 | 7.43 | 14.66 | 3.28 | 0.18-48.84 |
| MDS | 8 | 2.08 | 1.12 | 1.93 | 0.53-3.76 |
| MDS secondary to other malignancies | 3 | 3.97 | 1.31 | 4.17 | 2.57-5.17 |
| Sum of all myeloid malignancies* | 90 | 2.70 | 1.85 | 2.21 | 0.18-48.84 |
| Healthy controls | 20 | 1.17 | 1.00 | 0.87 | 0.27-4.51 |
| Diagnosis . | No. . | Mean . | SD . | Median . | Range . |
|---|---|---|---|---|---|
| De novo AML | 39 | 4.00 | 7.30 | 2.34 | 0.46-46.42 |
| AML secondary to MDS | 30 | 2.48 | 1.57 | 2.01 | 0.77-6.56 |
| AML secondary to other malignancies | 10 | 7.43 | 14.66 | 3.28 | 0.18-48.84 |
| MDS | 8 | 2.08 | 1.12 | 1.93 | 0.53-3.76 |
| MDS secondary to other malignancies | 3 | 3.97 | 1.31 | 4.17 | 2.57-5.17 |
| Sum of all myeloid malignancies* | 90 | 2.70 | 1.85 | 2.21 | 0.18-48.84 |
| Healthy controls | 20 | 1.17 | 1.00 | 0.87 | 0.27-4.51 |
AML indicates acute myeloid leukemia; and MDS, myelodysplastic syndrome.
Circulating Ang-2 serum levels were significantly elevated in patients compared with healthy controls (P < .001).
Association between serum levels of Ang-2 and clinicopathologic features
No statistically significant association was found between serum levels of Ang-2 and patient's sex (P = .651). However, a weak inverse correlation was observed between Ang-2 levels and age at time of HSCT (r = −0.211, P = .046) using the Spearman correlation. This association persisted when the Pearson correlation was used for logAng-2 values (r = −0.215, P = .042).
We also tested whether pre-HSCT Ang-2 levels differed with regard to response to prior chemotherapy and stage at transplantation, respectively. Levels of Ang-2 in healthy controls (n = 20: median, 0.87 ng/mL; range, 0.27-4.51 ng/mL) were significantly lower than pre-HSCT Ang-2 levels in patients who achieved first or second complete remission (CR, n = 17: median, 2.35 ng/mL; range, 0.81-4.19 ng/mL), patients who were untreated (n = 16: median, 2.29 ng/mL; range, 0.53-5.17 ng/mL), patients with primary induction failure (PIF, n = 28: median, 2.11 ng/mL; range, 0.18-6.56 ng/mL), and patients who had a relapse (n = 29: median, 2.21 ng/mL; range, 0.46-48.84 ng/mL). Ang-2 levels did not differ between patients with different disease stages at transplantation (P = .798). Similar results were obtained when respective Ang-2 levels were compared by ANOVA (P = .169; Figure 1B).
The cytogenetics of the 90 patients was good risk in 3, standard risk in 35, high risk in 49, and of unknown significance in 3 patients (Southwest Oncology Group/Eastern Cooperative Oncology Group Study5 criteria and FLT3 mutations45,46 ). Pre-HSCT Ang-2 levels were not different between patients with good risk/standard risk (n = 38: median, 2.10 ng/mL; range, 0.18-46.42 ng/mL) and high risk cytogenetics (n = 49: median, 2.35 ng/mL; range, 0.53-48.84 ng/mL; P = .221; Figure 1C).
Furthermore, we tested whether pre-HSCT Ang-2 levels could predict the incidence of acute or chronic GvHD during follow-up. No association was detected between Ang-2 and the occurrence of acute (P = .614) or chronic GVHD (P = .586). The same results were obtained when logAng-2 was analyzed (P = .977 and P = .896, respectively).
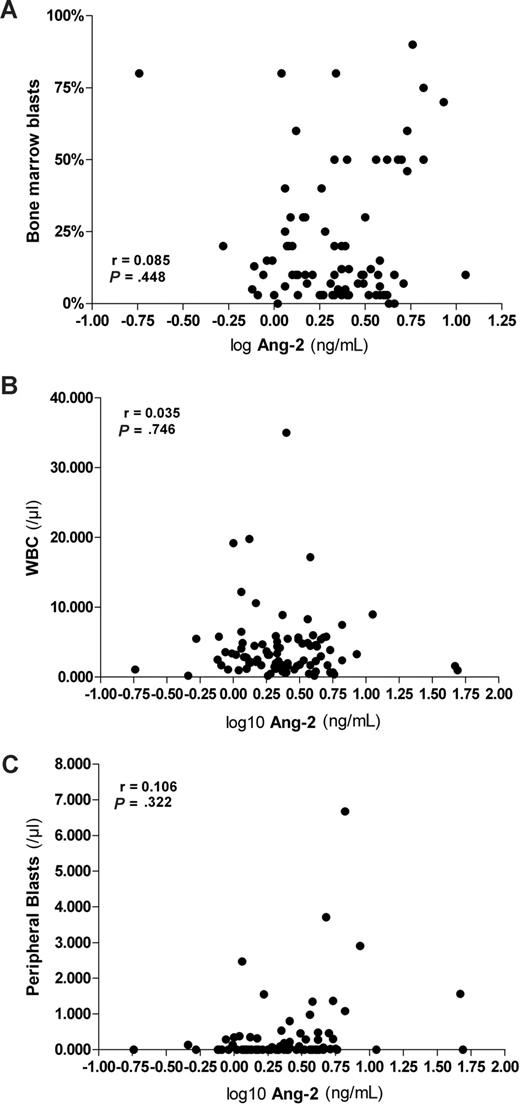
The bone marrow of HSCT patients studied was infiltrated by leukemic blasts in 60 of 81 analyzed patients, as shown by bone marrow aspiration. The median (range) percentage was 10% (0%-90%). Neither serum Ang-2 levels nor logAng-2 correlated with the percentage of bone marrow infiltration by leukemic blasts (r = 0.046, P = .686 and r = 0.085, P = .448; Figure 2A). Likewise, neither serum Ang-2 levels nor logAng-2 correlated with white blood cell count (r = −0.027, P = .802 and r = −0.035, P = .746; Figure 2B). The median of the absolute peripheral leukemic blast count (n = 90) was 0/μL (range, 0-34 560/μL). We detected a weak correlation between Ang-2 levels and the absolute peripheral leukemic blast count using the Spearman correlation (r = 0.298, P = .004). However, this association did not remain significant when the Pearson correlation was used for logAng-2 values (r = 0.106, P = .322; Figure 2C).
Pre-HSCT serum levels of Ang-2 lack association with percentage of bone marrow blasts, white blood cells, and peripheral blasts. (A) Pre-HSCT logAng-2 levels did not correlate with the percentage of bone marrow infiltration by leukemic blast (r = 0.085, P = .448), (B) white blood count (r = −0.035, P = .746), and (C) absolute peripheral leukemic blast count (r = 0.106, P = .322) using the Pearson correlation.
Pre-HSCT serum levels of Ang-2 lack association with percentage of bone marrow blasts, white blood cells, and peripheral blasts. (A) Pre-HSCT logAng-2 levels did not correlate with the percentage of bone marrow infiltration by leukemic blast (r = 0.085, P = .448), (B) white blood count (r = −0.035, P = .746), and (C) absolute peripheral leukemic blast count (r = 0.106, P = .322) using the Pearson correlation.
Serum levels of Ang-2 and disease-free survival
To determine the relationship of pre-HSCT Ang-2 levels and disease-free survival, univariate Cox proportional hazards analyses were initially performed. In our cohort of patients with high-risk myeloid malignancies, age and sex did not show prognostic significance for disease-free survival (Table 3). The same was true for diagnostic subtypes, cytogenetics, and disease stage at transplantation, respectively. In addition, neither donor type nor cytomegalovirus status showed prognostic significance (Table 3).
Univariate and multivariate analysis for disease-free survival
| Variable . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age, y | 1.01 | 0.98-1.04 | .446 | |||
| Sex | 1.36 | 0.76-2.42 | .304 | |||
| Diagnosis* | — | — | .226 | |||
| Cytogenetics (high risk vs others) | 1.04 | 0.58-1.87 | .888 | |||
| Stage at transplantation* | — | — | .120 | |||
| Donor type* | — | — | .648 | |||
| CMV status* | — | — | .132 | |||
| CD34+ cell count (106/kg steps) | 1.01 | 0.93-1.10 | .797 | |||
| Marrow infiltration (10% steps) | 1.23 | 1.09-1.40 | .001† | 1.14 | 1.01-1.29 | .033† |
| Peripheral blast count | 1.02 | 1.01-1.03 | .001† | 1.01 | 0.99-1.03 | .237 |
| Chemotherapy cycles before transplantation | 1.43 | 1.10-1.86 | .008† | 1.38 | 1.01-1.08 | .043† |
| Angiopoietin-2 (ng/mL) (0.1 ng steps) | 1.03 | 1.00-1.06 | .034† | |||
| Angiopoietin-2 (greater vs less than median) | 2.26 | 1.24-4.11 | .008† | 2.46 | 1.27-4.76 | .005† |
| Variable . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age, y | 1.01 | 0.98-1.04 | .446 | |||
| Sex | 1.36 | 0.76-2.42 | .304 | |||
| Diagnosis* | — | — | .226 | |||
| Cytogenetics (high risk vs others) | 1.04 | 0.58-1.87 | .888 | |||
| Stage at transplantation* | — | — | .120 | |||
| Donor type* | — | — | .648 | |||
| CMV status* | — | — | .132 | |||
| CD34+ cell count (106/kg steps) | 1.01 | 0.93-1.10 | .797 | |||
| Marrow infiltration (10% steps) | 1.23 | 1.09-1.40 | .001† | 1.14 | 1.01-1.29 | .033† |
| Peripheral blast count | 1.02 | 1.01-1.03 | .001† | 1.01 | 0.99-1.03 | .237 |
| Chemotherapy cycles before transplantation | 1.43 | 1.10-1.86 | .008† | 1.38 | 1.01-1.08 | .043† |
| Angiopoietin-2 (ng/mL) (0.1 ng steps) | 1.03 | 1.00-1.06 | .034† | |||
| Angiopoietin-2 (greater vs less than median) | 2.26 | 1.24-4.11 | .008† | 2.46 | 1.27-4.76 | .005† |
HR indicates hazard ratio; CI, confidence interval; and —, not applicable.
HR and CI for categorical variables are only displayed in case of multivariate significance.
Circulating Ang-2 serum levels were significantly elevated in patients compared with healthy controls (P < .001).
Among the tested variables, bone marrow infiltration (P = .001), peripheral blast counts (P = .001), number of chemotherapy cycles before HSCT (P = .008), and amount of circulating Ang-2 (P = .034) displayed prognostic significance (Table 3). When using the Ang-2 median (2.22 ng/mL) as a dichotomous cutoff value, its prognostic impact was even more pronounced (P = .008). Of note, the 3-year disease-free survival of patients among the low Ang-2 group was 54%, whereas it was 0% in the group of patients with high Ang-2 expression.
Subsequently, the following variables were found to be statistically significant at a 10% level in the univariate analysis and subjected to multivariate Cox regression analysis: marrow infiltration, peripheral blast count, number of chemotherapy cycles before HSCT, and circulating Ang-2 (as a dichotomous variable using the Ang-2 median as cutoff). Except for peripheral blast count (P = .237), all other variables remained significant in the multivariate setting (P = .033, P = .043, and P = .005, respectively; WALD test). Thus, marrow infiltration, number of chemotherapy cycles before HSCT, and circulating Ang-2 were identified as independent prognostic factors for disease-free survival. The hazard for relapse in our cohort was 2.5-fold in the high Ang-2 group compared with the low Ang-2 group (Table 3).
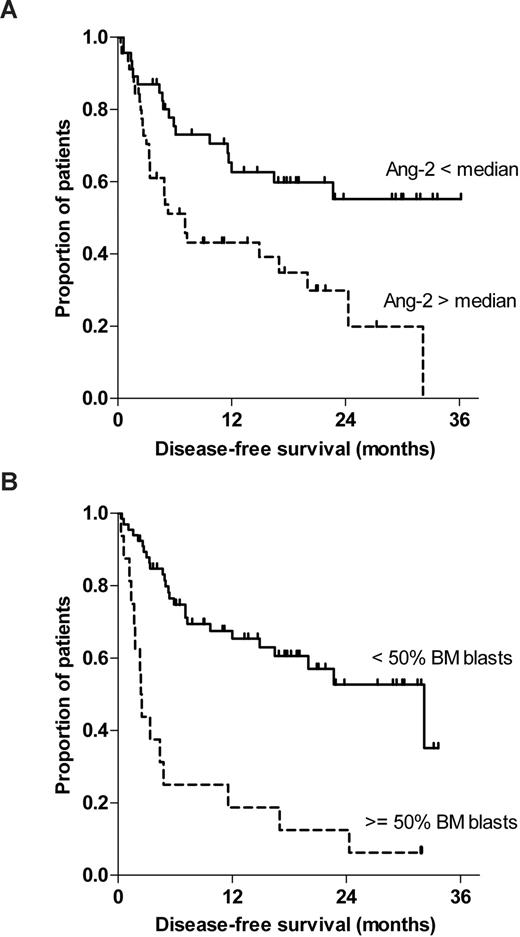
To exclude any confounding effect of prophylactic donor lymphocyte infusion (DLI), we reperformed a multivariate Cox regression analysis incorporating DLI as an additional time-dependent indicator variable. However, DLI was not an additional significant prognostic factor for disease-free survival, nor did it affect the significance of the other variables. Figure 3 illustrates the Kaplan-Meier curves of disease-free survival stratified to Ang-2 (less than vs more than median; Figure 3A) and bone marrow infiltration (< 50% vs > 50%). Log-rank test confirmed statistical significance for Ang-2 (P = .011) and bone marrow infiltration (P < .001; Figure 3B).
Pre-HSCT serum levels of Ang-2 and percentage of bone marrow blasts are predictors for disease-free survival after HSCT for high-risk myeloid malignancy. This figure illustrates the Kaplan-Meier curves of disease-free survival stratified to (A) Ang-2 (less than vs greater than median) and (B) bone marrow infiltration (< 50% vs > 50%) before allogeneic HSCT. Log-rank test confirmed statistical significance for Ang-2 (P = .011) and bone marrow infiltration (P < .001).
Pre-HSCT serum levels of Ang-2 and percentage of bone marrow blasts are predictors for disease-free survival after HSCT for high-risk myeloid malignancy. This figure illustrates the Kaplan-Meier curves of disease-free survival stratified to (A) Ang-2 (less than vs greater than median) and (B) bone marrow infiltration (< 50% vs > 50%) before allogeneic HSCT. Log-rank test confirmed statistical significance for Ang-2 (P = .011) and bone marrow infiltration (P < .001).
Serum levels of Ang-2 and relapse-free survival, overall survival, and nonrelapse mortality
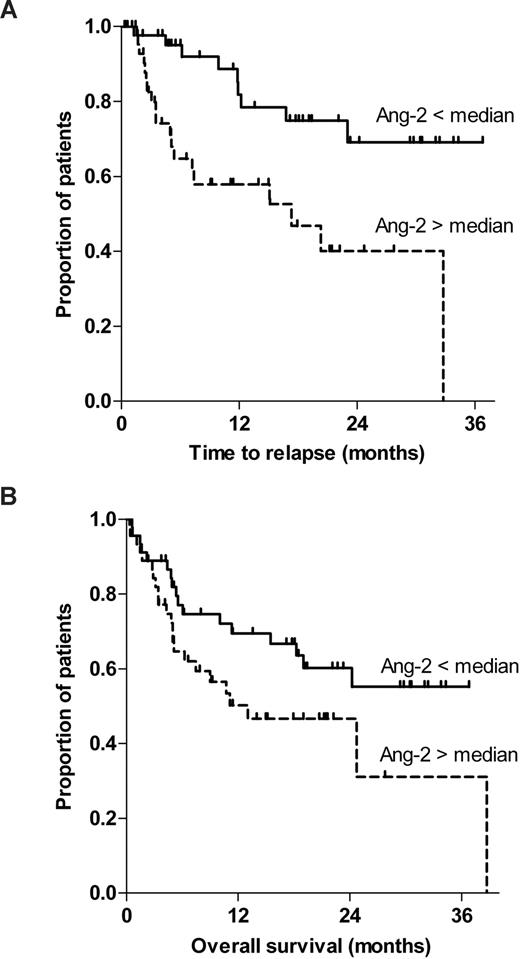
In addition, we tested whether Ang-2 levels predicted disease relapse or nonrelapse mortality. Of note, Ang-2 was identified as a strong predictor for disease relapse as shown by Kaplan-Meier curves stratified to Ang-2 (lower vs higher than median; log-rank test, P = .002; Figure 4A). Consistent with this result, Ang-2 did not predict nonrelapse mortality (log-rank test, P = .748).
Pre-HSCT serum levels of Ang-2 predict time to relapse but lack association with overall survival. (A) This figure illustrates the Kaplan-Meier curves of time to relapse stratified to Ang-2 (less than vs greater than median; log-rank test, P = .002). (B) The Kaplan-Meier curves of overall survival stratified to Ang-2 (less than vs greater than median) are shown (log-rank test, P = .082).
Pre-HSCT serum levels of Ang-2 predict time to relapse but lack association with overall survival. (A) This figure illustrates the Kaplan-Meier curves of time to relapse stratified to Ang-2 (less than vs greater than median; log-rank test, P = .002). (B) The Kaplan-Meier curves of overall survival stratified to Ang-2 (less than vs greater than median) are shown (log-rank test, P = .082).
In contrast, Ang-2 did not predict overall survival in our cohort, although a trend was observed (log-rank test, P = .082; Figure 4B). Neither Ang-2 as continuous or dichotomous variable (less than or more than median, P = .092) nor primary (4.18 ng/mL, P = .078) or secondary (2.15 ng/mL, P = .657) Ang-2 cutoffs as defined by recursive partitioning could predict overall survival in a multivariate Cox regression model, although a clear trend was observed. Only pre-HSCT percentage of bone marrow infiltration remained statistically significant with regard to overall survival (hazard ratio (HR) = 1.23 for 10% steps, P = .001, 95% confidence interval (CI), 1.091-1.395).
Identification of Ang-2 cutoff values and risk stratification by regression tree analysis
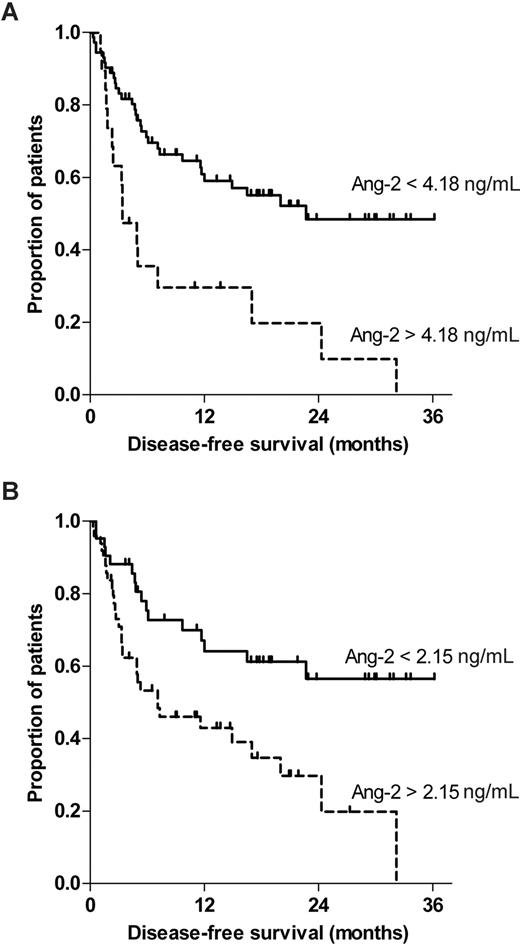
In an explorative approach, we wanted to detect optimal cutoff values of Ang-2 with regard to disease-free survival. Recursive partitioning (decision tree analysis) was preferable to the classic Cox regression approach47 for the following reasons: First, recursive partitioning is directly focused on the derivation and characterization of clearly separable risk classes rather than on fitting regression equations to the data. Second, interactions between the predictors, if present in the data, are entered automatically into the model. Using recursive partitioning (decision tree analysis), 2 cutoff values for Ang-2 were identified (Figure 5). First, 15 of 18 patients (83%) with circulating Ang-2 levels greater than the primary cutoff (4.18 ng/mL) relapsed during follow-up (Figure 5A). Interestingly, a secondary cutoff (2.15 ng/mL) close to the median (2.22 ng/mL) was detected by recursive partitioning, which separated 42 patients with 16 (38%) relapses from 30 patients with 15 (50%) relapses (Figure 5B). To analyze the individual impact on disease-free survival, each calculated cutoff was subjected to log-rank test individually. The Kaplan-Meier curves of the primary (4.18 ng/mL) and the secondary (2.15 ng/mL) Ang-2 cutoffs are shown in Figure 5A and 5B, respectively (log-rank test, P < .001 and P = .003, respectively).
Regression tree analysis recursively identified 2 different Ang-2 cutoffs that predicted disease-free survival after HSCT for high-risk myeloid malignancy. The impact of the recursively identified (A) primary Ang-2 cutoff (< 4.18 vs > 4.18 ng/mL) and (B) a secondary cutoff (< 2.15 vs > 2.15 ng/mL) on disease-free survival were separately analyzed by log-rank test and displayed by Kaplan-Meier curves (P < .001 and P = .003, respectively).
Regression tree analysis recursively identified 2 different Ang-2 cutoffs that predicted disease-free survival after HSCT for high-risk myeloid malignancy. The impact of the recursively identified (A) primary Ang-2 cutoff (< 4.18 vs > 4.18 ng/mL) and (B) a secondary cutoff (< 2.15 vs > 2.15 ng/mL) on disease-free survival were separately analyzed by log-rank test and displayed by Kaplan-Meier curves (P < .001 and P = .003, respectively).
Combined prediction model for disease-free survival after allo-HSCT
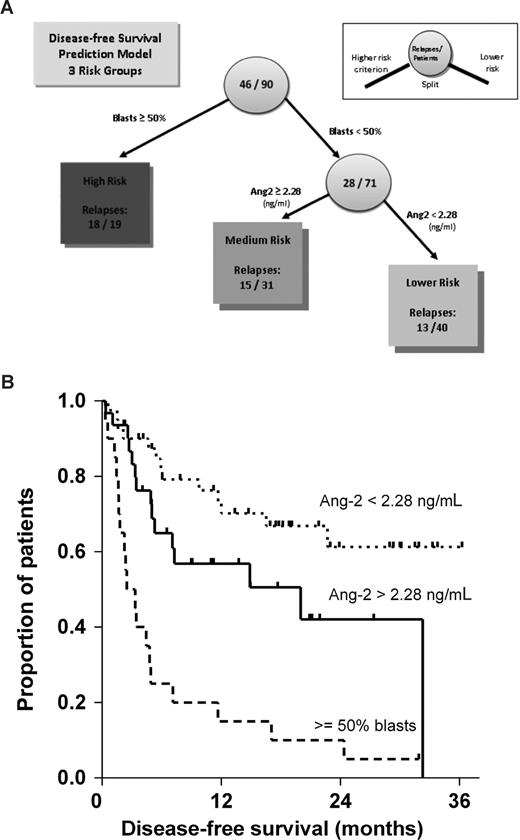
Finally, we incorporated all variables with significant prognostic impact on disease-free survival (Ang-2, bone marrow infiltration [≥ 50% vs < 50%] and chemotherapy cycles before HSCT) into a regression tree analysis model. As a result, at a cutoff point of 2.28 ng/mL (again close to the median of 2.22 ng/mL), Ang-2 separated patients with bone marrow infiltration less than 50% into subgroups with better (13 of 40 relapses, 32.5%) and moderate (15 of 31 relapses, 48.4%) disease-free survival. The subgroup of patients with bone marrow infiltration more than or equal to 50% (18 of 19 relapses, 94.7%) was not subdivided further. Figure 6A illustrates the combined prediction model for disease-free survival in our cohort as identified by regression tree analysis.
Combined prediction model for disease-free survival after allo-HSCT as identified by regression tree analysis. (A) Combined prediction model for disease-free survival in our cohort as identified by regression tree analysis. Numbers in circles represent the number of patients at that node. Split to the left represents higher-risk criterion. Numbers in boxes represent the number of relapses that were predicted by the split variable in the respective risk group. (B) Subsequently, the impact of the identified splits (bone marrow infiltration > 50%, and the Ang-2 cut-off point of 2.28 ng/mL) on disease-free survival were analyzed. Overall comparison (Mantel-Cox) between the 3 risk groups was significant (P < .001). In addition, pair-wise log-rank test was calculated for the 2 split points in a consecutive fashion and confirmed the independent statistical significance between each of the 3 risk groups (bone marrow infiltration ≥ 50% vs < 50%, P < .001, n = 90; Ang-2 > 2.28 vs < 2.28 ng/mL, P = .049, n = 71). The corresponding Kaplan-Meier curves of all 3 clearly distinguishable risk groups are shown in Figure 6.
Combined prediction model for disease-free survival after allo-HSCT as identified by regression tree analysis. (A) Combined prediction model for disease-free survival in our cohort as identified by regression tree analysis. Numbers in circles represent the number of patients at that node. Split to the left represents higher-risk criterion. Numbers in boxes represent the number of relapses that were predicted by the split variable in the respective risk group. (B) Subsequently, the impact of the identified splits (bone marrow infiltration > 50%, and the Ang-2 cut-off point of 2.28 ng/mL) on disease-free survival were analyzed. Overall comparison (Mantel-Cox) between the 3 risk groups was significant (P < .001). In addition, pair-wise log-rank test was calculated for the 2 split points in a consecutive fashion and confirmed the independent statistical significance between each of the 3 risk groups (bone marrow infiltration ≥ 50% vs < 50%, P < .001, n = 90; Ang-2 > 2.28 vs < 2.28 ng/mL, P = .049, n = 71). The corresponding Kaplan-Meier curves of all 3 clearly distinguishable risk groups are shown in Figure 6.
Finally, we tested the combined impact of the recursively identified split values (bone marrow infiltration less than 50%, and the Ang-2 cut point of 2.28 ng/mL) on disease-free survival. Overall comparison (Mantel-Cox) between the 3 risk groups was significant (P < .001). In addition, pairwise log-rank test was calculated for the 2 split points in a consecutive fashion and confirmed the independent statistical significance between each of the 3 risk groups (bone marrow infiltration ≥ 50% vs < 50%, P < .001; n = 90; Ang-2 > 2.28 vs < 2.28 ng/mL, P = .049; n = 71). The corresponding Kaplan-Meier curves of all 3 clearly distinguishable risk groups are shown in Figure 6B.
Discussion
Allogeneic HSCT as a treatment modality includes high doses of chemoradiotherapy to eradicate the leukemia, the replacement of the diseased bone marrow with a bone marrow free of leukemia from a healthy donor, and the benefit of a graft-versus-leukemia effect.48 As a consequence, the immediate morbidity and mortality associated with this treatment are high. Unfortunately, “a priori” predictors for overall survival and disease recurrence are rare in allogeneic HSCT for patients with high-risk AML and MDS.49 So far, only a lower bone marrow blast count and a favorable or intermediate karyotype have been identified as prognostic factors for relapse in allogeneic HSCT for myeloid malignancies.14,50,51
In this study, we were able to detect elevated pretransplant Ang-2 levels in serum of patients who underwent HSCT for high-risk AML or MDS compared with healthy controls. These findings are in line with 2 recent observations of elevated serum Ang-2 levels in de novo AML.15,16 Schliemann et al16 and Lee et al15 independently demonstrated that high serum levels of Ang-2 in newly diagnosed AML patients correlated with inferior long-term survival. Increased expression of serum Ang-2 was also detected in other lymphohematopoietic diseases, such as chronic myeloid leukemia and multiple myeloma.52
To our knowledge, this is the first report on circulating Ang-2 as a prognostic factor in the setting of allogeneic HSCT for high-risk myeloid malignancies. Of note, Cox regression analysis demonstrated that a high pretransplantation level of Ang-2 represents an unfavorable prognostic factor for disease-free survival after allogeneic HSCT in high-risk patients. Multivariate analysis revealed that the prognostic impact of Ang-2 was independent from established prognostic markers, such as percentage of bone marrow infiltration and number of chemotherapy cycles before HSCT. Strikingly, Ang-2 displayed a prognostic value at least equivalent to that for the percentage of marrow infiltration by leukemic blasts in our cohort. In addition, Ang-2 was identified as a powerful predictor for time to relapse (P = .002). Interestingly, Ang-2 levels did not predict nonrelapse mortality, nor overall survival, as defined by multivariate Cox regression model and recursive partitioning, respectively, although a trend was observed (P = .092 and P = .078). In a regression tree analysis model, marrow infiltration of more than or equal to 50% identified a subgroup of patients with very poor prognosis. Circulating Ang-2 was even a strong prognostic marker in patients with marrow infiltration of less than 50%. Other established risk factors, such as cytogenetics and age, were not significant for relapse in our analysis, presumably because the analyzed patients are from a selected high-risk collective. Thus, Ang-2 expression might serve as a powerful independent biomarker to pre-estimate the risk of relapse in the setting of HSCT.
Emerging data indicate a crucial involvement of angiogenesis in the pathophysiology of hematologic malignancies. The importance of angiogenesis for solid tumor growth is well established.23,24 Likewise, patients with AML show significantly increased angiogenesis in the neoplastic bone marrow.25,26,53 The association between circulating Ang-2 and tumor size/progression has recently been found in patients with soft tissue sarcoma,54 angiosarcoma,55 breast cancer,56 non–small-cell lung cancer,57 multiple myeloma, and chronic myeloid leukemia.52 It is thus conceivable that circulating pre-HSCT Ang-2 closely reflects the magnitude of leukemia-associated neo-angiogenesis in the bone marrow.16 In line with this conception, serum levels of Ang-2 predicted time to relapse but not overall survival in the present study. Although we cannot rule out the possibility that circulating Ang-2 might have derived from leukemic blasts, it is conceivable that the predominant proportion of circulating Ang-2 in our cohort derived from bone marrow endothelial cells because of extensive neo-angiogenesis.27,28 Schliemann et al found a strong correlation between circulating Ang-2 levels and both the total white blood count and the percentage of bone marrow infiltration in de novo AML.16 These findings are in apparent contrast to the results of the present study because part of the leukemic patients included in the present study were in CR, they had much lower blast counts compared with patients studied by Schliemann et al.16 Because leukemic blasts were absent in the majority of our patients, we hardly detected a correlation between serum and Ang-2 levels and leukemic blast counts using the Spearman correlation, whereas white blood cells did not correlate with Ang-2 levels.
Increased bone marrow angiogenesis might not only serve as a prognostic marker but has been recognized as a central player in pathophysiology and will become a crucial target in the treatment of hematologic malignancies.25,26 Recently, the efficacy of thalidomide and 2 small molecular tyrosine kinase inhibitors (SU5416 and SU11248) in primary refractory or recurrent AML has been demonstrated by phase 1/2 trials.58-60 Of note, in patients who responded to therapy, a concomitant significant reduction in microvascular density was observed. Furthermore, experimental models imply that there is a paracrine feedback loop between AML blasts and endothelial cells involving VEGF.27 Such a paracrine feedback loop has not been thoroughly demonstrated for the Ang/Tie receptor-ligand system but would provide the rational for Ang-2 selective inhibition of bone marrow angiogenesis. At least in endothelial cells, Ang-2 has been shown to act through an internal autocrine loop mechanism.22,61
So far, the marked therapeutic antiangiogenic efficacy of selective Ang-2 blockade has already been demonstrated in murine models of colon cancer62 and a phase 1 trial evaluating a recombinant Fc protein directed against the action of the angiopoietins in advanced solid cancer has been completed recently (www.clinicaltrials.gov/ct/show/NCT00102830). Collectively, these data constitute the intriguing concept that pre-HSCT Ang-2 (as a new biomarker) cannot only identify patients at risk for relapse but might be a rational drug target for additional antiangiogenic therapy at the same time.
In conclusion, the results from this study clearly demonstrate that circulating Ang-2 is a strong and independent prognostic factor in the setting of allogeneic HSCT for patients with high-risk myeloid diseases. Given that very few predictors for disease-free survival exist in clinical practice, measurement of circulating Ang-2 concentrations may soon become part of the clinical routine as a readily available biomarker to pre-estimate the risk of relapse in the setting of HSCT. As shown by regression tree analysis, the combination of several predictors for relapse (Ang-2 and bone marrow infiltration) might help physicians to individualize the posttransplant treatment and may open new perspectives for a rational use of immunosuppression and administration of prophylactic DLI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the doctors and nurses in our transplantation unit and outpatient clinic for their dedicated work, Elke Dammann for the data collection process, and Michael Morgan for critical reading of the manuscript.
C.K. is a scholar of the Deutsche Forschungsgemeinschaft (grant KO 3582/1-1).
Authorship
Contribution: P.K. and C.K. had the initial idea, designed and performed the research, analyzed the results, made the figures, and wrote the manuscript; H. Hecker performed and supervised the statistical analysis and performed regression tree analysis and reviewed the manuscript; J.H. and R.H. established the Ang-2 ELISA assay, performed the experiments, and reviewed and contributed to the manuscript; W.V. stored and provided samples and reviewed the manuscript; S.B., B.H., J.K., M.E., and S.D. identified patients, provided clinical data, participated in the design of the study, and reviewed the manuscript; G.G. contributed cytogenetic data and reviewed the manuscript; and H. Haller and A.G. supervised the project and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnold Ganser, Department of Hematology, Hemostasis, Oncology, and Stem-Cell Transplantation, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: ganser.arnold@mh-hannover.de.
References
Author notes
*P.K. and C.K. contributed equally to the manuscript; and both are considered first authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal