Abstract
Genetic causes of hypochromic microcytic anemia include thalassemias and some rare inherited diseases such as DMT1 deficiency. Here, we show that iron deficiency anemia with poor intestinal absorption and defective iron utilization of IV iron is caused by inherited mutations in TMPRSS6, a liver-expressed gene that encodes a membrane-bound serine protease of previously unknown role that was recently reported to be a regulator of hepcidin expression.
Introduction
Hypochromic microcytic anemia is quite common in children with poor iron intake.1 Genetic causes include thalassemias2 and some rare inherited forms with defective absorption and use of iron.3,4 Recently, several groups,5-7 including ours,7 have reported genetic defects of the iron transporter DMT1 in such patients. It is noteworthy that DMT1 mutations causing microcytic anemia have been associated with rather high serum iron and transferrin saturation as well as increased hepatic iron stores.8 Here we report the case of a young patient with microcytic iron-refractory iron deficiency anemia (IRIDA) who is a compound heterozygote for 2 nonsense mutations in the transmembrane serine protease TMPRSS6. Mutations in this gene have been recently described in the Mask mouse, a mouse model of IRIDA,9,10 and TMPRSS6 knock-out mice also display the same phenotype.11 Our observations confirm a very recent communication reporting germ line mutations of TMPRSS6 in IRIDA patients12
Methods
The proband was born to nonconsanguinous English parents and was 18 months old when microcytic hypochromic anemia was first diagnosed. He presented with rotavirus gastroenteritis and he was incidentally found to have a hemoglobin concentration of 60 g/L, mean corpuscular volume of 47 fL, and serum iron below 5 μmol/L. He is one of dizygotic twins and has one elder sister. In retrospect, he was thought to be mildly symptomatic of anemia as parents felt he was more lethargic than his twin sister. Both parents are normal and he is the only affected sibling (Table 1). Serum haptoglobin concentration was normal. Thereafter, serum iron was consistently low (< 5 μmol/L), transferrin saturation was less than 5%, and serum ferritin was usually in the lower normal range. Three attempts were undertaken to correct the iron deficiency with 200 mg oral iron per day for either 6 months when he was 21 months old or for 3 months at the age of 3 years and once again at the age of 6 years. He failed to respond to oral iron, with no improvement in hemoglobin levels and serum iron remaining below 5 μmol/L. At the age of 7 years, iron absorption was assessed by administration of oral ferrous iron at 3 mg/kg followed by measurement of serum iron at 3 hours. Defective iron absorption was demonstrated by a rise of 13 μmol/L in serum iron compared with a minimal expected rise of 18 μmol/L for children with comparable IDA.13 Consequently, he was given a course of intravenous iron (iron sucrose 100 mg weekly during 3 consecutive weeks). Serum ferritin rose from 11 μg/L to 109 μg/L and hemoglobin rose from 68 g/L before the treatment to a maximum of 98 g/L. Microcytosis with pencil cells and hypochromia persisted, however. At that time, bone marrow was mildly hypocellular with ragged erythroblasts, nuclear-cytoplasmic asynchromy, intercytoplasmic bridging, and no sideroblast. Iron was detectable on trephine biopsy.
Biologic parameters of the patient and of his relatives at the time of the present study (2008)
| . | Normal values . | Patient . | Father . | Mother . | Twin sister . | Elder sister . |
|---|---|---|---|---|---|---|
| Age, y | 14 | 48 | 47 | 14 | 16 | |
| Hb, g/L | 120-155 | 86 | 151 | 127 | 144 | 142 |
| Hematocrit | 0.36-0.45 | 0.30 | 0.44 | 0.38 | 0.43 | 0.42 |
| MCV, fL | 80-90 | 54.3 | 85.7 | 85.8 | 86 | 79.1 |
| Reticulocytes, 109/L | 50-100 | 30 | ND | ND | ND | ND |
| Iron status | ||||||
| Serum iron (μmol/L) | 14.5-26.0 | 2.44 | 22 | 14.5 | 12 | 19 |
| Serum ferritin (μg/L) | 30-300 (M), 20-150 (F) | 9 | 88 | 10.4 | 9 | 20.2 |
| Soluble transferrin receptors (mg/L) | 0.83-1.76 | 6.52 | 1.44 | 1.52 | 1.6 | 1.17 |
| Plasma hepcidin (ng/mL) | *29-254 (M), *16-288 (F) | 70 | 191 | 24 | 66 | 86 |
| . | Normal values . | Patient . | Father . | Mother . | Twin sister . | Elder sister . |
|---|---|---|---|---|---|---|
| Age, y | 14 | 48 | 47 | 14 | 16 | |
| Hb, g/L | 120-155 | 86 | 151 | 127 | 144 | 142 |
| Hematocrit | 0.36-0.45 | 0.30 | 0.44 | 0.38 | 0.43 | 0.42 |
| MCV, fL | 80-90 | 54.3 | 85.7 | 85.8 | 86 | 79.1 |
| Reticulocytes, 109/L | 50-100 | 30 | ND | ND | ND | ND |
| Iron status | ||||||
| Serum iron (μmol/L) | 14.5-26.0 | 2.44 | 22 | 14.5 | 12 | 19 |
| Serum ferritin (μg/L) | 30-300 (M), 20-150 (F) | 9 | 88 | 10.4 | 9 | 20.2 |
| Soluble transferrin receptors (mg/L) | 0.83-1.76 | 6.52 | 1.44 | 1.52 | 1.6 | 1.17 |
| Plasma hepcidin (ng/mL) | *29-254 (M), *16-288 (F) | 70 | 191 | 24 | 66 | 86 |
ND indicates not determined; M, male; F, female.
5% to 95 % range (T. Ganz, G. Olbina, D. Girelli, E. Nemeth, M.W., unpublished data).
Since that time the patient has been off treatment and remains well, growing satisfactorily. Informed consent of the parents and blood samples of all family members were obtained for genetic analysis in accordance with the Declaration of Helsinki. Genomic DNA was extracted and the DMT1 gene was explored as previously described.7 Each of the TMPRSS6 18 exons and intron exon junctions was amplified by PCR with a set of primers complementary to flanking intron sequences (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The PCR fragments were sequenced using a Big Dye terminator kit (Applied Biosystems, Courtaboeuf, France).
A new experimental enzyme-linked immunosorbent assay (ELISA) was used to measure plasma hepcidin in frozen samples stored at −80°C. Duplicate 5 μL samples were diluted 1:20 in PBS, and the refolded, bioactive form of hepcidin was measured using previously described polyclonal antibodies14 in a competitive ELISA (Intrinsic LifeSciences, La Jolla, CA), using HPLC-purified, synthetic, bioactive hepcidin as a standard.
Results and discussion
In search of rare causes of genetic microcytic anemia, we first sequenced the DMT1 gene in a patient with IRIDA without finding any mutation. We then tested the TMPRSS6 gene because of the similarities in the hematologic profile between our patient and the recently described Mask mouse.9,10 This mouse strain presents iron deficiency and microcytic anemia related to the inability to repress hepcidin, a key regulator of iron metabolism. The Mask phenotype was ascribed to a homozygous loss of function mutation in the Tmprss6 gene, a gene mostly expressed in the liver, encoding a membrane-bound serine protease of previously unknown role.15,16
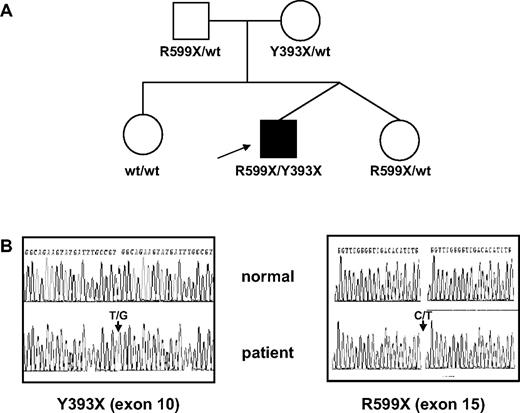
In our patient, 2 nonsense mutations were identified by sequencing the TMPRSS6 gene: one heterozygous cDNA 1179T>G substitution in exon 10 introducing a nonsense Y393X codon in the protein and a cDNA 1795C>T substitution in exon 15 introducing another protein nonsense codon R599X (cDNA sequence reference AY05538417 ; Figure 1). These mutations are both predicted to delete the serine protease domain from the encoded protein unless the mRNA harbouring premature translation termination codon is rapidly degraded through the nonsense-mediated RNA decay surveillance pathway.18 Analysis of the parents' DNA revealed that each parent is heterozygous for one of the nonsense mutations, confirming that each mutation in the proband has targeted a different allele, thus resulting in the absence of normal gene product. The twin sister is heterozygous for the R599X protein substitution, while the elder sister does not carry any TMPRSS6 mutation (Figure 1). A very recent communication reports several cases of patients with TMPRSS6 mutations and inappropriately normal hepcidin level with regard to the degree of anemia.12 Hepcidin is a major regulator of iron homeostasis; it binds to ferroportin and induces its internalization and degradation. Therefore, high hepcidin levels prevent duodenal iron absorption as well as recycling of heme iron by macrophages.19 In our patient, plasma hepcidin levels were within the normal range established using a new experimental competitive ELISA, despite IDA (Table 1). Undetectable hepcidin levels have been found in 18 of 19 IDA patients (M.W., unpublished data, May 2008) and were also shown by mass spectrometry to be unmeasurable in IDA patients.20 Thus, the proband had hepcidin levels that were inappropriately high relative to his iron status. Several other observations are consistent with a defective hepcidin down-regulation. First, oral iron supplementation was not effective in correcting anemia. Second, absorption of a bolus of ferrous iron increased serum iron levels by 13 μmol/L rather than the predicted minimum of 18 μmol/L, suggesting a partial block in intestinal iron absorption.14 Finally, our patient had constantly low serum iron concentrations despite the presence of detectable iron stores in bone marrow after intravenous iron therapy and serum ferritin values within the normal range.
TMPRSS6 genotypes in the family. (A) Pedigree of the family. (B) Sequencing traces from exon 10 and exon 15 of TMPRSS6. Arrows indicate heterozygous nucleotide substitutions leading to Y393X and R599X nonsense codons. wt indicates wild-type.
TMPRSS6 genotypes in the family. (A) Pedigree of the family. (B) Sequencing traces from exon 10 and exon 15 of TMPRSS6. Arrows indicate heterozygous nucleotide substitutions leading to Y393X and R599X nonsense codons. wt indicates wild-type.
This patient presents distinctive features from the previously reported patients with DMT1 mutations.5-7 The anemia was detected at birth in DMT1 patients while here the diagnosis was made at 18 months, suggesting that iron transfer from the mother to the fetus is not altered by TMPRSS6 mutations. Similarly, in the paper by Finberg et al, none of the anemia was diagnosed at birth.11 Furthermore, DMT1 patients always had high serum iron values,8 in contrast with the strikingly low values found in our patient and they developed severe liver iron overload at a young age
Sixteen different genes of the TMPRSS family are known in 2008 and mutations in TMPRSS1, 2, 3, and 5 are apparently involved in nonsyndromic deafness21 and in the pathogenesis of cancers.22,23 The role of TMPRSS6 in down-regulating hepcidin expression in response to IDA remains to be established.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Gordana Olbina (Intrinsic LifeSciences) for performing the hepcidin assays.
This work was supported by an ANR-GIS Institut des Maladies Rares grant to Carole Beaumont (RPV07047HSA).
Authorship
Contributions: F.G. and C.K. designed and performed sequencing experiments and analyzed data; S.L. diagnosed the patients; M.W. performed hepcidin assays; and C.B. and B.G. designed the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carole Beaumont, Inserm U773, Université Paris Diderot, site Bichat, 16 rue Henri Huchard, 75018 Paris, France; e-mail: carole.beaumont@inserm.fr.