Abstract
The production of immunoglobulin E (IgE) is tightly regulated. This is evidenced by the fact that it comprises less than 0.0001% of serum Ig, and aberrant production causes atopic conditions, including allergy, rhinitis, and anaphylaxis. Interleukin-4 (IL-4) is a well-characterized inducer of IgE by human and murine B cells, whereas interferon-γ can antagonize this effect. IL-21 has also been recognized for its ability to suppress IL-4–induced IgE production by murine B cells. Here, we identified IL-21 as an inducer of IgE production by CD40L-stimulated human naive B cells. Furthermore, there was a striking synergy between IL-4 and IL-21 on inducing IgE secretion by CD40L-stimulated human B cells, such that the levels detected under these conditions exceeded those induced by IL-4 or IL-21 alone by more than 10-fold. IL-21 induced activation of STAT3 and analysis of B cells from patients with loss-of-function STAT3 mutations revealed that the ability of IL-21 to induce IgE secretion, and augment that driven by IL-4, was STAT3-dependent. These findings highlight a fundamental difference between the regulation of IgE production by human and murine B cells and have implications for the dysregulated production of IgE in conditions characterized by extremely high levels of serum IgE.
Introduction
In normal individuals, the concentration of serum immunoglobulin E (IgE) is approximately 100 ng/mL, 104 to 105 times lower than IgG and IgA, making it the least abundant serum Ig.1 However, aberrant production of IgE, often resulting from dysregulated Th2 or deficient Th1 responses, is associated with numerous diseases, including atopy, allergy, asthma, eczema/dermatitis, and parasitic infections.2 Furthermore, extraordinarily high levels of IgE are present in patients with hyper-IgE syndrome (HIES), Ommen syndrome, Wiskott-Aldrich syndrome, Comel-Netherton syndrome, and immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, diseases also associated with eczema, allergy, and atopy.2,3 Thus, to maintain IgE at normal (nonpathogenic) levels, its production must be strictly regulated. Identification of modulators of IgE production is therefore critical for understanding the pathogenesis of, and designing novel therapeutics for, conditions such as allergy and atopy.
It is well established that interleukin-4 (IL-4) induces IgE production by human and murine B cells.4,5 Human B cells also produce IgE when stimulated with IL-13.2,6,7 Several factors, such as interferon-γ (IFN-γ), IFN-α, transforming growth factor-β, IL-10, and prostaglandins, have been identified that antagonize IL-4-induced IgE production by human and murine B cells.2,4,5,8-10 Recent studies have also revealed that IL-21 inhibits IL-4–induced IgE secretion by murine B cells.11-15 The increased levels of serum IgE in IL-21R– or IL-21–deficient mice11,13 most probably results from the ability of IL-21 to directly inhibit isotype switching to IgE by their B cells.12 Despite these findings, the effects of IL-21 on IgE secretion by human B cells remain incompletely characterized. On one hand, IL-21 inhibited IL-4–induced IgE secretion in cultures of total peripheral blood mononuclear cells (PBMCs)16,17 by inducing apoptosis of B cells committed to producing IgE and/or inducing IFN-γ production by non-B cells in the PBMCs.14,17 On the other hand, IL-21 had no effect on IgE secretion by human B cells stimulated with anti-CD40 monoclonal antibody (mAb),18,19 yet it did enhance IgE secretion induced by anti-CD40 mAb and IL-4.16-18,20
Because of conflicting reports on the role of IL-21 in switching to IgE by human B cells, and potential differences in regulating IgE by human and murine B cells, we examined the effect of IL-21 on IgE secretion by human naive B cells. IL-21 induced IgE secretion by CD40L-stimulated naive B cells. Furthermore, addition of both IL-4 and IL-21 to CD40L-stimulated human B cells resulted in potent synergy, inducing 10- to 100-fold higher levels of IgE than those induced by IL-4 or IL-21 alone. One explanation for the interplay between IL-4 and IL-21 resulting in secretion of large amounts of IgE was the concomitant phosphorylation and activation of STAT3 and STAT6 in the majority of stimulated B cells. Indeed, the synergistic effect of IL-21 and IL-4 on IgE secretion was attenuated when B cells from patients with loss-of-function STAT3 mutations were examined. These results suggest that both IL-4 and IL-21 positively regulate IgE production by human B cells. Aberrant production of, or signaling by, either of these cytokines may underlie excessive production of IgE in certain diseases. Thus, targeting IL-21 may be a means of treating these disorders.
Methods
mAbs and reagents
The following mAbs were used: fluorescein isothiocyanate-anti-CD20, allophycocyanin-anti-CD10 (BD Biosciences, San Jose, CA); phycoerythrin-anti-CD23 (eBioscience, San Diego, CA); phycoerythrin-anti-CD27, anti-STAT1, anti-STAT3, Alexa488-anti-STAT5, Alexa647-anti–STAT6 (BD Biosciences PharMingen, San Diego, CA); and neutralizing anti–IL-6 mAb (R&D Systems, Minneapolis, MN). Membranes of insect cells infected with baculovirus expressing recombinant human CD40L have been described.21 Human IL-4 and neutralizing anti–IL-4 mAb (MP4-25D2) were provided by Dr Rene de Waal Malefyt (DNAX Research, Palo Alto, CA). Human IL-21 was from PeproTech (Rocky Hill, NJ).
Lymphocyte purification and phenotyping
Cord blood (CB) and peripheral blood (PB) were collected from healthy donors and patients with HIES resulting from STAT3 mutations.22,23 The clinical details, STAT3 mutations, and serum IgE levels of HIES patients are described elsewhere.24 B cells were isolated from PBMCs by Negative Isolation (Dynal Biotech, Oslo, Norway) and then sorted into transitional (CD20+CD10+CD27−), naive (CD20+CD10−CD27−), and memory (CD20+CD10−CD27+) populations.25-27 Expression of CD23 on naive and memory B cells from normal donors and HIES patients was determined using mAb to CD20, CD27, and CD23 and then gating on CD20+CD27− and CD20+CD27+ B cells, respectively.25,26 CD4+ T cells were isolated using CD4-Beads (Dynal Biotech).28 The purity for each population was more than 98%. All studies were approved by Sydney South West Area Health Service Human Research Ethics committee (X03-0317, X04-0122, and X06-0013), in accordance with the Declaration of Helsinki.
Lymphocyte cultures
B cells (0.5-50 × 103/200 μL) were cultured with CD40L alone or with IL-4 (100 U/mL), IL-21 (5-100 ng/mL), or IL-4 plus IL-21, in the absence or presence of a neutralizing anti–IL-4 or IL-6 mAb (20 μg/mL). To examine the role of cell division in IgE secretion, CB B cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE)21,29 and cultured with CD40L, CD40L/IL-4, CD40L/IL-21, or CD40L/IL-4/IL-21. After 5 days, cells were harvested, sorted into distinct division intervals, and recultured (∼10-20 × 103 cells/200 μL) for 2 days with CD40L (for initial cultures with CD40L or CD40L/IL-4) or CD40L plus IL-21 (for initial cultures with CD40L/IL-21 or CD40L/IL-4/IL-21). IL-4 was excluded from secondary cultures as it induced apoptosis of preactivated B cells (not shown). CD4+ T cells (30 × 103 cells/well/200 μL) were cultured with anti-CD3 mAb (5 μg/mL; SpvT3) with or without in anti-CD28 mAb (750 ng/mL; BD Biosciences PharMingen), IL-2 (20 U/mL; Pierce Endogen, Rockford, IL) or IL-21 (50 ng/mL).28 IFN-γ and IL-10 secretion was measured by enzyme-linked immunosorbent assay.24
Detection of secreted IgE
Microtiter plates were coated with anti–human IgE mAb (Southern Biotechnology, Birmingham, AL) and blocked with 2% fetal calf serum/phosphate-buffered saline (FCS/PBS). Culture supernatants and IgE myeloma standard (Calbiochem, San Diego, CA) were added to the wells and incubated for 2 hours at 37°C before adding biotin-conjugated anti–human IgE mAb (Southern Biotechnology). Bound Ab was detected with streptavidin-horseradish peroxidase (Jackson Immuno-Research, West Grove, PA) and visualized with tetramethylbenzidine (Sigma-Aldrich, St Louis, MO).
Expression of phosphorylated STATs
B cells were unstimulated or stimulated with CD40L alone or with IL-4, IL-21, or IL-4/IL-21 for 16 hours, then harvested, fixed in 2% formaldehyde, permeabilized in 90% methanol, and labeled with antiphospho-STAT1, STAT3, STAT5, or STAT6 mAb.30
Results
IL-21 induces CD40L-stimulated human cord blood B cells to secrete IgE
Previous studies found that IL-21 did not induce IgE secretion by anti-CD40 mAb-treated human B cells,18,19 yet it could increase IgE secretion by anti-CD40/IL-4–stimulated PBMCs.16-18,20 The findings from PBMCs are difficult to interpret because the net outcome of IL-21 stimulation could reflect a direct effect on B cells and/or an indirect effect on CD4+ T cells, NK cells, or NKT cells, which can all respond to IL-2131-34 and modulate Ig production.2,35,36 For these reasons, we stimulated naive CB B cells with CD40L plus or minus IL-21 and examined IgE secretion. CB B cells were also cultured with CD40L/IL-4, a known inducer of IgE secretion.4,7,37
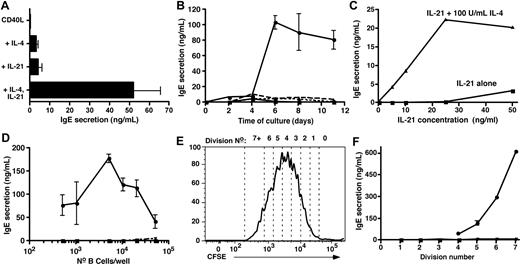
Culture of CB B cells with CD40L/IL-4, but not CD40L alone, yielded low but detectable amounts of IgE (Figure 1A). CB B cells also produced IgE when cultured with CD40L/IL-21 (Figure 1A). Strikingly, IgE production by CB B cells stimulated with CD40L and IL-4 or IL-21 was augmented 10- to 20-fold in the presence of both cytokines (Figure 1A). Thus, IL-21- like IL-4 can induce human CB B cells to produce IgE, and these 2 cytokines synergize to induce very high levels of IgE.
IL-21 induces IgE secretion by human naive cord blood B cells and synergizes with IL-4 to induce high-level production of IgE. (A-D) CB B cells were cultured at (A-C) 10 × 103 cells/200 μL per well; (D) 5 × 102 to 50 × 103/well with (A,B) CD40L alone (■) or with IL-4 (100 U/mL; ▴), IL-21 (50 ng/mL; ▾), or both IL-4 and IL-21 (●). (C) CD40L and increasing concentrations of IL-21 (0-50 ng/mL) only (■) or together with 100 U/mL IL-4 (▴). Culture supernatants were collected after 10 to 14 days (A,C,D) or at the indicated times (B) and the amount of secreted IgE determined by Igϵ heavy chain-specific enzyme-linked immunosorbent assay. The results in panel A represent the mean plus or minus SEM of data from 8 independent experiments; those results in panels B, C, and D are representative of multiple experiments performed using B cells from different CB donors. (E,F) CB B cells were labeled with CFSE and then cultured with CD40L alone (■) or together with IL-4 (▴), IL-21 (▾), or IL-4 plus IL-21 (●). After 5 days, the cells were resorted into different division intervals based on dilution of CFSE (E; representing the division profile of cells stimulated with CD40L/IL-21), and equal numbers of cells then recultured for a further 2 days. After this time, secretion of (F) IgE was determined.
IL-21 induces IgE secretion by human naive cord blood B cells and synergizes with IL-4 to induce high-level production of IgE. (A-D) CB B cells were cultured at (A-C) 10 × 103 cells/200 μL per well; (D) 5 × 102 to 50 × 103/well with (A,B) CD40L alone (■) or with IL-4 (100 U/mL; ▴), IL-21 (50 ng/mL; ▾), or both IL-4 and IL-21 (●). (C) CD40L and increasing concentrations of IL-21 (0-50 ng/mL) only (■) or together with 100 U/mL IL-4 (▴). Culture supernatants were collected after 10 to 14 days (A,C,D) or at the indicated times (B) and the amount of secreted IgE determined by Igϵ heavy chain-specific enzyme-linked immunosorbent assay. The results in panel A represent the mean plus or minus SEM of data from 8 independent experiments; those results in panels B, C, and D are representative of multiple experiments performed using B cells from different CB donors. (E,F) CB B cells were labeled with CFSE and then cultured with CD40L alone (■) or together with IL-4 (▴), IL-21 (▾), or IL-4 plus IL-21 (●). After 5 days, the cells were resorted into different division intervals based on dilution of CFSE (E; representing the division profile of cells stimulated with CD40L/IL-21), and equal numbers of cells then recultured for a further 2 days. After this time, secretion of (F) IgE was determined.
The effects of IL-21 on IgE secretion are time-, dose-, and density-dependent
To investigate further the effects of IL-21 on IgE secretion, CB B cells were stimulated with CD40L alone or with IL-4 and/or IL-21 and IgE measured after different times. Low levels of IgE were produced after 4 to 6 days of in vitro culture with IL-4 or IL-21, whereas IL-4/IL-21 resulted in detectable IgE levels by 2 to 4 days (Figure 1B). Whereas the amount of IgE induced by CD40L/IL-4 or CD40L/IL-21 remained low for the entire culture period, the combination of IL-4 and IL-21 resulted in a large increase in IgE after 4 to 6 days (Figure 1B). Next, CB B cells were stimulated with CD40L and increasing doses of IL-21. IL-21 induced IgE secretion when used at 50 ng/mL (Figure 1C). When CB B cells were stimulated with CD40L, a fixed dose of IL-4 (100 U/mL), and increasing concentrations of IL-21, the synergistic effect of these 2 cytokines was clear. Under these conditions, CB B cells secreted IgE in response to doses of IL-21 that had minimal effect when used alone (ie, 5-25 ng/mL of IL-21; Figure 1C). Thus, although IL-4 or IL-21 could induce CD40L-stimulated B cells to secrete IgE, the addition of both IL-4 and IL-21 increased the sensitivity of CB B cells to the stimulatory effects of these cytokines, as evidenced by a requirement for a reduced concentration of IL-21 to induce IgE secretion.
It was recently reported that IL-4 and IL-21 maximally induced IgE production by CD40L-stimulated IgD+ tonsil B cells when cultured at densities of approximately 3 × 103 cells/well, whereas higher cell densities inhibited IgE production. In contrast, the greatest effect of IL-4 alone occurred at approximately 10 × 103 cells/well.20 The effect of IL-21 alone on IgE secretion was not examined in this study.20 To investigate whether IL-4 and IL-21 exhibited similar density-dependent effects in our system, increasing numbers (5 × 102 to 104) of CB B cells were cultured with CD40L plus or minus IL-4, IL-21, and IL-4/IL-21. IgE production was detected in cultures seeded with 25 to 50 × 103 B cells and stimulated with CD40L/IL-21 (Figure 1D). In contrast, IgE secretion was detected when as few as 500 B cells were cultured with CD40L/IL-4/IL-21 (Figure 1D). The greatest amounts of IgE were noted in cultures established with 5 to 10 × 103 CB B cells, and IgE secretion was dramatically reduced in cultures containing more than 20 × 103 B cells. Thus, consistent with Caven et al,20 induction of high levels of IgE by CD40L/IL-4/IL-21–stimulated B cells is modulated by cell density.
Differentiation of human B cells to IgE-secreting cells is linked to cell division
Isotype switching is linked to cell division.21,29,38 It was therefore of interest to assess the role of division in switching to IgE by human B cells. CFSE-labeled CB B cells were cultured with CD40L plus or minus IL-4, IL-21, or IL-4/IL-21. After 5 days, cells were sorted from individual division intervals (Figure 1E), recultured for a further 2 days, and IgE secretion assessed. Dividing B cells isolated after primary culture with CD40L did not produce detectable levels of IgE, whereas initial culture with CD40L/IL-4 or CD40L/IL-21 resulted in the detection of trace amounts (IL-4, < 5 ng/mL; IL-21, 5-10 ng/mL) of IgE from cells that had undergone more than 2 divisions (Figure 1F). However, CB B cells initially cultured with CD40L/IL-4/IL-21 secreted substantially higher levels of IgE on a per-division basis. IgE secretion was detected once B cells had undergone 4 divisions before sorting, and the amount produced increased with each subsequent division (Figure 1F). The levels of IgE secreted by highly divided B cells exceeded that detected in bulk cultures of total CB B cells by 5- to 10-fold. These data demonstrate that differentiation of human naive B cells into IgE-secreting B cells is linked to cell division.
IL-21 induces IgE production by distinct B cell subsets
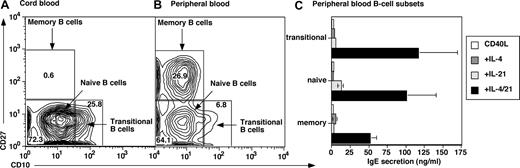
Although both CB (Figure 1) and PB B cells (not shown) produced IgE after stimulation with CD40L/IL-21, the composition of these B-cell populations is distinct: CB is enriched for transitional B cells, whereas PB contains a substantial fraction of CD27+ (ie, memory) B cells25-27 (Figure 2A,B). Thus, the B-cell subset(s) that produce IgE was determined by sorting PB transitional, naive, and memory B cells (Figure 2A,B). Transitional and naive B cells secreted low levels of IgE after stimulation with CD40L/IL-4 or CD40L/IL-21 (Figure 2C), and the combination of CD40L/IL-4/IL-21 increased IgE secretion by 10- to 30-fold over that induced by either cytokine alone. Memory B cells also produced substantial amounts of IgE when stimulated with CD40L/IL-4/IL-21 (Figure 2C). Thus, all subsets of mature B cells can produce increased levels of IgE after stimulation with CD40L, IL-4, and IL-21.
IL-21 induces IgE secretion by human transitional, naive, and memory B cells. Human CB (A) and PB (B) MNCs were labeled with mAb specific for CD20, CD10, and CD27. The frequencies of transitional (CD10+CD27−), naive (CD10−CD27−), and memory (CD10−CD27+) cells were determined. Values represent the mean percentages of B-cell subsets in 12 CB and 8 PB samples. (C) Transitional, naive, and memory B cells were sort-purified and then cultured (∼ 20 × 103 cells/200 μL per well) with CD40L alone (□) or in combination with IL-4 (100 U/mL;  ), IL-21 (50 ng/mL;
), IL-21 (50 ng/mL;  ), or IL-4 plus IL-21 (■). After 12 to 14 days, supernatants were harvested and the amount of secreted IgE determined. The results are the means plus or minus SEM of data from 4 independent experiments using B-cell subsets isolated from 4 different blood donors.
), or IL-4 plus IL-21 (■). After 12 to 14 days, supernatants were harvested and the amount of secreted IgE determined. The results are the means plus or minus SEM of data from 4 independent experiments using B-cell subsets isolated from 4 different blood donors.
IL-21 induces IgE secretion by human transitional, naive, and memory B cells. Human CB (A) and PB (B) MNCs were labeled with mAb specific for CD20, CD10, and CD27. The frequencies of transitional (CD10+CD27−), naive (CD10−CD27−), and memory (CD10−CD27+) cells were determined. Values represent the mean percentages of B-cell subsets in 12 CB and 8 PB samples. (C) Transitional, naive, and memory B cells were sort-purified and then cultured (∼ 20 × 103 cells/200 μL per well) with CD40L alone (□) or in combination with IL-4 (100 U/mL;  ), IL-21 (50 ng/mL;
), IL-21 (50 ng/mL;  ), or IL-4 plus IL-21 (■). After 12 to 14 days, supernatants were harvested and the amount of secreted IgE determined. The results are the means plus or minus SEM of data from 4 independent experiments using B-cell subsets isolated from 4 different blood donors.
), or IL-4 plus IL-21 (■). After 12 to 14 days, supernatants were harvested and the amount of secreted IgE determined. The results are the means plus or minus SEM of data from 4 independent experiments using B-cell subsets isolated from 4 different blood donors.
IL-21–induced IgE production is independent of endogenous IL-4 but partially dependent on IL-6
The finding that IL-21 induced IgE production by human B cells was surprising because IL-21 inhibits IgE secretion by murine B cells.11-15 It was therefore important to determine whether IL-21 had a direct effect on IgE secretion by human B cells. Although T cells are a main source of IL-4, it can also be produced by B cells.39 Thus, one mechanism whereby IL-21 could indirectly induce IgE secretion would be by promoting IL-4 production, which would then act in an autocrine manner. To examine this possibility, CB B cells were cultured in the presence of a neutralizing IL-4 mAb. Whereas IgE secretion by CD40L-stimulated CB B cells induced by exogenous IL-4 was abrogated by anti–IL-4 mAb, IL-21–induced IgE was unaffected (Table 1, experiments 1, 2). Furthermore, the synergistic effect of IL-4 and IL-21 on IgE production by CD40L-stimulated CB B cells was reduced to the effect of IL-21 alone by anti–IL-4 mAb (Table 1, experiments 1, 2). The combined effect of IL-4 and IL-21 on IgE secretion was also unrelated to alterations in expression of their receptors because neither IL-4 nor IL-21 enhanced the level of expression of the IL-21R or IL-4R, respectively, induced by CD40L on stimulated B cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The ability of IL-21 to induce IgE secretion by CD40L-activated human B cells is independent of endogenous IL-4 but partially dependent on endogenous IL-6
| Culture . | IgE secretion (ng/mL) . | |||
|---|---|---|---|---|
| No mAb . | Control mAb . | Anti-IL-4 mAb . | Anti-IL-6 mAb . | |
| Experiment 1 | ||||
| CD40L | <0.1 | ND | ND | — |
| CD40L IL-4 | 7.6 ± 0.32 | ND | <0.1 | — |
| CD40L IL-21 | 3.9 ± 0.9 | 3.02 ± 0.24 | 4.28 ± 0.9 | — |
| CD40L IL-4/IL-21 | 41.8 ± 2.5 | 34.8 ± 4.7 | 4.1 ± 0.47 | — |
| Experiment 2 | ||||
| CD40L | <0.1 | ND | ND | — |
| CD40L IL-4 | 1.90 ± 0.3 | ND | <0.1 | — |
| CD40L IL-21 | 4.9 ± 0.9 | 3.7 ± 0.46 | 4.2 ± 1.1 | — |
| CD40L IL-4/IL-21 | 73.1 ± 13.0 | 84.0 ± 3.8 | 7.6 ± 2.3 | — |
| Experiment 3 | ||||
| CD40L | <0.1 | ND | — | <0.1 |
| CD40L IL-4 | <0.1 | <0.1 | — | <0.1 |
| CD40L IL-21 | <0.1 | <0.1 | — | <0.1 |
| CD40L IL-4/IL-21 | 48.6 ± 10 | 40.0 ± 8.0 | — | 24.0 ± 3.7 |
| Experiment 4 | ||||
| CD40L | <0.1 | ND | — | <0.1 |
| CD40L IL-4 | <0.1 | ND | — | <0.1 |
| CD40L IL-21 | 6.4 ± 2.2 | ND | — | 4.4 ± 1.8 |
| CD40L IL-4/IL-21 | 170 ± 2.0 | ND | — | 80.6 ± 18.2 |
| Culture . | IgE secretion (ng/mL) . | |||
|---|---|---|---|---|
| No mAb . | Control mAb . | Anti-IL-4 mAb . | Anti-IL-6 mAb . | |
| Experiment 1 | ||||
| CD40L | <0.1 | ND | ND | — |
| CD40L IL-4 | 7.6 ± 0.32 | ND | <0.1 | — |
| CD40L IL-21 | 3.9 ± 0.9 | 3.02 ± 0.24 | 4.28 ± 0.9 | — |
| CD40L IL-4/IL-21 | 41.8 ± 2.5 | 34.8 ± 4.7 | 4.1 ± 0.47 | — |
| Experiment 2 | ||||
| CD40L | <0.1 | ND | ND | — |
| CD40L IL-4 | 1.90 ± 0.3 | ND | <0.1 | — |
| CD40L IL-21 | 4.9 ± 0.9 | 3.7 ± 0.46 | 4.2 ± 1.1 | — |
| CD40L IL-4/IL-21 | 73.1 ± 13.0 | 84.0 ± 3.8 | 7.6 ± 2.3 | — |
| Experiment 3 | ||||
| CD40L | <0.1 | ND | — | <0.1 |
| CD40L IL-4 | <0.1 | <0.1 | — | <0.1 |
| CD40L IL-21 | <0.1 | <0.1 | — | <0.1 |
| CD40L IL-4/IL-21 | 48.6 ± 10 | 40.0 ± 8.0 | — | 24.0 ± 3.7 |
| Experiment 4 | ||||
| CD40L | <0.1 | ND | — | <0.1 |
| CD40L IL-4 | <0.1 | ND | — | <0.1 |
| CD40L IL-21 | 6.4 ± 2.2 | ND | — | 4.4 ± 1.8 |
| CD40L IL-4/IL-21 | 170 ± 2.0 | ND | — | 80.6 ± 18.2 |
B cells purified from different CB samples were cultured (∼10-20 × 103 cells per 200 μL per well) with CD40L alone or together with IL-4, IL-21, or both IL-4 and IL-21 in the absence or presence of a control mAb, neutralizing anti–IL-4 mAb (experiments 1, 2) or neutralizing anti-IL-6 mAb (experiments 3, 4). After 8 to 10 days, supernatants were harvested and the amount of secreted IgE determined by ELISA. These results represent the means plus or minus the SEM of replicate cultures.
ND indicates not done; and —, not applicable.
Because endogenous IL-6 can enhance IgE production induced by IL-4,40 we investigated whether IL-6 also contributed to IgE production induced by IL-21 or IL-4/IL-21. Culturing CB B cells in the presence of neutralizing anti–IL-6 mAb did not affect IgE induced by CD40L/IL-21 (Table 1, experiments 3, 4). However, blocking endogenous IL-6 reduced CD40L/IL-4/IL-21-induced IgE secretion by approximately 50% (Table 1, experiments 3, 4). These results established that the ability of IL-21 to induce IgE secretion by CB B cells is independent of endogenous production of both IL-4 and IL-6, whereas the combined effect of IL-4 plus IL-21 is partially dependent on the action of autocrine IL-6.
Complementary phosphorylation of STAT proteins by IL-21 and IL-4 in naive B cells
Cytokine signaling induces phosphorylation of JAKs that associate with cytokine receptors and phosphorylate tyrosines in their cytoplasmic domains, which act as docking sites to recruit various STATs that undergo JAK-mediated tyrosine phosphorylation, dimerization, and activation.2,41 Although receptors for IL-4 and IL-21 share γc, engagement of the IL-4R and IL-21R complexes activates distinct signaling pathways. Thus, IL-4 activates STAT6,41,42 whereas IL-21 can activate STAT1, STAT3, and STAT5.12,43-47 We therefore explored the possibility that the synergistic effect of IL-4 and IL-21 on IgE production was achieved by concomitant activation of distinct STAT proteins in CD40L-stimulated B cells.
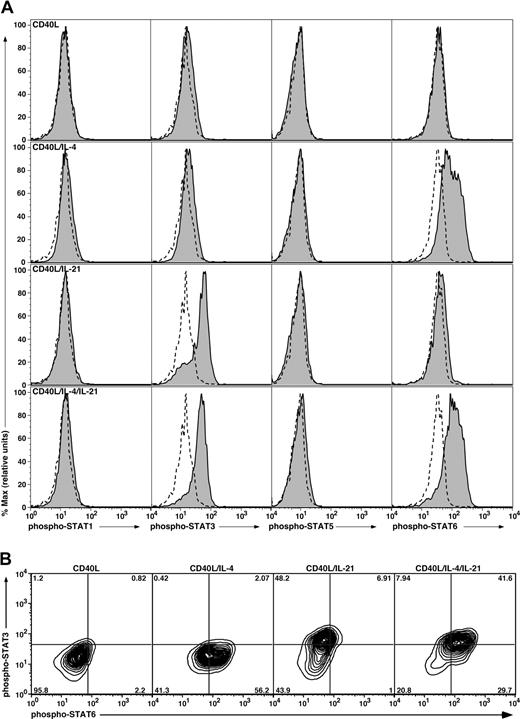
The phosphorylation status of STAT1, 3, 5, and 6 in CB B cells was assessed by flow cytometry using phospho-STAT mAbs. Stimulation of CB B cells with CD40L alone for 16 hours failed to induce phosphorylation of any of the STATs examined (Figure 3A,B). However, inclusion of IL-4 with CD40L resulted in phosphorylation of STAT6 in more than 50% of cells (Figure 3A,B). On the other hand, IL-21 induced phosphorylation of only STAT3 in activated CB B cells (Figure 3A). Similar to the effect of IL-4 on phospho-STAT6, more than 50% of IL-21–stimulated B cells expressed phospho-STAT3 (Figure 3B). The combination of IL-4 and IL-21 resulted in expression of both phospho-STAT3 and phospho-STAT6 by more than 40% of CD40L-stimulated CB B cells, and a greater frequency of cells expressing phospho-STAT6 (58% vs 71%; Figure 3A bottom panel; 3B). A recent study found that IL-21 rapidly and transiently activated STAT5 in human B cells that had been preactivated with CD40L for 36 hours.47 Thus, we examined the kinetics of STAT phosphorylation in CD40L-stimulated B cells. IL-21 induced phosphorylation of STAT3 in CD40L-stimulated B cells within approximately 30 minutes of stimulation, and the levels of expression increased over the 18-hour stimulation period (Figure S2). In contrast, IL-21 had negligible effect on expression of phospho-STAT1 or STAT5 in the B cells at any of the times examined (Figure S2). Thus, IL-4 and IL-21 may achieve their synergistic effect by simultaneously activating target genes downstream of STAT3 and STAT6 in the majority of stimulated B cells.
Induction of phosphorylation of STAT proteins in stimulated CB B cells. (A) CB B cells were cultured in the absence or presence of CD40L with or without IL-4, IL-21, or both cytokines. After 16 hours, the cells were harvested, fixed, and permeabilized, and expression of phospho-STAT1, 3, 5, and 6 determined by immunofluorescence and flow cytometry. Solid histograms represent expression of phosphorylated STAT1, 3, 5, or 6 in the presence of the indicated stimulus; the dashed outline histograms represents expression of phosphorylated STATs in cells cultured in the absence of any exogenous stimulus. (B) Coexpression of STAT3 and STAT6 was determined for CB B cells stimulated with CD40L alone or together with IL-4, IL-21, or IL-4 and IL-21. The values represent the percentage of cells in each quadrant of the contour plot.
Induction of phosphorylation of STAT proteins in stimulated CB B cells. (A) CB B cells were cultured in the absence or presence of CD40L with or without IL-4, IL-21, or both cytokines. After 16 hours, the cells were harvested, fixed, and permeabilized, and expression of phospho-STAT1, 3, 5, and 6 determined by immunofluorescence and flow cytometry. Solid histograms represent expression of phosphorylated STAT1, 3, 5, or 6 in the presence of the indicated stimulus; the dashed outline histograms represents expression of phosphorylated STATs in cells cultured in the absence of any exogenous stimulus. (B) Coexpression of STAT3 and STAT6 was determined for CB B cells stimulated with CD40L alone or together with IL-4, IL-21, or IL-4 and IL-21. The values represent the percentage of cells in each quadrant of the contour plot.
STAT3 is required for IL-21–mediated induction of IgE secretion by human B cells
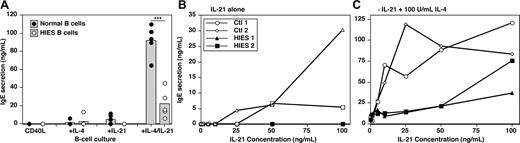
Heterozygous mutations in STAT3 have recently been identified in individuals with HIES.22,23 Although serum IgE levels are increased in HIES patients with STAT3 mutations, B cells from these patients represent a model system to determine whether the ability of IL-21 to induce IgE secretion by CD40L-stimulated B cells was STAT3-dependent. We identified 5 HIES patients with heterozygous mutations in either the DNA-binding or SH2-domains of STAT3.24 Total B cells were isolated from HIES patients and normal controls. As expected, B cells from normal donors produced low amounts of IgE in response to CD40L and either IL-4 or IL-21, and 10- to 20-fold higher levels in the presence of both cytokines (Figure 4A). Whereas the ability of B cells from HIES patients to produce IgE in response to CD40L/IL-4 was intact, they completely failed to produce IgE after stimulation with CD40L/IL-21 (Figure 4A). Furthermore, the synergistic effect of IL-4 plus IL-21 on IgE secretion by CD40L-stimulated B cells was significantly reduced by STAT3 mutations, as revealed by 3- to 10-fold less IgE secreted by HIES B cells stimulated with CD40L/IL-4/IL-21 relative to normal B cells (Figure 4A). Thus, functional STAT3 is required for IL-21 to induce high quantities of IgE induced by costimulation of human B cells with CD40L and IL-4.
IL-21-induced IgE secretion by human B cells is STAT3-dependent. (A) PB B cells from 5 healthy donors (●) and 5 HIES patients with heterozygous STAT3 mutations (○) were cultured (∼ 20 × 103 cells/200 μL per well) in quadruplicate with CD40L, CD40L/IL-4 (100 U/mL), CD40L/IL-21 (50 ng/mL), or CD40L/IL-4/IL-21 for 10 to 12 days. Each point represents IgE secretion by an individual normal donor or HIES patient; the gray column represents the mean. ***P = .001 (unpaired Student t test). (B,C) B cells from 2 donors (◇,○) and 2 HIES patients (■,▴) were cultured with CD40L and increasing concentrations of IL-21 (0-100 ng/mL) in the absence (B) or presence (C) of IL-4 (100 U/mL). IgE secretion was determined after 12 days. Note the different scales of the y-axes in panels B and C.
IL-21-induced IgE secretion by human B cells is STAT3-dependent. (A) PB B cells from 5 healthy donors (●) and 5 HIES patients with heterozygous STAT3 mutations (○) were cultured (∼ 20 × 103 cells/200 μL per well) in quadruplicate with CD40L, CD40L/IL-4 (100 U/mL), CD40L/IL-21 (50 ng/mL), or CD40L/IL-4/IL-21 for 10 to 12 days. Each point represents IgE secretion by an individual normal donor or HIES patient; the gray column represents the mean. ***P = .001 (unpaired Student t test). (B,C) B cells from 2 donors (◇,○) and 2 HIES patients (■,▴) were cultured with CD40L and increasing concentrations of IL-21 (0-100 ng/mL) in the absence (B) or presence (C) of IL-4 (100 U/mL). IgE secretion was determined after 12 days. Note the different scales of the y-axes in panels B and C.
Whereas the magnitude of the IgE response to CD40L/IL-4/IL-21 by HIES B cells was less than control B cells, detectable amounts of IgE were still secreted by HIES B cells that exceeded that induced by CD40L/IL-4. This suggested some residual STAT3 function in HIES B cells. Because the active form of STAT proteins consists of homodimers,41 heterozygous mutations would be expected to result in approximately 25% of STAT3 dimers comprising wild-type (STAT3WT:STAT3WT) protein, 50% comprising one WT and one mutant molecule (STAT3WT:STAT3MUT), and 25% being STAT3MUT:STAT3MUT. Thus, we hypothesized that the 25% STAT3WT:STAT3WT dimer activated in response to IL-21 would be sufficient to induce low levels of IgE when used with IL-4. To examine this in more detail, we cultured HIES B cells with CD40L and increasing doses of IL-21 in the absence or presence of 100 U/mL of IL-4. PB B cells from normal donors secreted low levels of IgE after stimulation with CD40L and more than 25 ng/mL of IL-21 (Figure 4B). Whereas less than 10 ng/mL of IL-21 alone did not result in IgE secretion (Figure 4B), these low doses increased IL-4–induced IgE production by 10-fold (Figure 4C). In contrast, B cells from HIES patients failed to produce IgE at any of the doses of IL-21 tested alone (Figure 4B), and the IL-4–induced production of IgE was barely affected by IL-21 when added at 1 to 25 ng/mL (Figure 4C). Despite this, higher doses of IL-21 were capable of augmenting IgE secretion by HIES B cells induced by IL-4 (Figure 4C). Thus, B cells with STAT3 mutations fail to respond to limiting doses of IL-21, most probably because of insufficient STAT3WT:STAT3WT dimers. However, this impairment is partially corrected by increasing the dose of IL-21 and presumably the content of STAT3WT:STAT3WT dimers.
Elevated expression of B-cell CD23 and impaired production of IFN-γ and IL-10 by CD4+ T cells suggests dysregulated IL-4 signaling in HIES
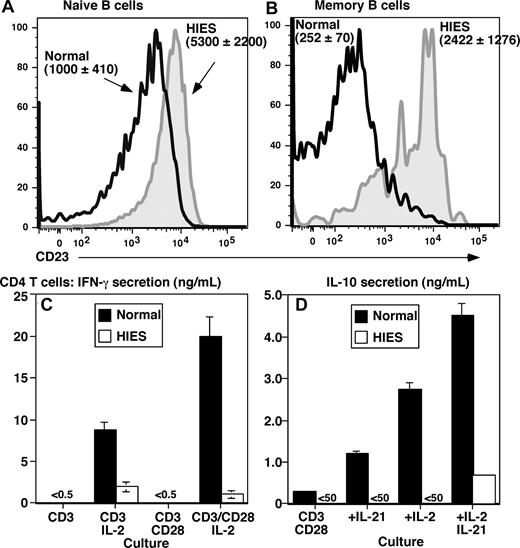
The finding that STAT3MUT B cells cannot produce high levels of IgE in vitro in response to IL-21 does not explain the extreme levels of serum IgE in HIES patients. Because IL-4 is a major regulator of IgE production,2 we sought to determine whether there may be heightened IL-4 signaling in HIES B cells. Because IL-4 strongly up-regulates CD23 on human B cells,6,48,49 we examined its expression. In normal donors, CD23 is expressed by naive but not memory B cells (Figure 5A,B).25,26 Strikingly, the level of CD23 on naive HIES B cells exceeded that on normal naive cells by 5-fold (Figure 5A). Furthermore, memory B cells from HIES patients expressed CD23 at a level that exceeded normal memory cells by approximately 10-fold (Figure 5B), such that its expression pattern resembled normal naive B cells.
Aberrant expression of CD23 on B cells and impaired production of IFN-γ and IL-10 by CD4+ T cells from STAT3 mutant patients suggests dysregulated IL-4 signaling in HIES. Expression of CD23 on ex vivo isolated naive (A; CD20+CD27−) and memory (B; CD20+CD27+) B cells from healthy donors (open black histogram) and HIES patients (solid gray histogram) was determined by flow cytometry. Panels A and B are representative histograms; the values are the mean fluorescence intensity plus or minus SEM of CD23 on naive and memory B cells from 5 controls and 4 HIES patients. (C,D) CD4+ T cells isolated from a normal donor (■) and HIES patient (□) were cultured for 5 days with immobilized anti-CD3 mAb in the absence or presence of IL-2, soluble anti-CD28 mAb, or IL-21. Secretion of (C) IFN-γ and (D) IL-10 was determined after 5 days of culture. The values represent the means plus or minus SD of triplicate cultures.
Aberrant expression of CD23 on B cells and impaired production of IFN-γ and IL-10 by CD4+ T cells from STAT3 mutant patients suggests dysregulated IL-4 signaling in HIES. Expression of CD23 on ex vivo isolated naive (A; CD20+CD27−) and memory (B; CD20+CD27+) B cells from healthy donors (open black histogram) and HIES patients (solid gray histogram) was determined by flow cytometry. Panels A and B are representative histograms; the values are the mean fluorescence intensity plus or minus SEM of CD23 on naive and memory B cells from 5 controls and 4 HIES patients. (C,D) CD4+ T cells isolated from a normal donor (■) and HIES patient (□) were cultured for 5 days with immobilized anti-CD3 mAb in the absence or presence of IL-2, soluble anti-CD28 mAb, or IL-21. Secretion of (C) IFN-γ and (D) IL-10 was determined after 5 days of culture. The values represent the means plus or minus SD of triplicate cultures.
IFN-γ and IL-10 antagonize the effects of IL-4 on IgE secretion9,10,49 and CD23 expression.49 Furthermore, PBMCs from HIES patients exhibit reduced expression and production of IFN-γ mRNA50 and protein.51-54 Thus, we speculated that production of IFN-γ and IL-10 by HIES CD4+ T cells may be impaired. CD4+ T cells from normal donors failed to produce IFN-γ in response to stimulation with anti-CD3 alone or in combination with anti-CD28 (Figure 5C). However, adding IL-2 to these cultures induced IFN-γ production, with higher amounts being produced by cells stimulated with anti-CD3/CD28 plus IL-2 (Figure 5C). Whereas HIES CD4+ T cells also secreted IFN-γ after stimulation with anti-CD3/IL-2, the levels were approximately 5-fold lower than those of normal CD4+ T cells. Furthermore, anti-CD28 mAbs failed to augment anti-CD3/IL-2–induced IFN-γ production, thereby revealing a 10-fold difference in IFN-γ secretion by anti-CD3/CD28/IL-2–stimulated normal and HIES CD4+ T cells (Figure 5C). HIES CD4+ T cells also failed to produce IL-10 in response to anti-CD3/CD28 stimulation (Figure 5D). Furthermore, whereas IL-10 production by normal CD4+ T cells could be increased by IL-2 or IL-21, these cytokines had no effect on HIES CD4+ T cells (Figure 5D). The inability of HIES CD4+ T cells to respond to IL-21 is consistent with IL-21 activating STAT3 in T cells.42 Thus, reduced production of IFN-γ and IL-10 by CD4+ T cells in vivo may contribute to sustained IL-4 signaling in HIES B cells, which may underlie increased levels of serum IgE in this disease.
Discussion
Secretion of IgE is a strictly regulated process, as evidenced by the fact that IgE comprises less than 0.0001% of all serum Ig,1 dysregulated IgE production can cause immunopathologies, such as allergy and atopy, and levels of IgE are extremely high in some human genetic diseases, such as HIES, Wiskott-Aldrich syndrome, Omenn's syndrome, and immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome.2,3 It is therefore important to identify regulators of IgE production. Studies in mice11-14 led to the conclusion that IL-21 has an important role in suppressing IgE production in vivo and may therefore have application in treating numerous IgE-mediated pathologies. Indeed, administration of IL-21 in vivo efficiently attenuated symptoms in animal models of allergic rhinitis and anaphylaxis.15,55
In striking contrast to its effect on murine B cells, we identified IL-21 as an inducer of IgE production by CD40L-stimulated human B cells. Furthermore, IL-4 and IL-21 synergized to increase IgE secretion by all B-cell subsets by more than 10-fold. Importantly, the synergy between IL-4 and IL-21 was evident at concentrations that failed to induce IgE secretion when used alone. Because B cells are probably exposed to limiting cytokine concentrations in vivo, such synergy at low doses of IL-4 and IL-21 implies that these cytokines may cooperate to regulate IgE production in vivo. In complementary studies, we have also found that combining IL-4 and IL-21 has diverse effects on isotype switching and Ig secretion by human naive B cells.56 IL-4 increased the frequency of IgG+ B cells induced in cultures of B cells stimulated with CD40L/IL-21; this predominantly resulted from IL-4 inducing the appearance of IgG1+ B cells. Despite this, secretion of IgG subclasses induced by IL-21 is not dramatically altered by IL-4. Furthermore, IL-4 inhibits IL-21–induced isotype switching to, and secretion of, IgA.56 Thus, the ability of IL-4 and IL-21 to synergize to induce secretion of large amounts of IgE is unique to this Ig isotype. Our results contrast previous studies, which found that IL-21 did not induce IgE secretion by human B cells.16-20 This probably reflects our use of multimeric CD40L as a B-cell stimulus, whereas other groups used soluble anti-CD40 mAb. Indeed, anti-CD40 mAb induces significantly less proliferation7 and IgE secretion by human B cells than CD40L (Figure S3). The fact that CD40L (Figure S3),57,58 but not anti-CD40,8,16,17,59,60 induced Igϵ germ line transcripts (GLT) in human B cells provides additional evidence for differences in the quality of signals delivered by CD40L and anti-CD40 mAb in the context of inducing IgE secretion.
Our results identified some of the requirements for IL-21–mediated induction of IgE production by human B cells, and the means by which signaling through the IL-21R and IL-4R interact to yield substantially greater levels of IgE. First, IgE production by B cells activated with CD40L, IL-4, and IL-21 was underpinned by cell division. This highlights the importance of signals that sustain or perpetuate cell division, such as continued presence of Ag or availability of T-cell help, in immune responses. Second, IL-21–induced IgE production was abolished in B cells with STAT3 mutations, thus revealing an obligatory role for STAT3 in this process. This is consistent with our finding that IL-21 induced activation of only STAT3 in human B cells. Thus, whereas STAT1 and STAT5 may mediate some effects of IL-21,12,43-47 loss of STAT3 function could not be compensated by other STATs indicating that STAT3 is necessary and sufficient for IL-21–induced IgE production. Third, the combination of IL-4 and IL-21 resulted in a large proportion of B cells expressing phosphorylated STAT6 and STAT3. Thus, stimulation with CD40L, IL-4, and IL-21 would activate a suite of STAT3 and STAT6 target genes in the same population of human B cells, thereby potentially contributing to the high levels of IgE observed. Fourth, IgE production induced by CD40L/IL-4/IL-21 was partially reduced by neutralizing endogenous IL-6. This is consistent with the finding that IL-4 induces production of IL-6 by CD40L-stimulated naive B cells, and this can be increased by IL-21.56 Because IL-6 activates STAT3,42 the involvement of IL-6 in this process would also contribute to impaired IgE secretion by STAT3 mutant B cells in response to these stimulatory conditions.
Activation of different STATs by IL-4 and IL-21 implies that these cytokines induce IgE production by distinct mechanisms. Indeed, IL-4, but not IL-21, induced Igϵ GLTs in human B cells (Figure S3).8,16,17,59 Thus, IL-4 probably induces IgE secretion by facilitating isotype switching through a process involving expression of GLTs,8,59 whereas IL-21 may achieve this by promoting expansion of naive B cells that either constitutively express Igϵ GLTs or acquire expression after CD40L stimulation.57,58 The use of different STAT signaling pathways by IL-4 and IL-21 may also explain why additional functions of these cytokines are not shared. For instance, IL-4 increases CD23 expression on human B cells,6,7,48 yet IL-21 had no effect (not shown). Thus, it will be important to identify IL-21–induced genes that mediate the effects of this cytokine.
Because IL-21 can activate STAT312,44,45 and suppress IgE secretion by murine B cells,11-15 it was postulated that the lack of a direct effect of IL-21 on inhibiting IgE secretion by human B cells because of loss-of-function STAT3 mutations would contribute to the elevated levels of IgE in HIES.23 However, the finding that IL-21 induced IgE secretion in a STAT3-dependent pathway implies that this scenario does not explain the abnormally high levels of serum IgE in HIES. Despite this, we can propose several mechanisms that may contribute to dysregulated IgE production in HIES. First, IL-21 can activate STAT3 in human NK and T cells and induce them to produce IFN-γ.17,31-34 Consequently, adding IL-21 to cultures of IL-4–stimulated human PBMCs indirectly inhibits IgE production in an IFN-γ–dependent manner.17 Thus, impaired production of IFN-γ by T and NK cells with STAT3 mutations may favor elevated production of IgE in HIES (Figure 6). A deficiency in IFN-γ–mediated regulation of B-cell activation in HIES is supported by several observations: 1) heightened expression of CD23 on HIES patients B cells (Figure 5A,B), and increased serum soluble CD23 in HIES,52 inasmuch that IFN-γ can inhibit IL-4–induced up-regulation of CD23 expression on B cells49 ; 2) reduced IFN-γ production by HIES PBMCs50,51,53,54 and CD4+ T cells (Figure 5D)61 ; 3) IFN-γ–mediated suppression of unusually high levels of IgE spontaneously produced by HIES PBMCs in vitro52,62 ; and 4) administration of IFN-γ reduced spontaneous IgE secretion by HIES patients B cells in vitro, and serum IgE levels.62 Our hypothesis that reduced IFN-γ production by CD4+ T cells contributes to aberrant IgE production in HIES is supported by the findings that patients with mutations in IL-12 or IFN-γR have significantly elevated serum IgE levels and increased incidence of clinical atopic disease compared with controls.63 Second, and similar to IFN-γ, it is possible that the regulatory effects of IL-10, which activates STAT342 and inhibits IL-4–induced IgE production,9,10 are compromised by STAT3 mutations. Indeed, STAT3 mutations abolished production of IL-10 by CD4+ T cells.24 This is consistent with STAT3 regulating IL-10 production by murine T cells.64 Notably, the ability of IL-21 to enhance IL-10 production was abrogated in HIES CD4+ T cells. Thus, it is probable that combined deficiencies in production of key cytokines that negatively regulate IL-4–induced IgE production by human B cells4,9,10 may contribute to dysregulation of IgE production in HIES. This would be further compounded by the inability of HIES B cells to respond to the regulatory effects of IL-10 because of STAT3 mutations. A third explanation for aberrant IgE production comes from the recent findings that STAT3 mutations prevent the generation of Th17 cells in HIES patients.24,61 It is possible that products of Th17 cells (IL-17, IL-22) suppress IgE secretion. However, this is unlikely because mature human B cells do not express receptors for these cytokines,30,65 and neither IL-17 nor IL-22 affected IgE secretion by human B cells (not shown).65 Because human Th17 cells remain incompletely characterized, it remains possible that another Th17 molecule acts to suppress IL-4–induced IgE production. Lastly, the function of negative regulators of IgE production may be impaired in HIES. For instance, bcl-6 suppresses IL-4–induced IgE secretion by antagonizing STAT6.2,66 Interestingly, bcl-6 also represses IRF-4, which induces CD23 expression on activated B cells.67 It is therefore possible that increased serum IgE levels and CD23 expression in HIES result from a reduction in expression and/or function of bcl-6 in HIES B cells. These scenarios reinforce the likelihood that extreme levels of IgE in conditions such as HIES are a manifestation of excessive IL-4/STAT6–mediated B-cell activation; and although IL-21 can induce IgE secretion by human B cells, it does not directly contribute to dysregulated IgE production in HIES. Further investigation into the consequences of STAT3 mutations on the behavior of CD4+ T cells, NK cells, and B cells with respect to cytokine production, effector function, and expression of transcriptional regulators of B-cell behavior will elucidate the mechanism(s) responsible for dysregulated IgE production in vivo in HIES. In the meantime, lymphocytes from HIES patients have provided a powerful system whereby we could demonstrate that (1) their B cells do not intrinsically produce aberrantly high levels of IgE, revealing the defect is B-cell extrinsic, (2) STAT3 is required for IL-21–mediated IgE production by human B cells, and (3) reduced production of cytokines involved in negatively regulating IgE production is a possible mechanism for dysregulated IgE production in HIES. Finally, our results reveal significant differences between the regulation of IgE secretion by human and murine B cells, and caution against using IL-21 therapeutically for suppressing IgE-mediated human diseases. Indeed, administration of IL-21 to patients could exacerbate their condition. Alternatively, neutralizing IL-21 together with IL-4 may be a means of treating patients with IgE-mediated conditions.
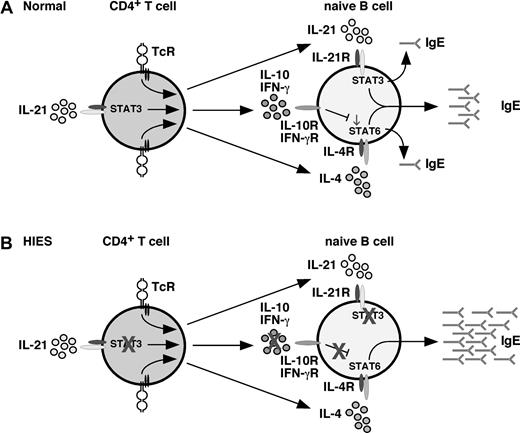
Model of cytokine-mediated regulation of IgE production: role of STAT3. (A) Under normal conditions, CD4+ T (and NK T) cells produce IL-4, IL-21, IL-10, and IFN-γ (among other cytokines) in response to stimulation through the TcR. Production of IFN-γ32-34 and IL-10 (Figure 5) can be enhanced by IL-21. IL-4, and IL-21 alone can induce IgE secretion by naive B cells; however, they act synergistically in a STAT3-dependent manner to promote secretion of high levels of IgE. Induction of IgE by IL-4 is negatively regulated by IL-10 and IFN-γ; the net result of the interplay between IL-4, IL-21, IL-10, and IFN-γ is production of a low basal level of IgE. (B) In HIES because of mutations in STAT3, the ability of (for example) IL-21 to heighten IL-10 and IFN-γ production by activated CD4+ T (and NKT) cells is abrogated; consequently, the suppressive effects of these cytokines on IgE production are attenuated. This is further compounded by the inability of HIES B cells to respond to the regulatory effects of IL-10, which acts through STAT3. Thus, despite the B cells being unable to respond to IL-21, their responses to the stimulatory effects of IL-4 are dysregulated, resulting in excessive production of IgE, a hallmark of HIES.
Model of cytokine-mediated regulation of IgE production: role of STAT3. (A) Under normal conditions, CD4+ T (and NK T) cells produce IL-4, IL-21, IL-10, and IFN-γ (among other cytokines) in response to stimulation through the TcR. Production of IFN-γ32-34 and IL-10 (Figure 5) can be enhanced by IL-21. IL-4, and IL-21 alone can induce IgE secretion by naive B cells; however, they act synergistically in a STAT3-dependent manner to promote secretion of high levels of IgE. Induction of IgE by IL-4 is negatively regulated by IL-10 and IFN-γ; the net result of the interplay between IL-4, IL-21, IL-10, and IFN-γ is production of a low basal level of IgE. (B) In HIES because of mutations in STAT3, the ability of (for example) IL-21 to heighten IL-10 and IFN-γ production by activated CD4+ T (and NKT) cells is abrogated; consequently, the suppressive effects of these cytokines on IgE production are attenuated. This is further compounded by the inability of HIES B cells to respond to the regulatory effects of IL-10, which acts through STAT3. Thus, despite the B cells being unable to respond to IL-21, their responses to the stimulatory effects of IL-4 are dysregulated, resulting in excessive production of IgE, a hallmark of HIES.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Royal Prince Alfred Hospital Birth Center for providing cord blood, Jerome Darakdjian for cell sorting, Rene de Waal Malefyt and Karen Fenimore-Pirog for reagents, and Dr Rob Brink and Prof Tony Basten for helpful discussion and critical review of this manuscript.
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Canberra, Australia). C.S.M. is a Peter Doherty Research Fellow, and S.G.T. is a Senior Research Fellow of the NHMRC.
Authorship
Contribution: D.T.A., C.S.M., and V.L.B. performed experiments and analyzed results; B.S.-N. and R.N. provided cord blood samples; M.W., D.A.F., and M.C.C. provided patient samples; D.A.F. and M.C.C. contributed to interpretation of the results and preparing the manuscript; and S.G.T. conceptualized and designed the research, performed experiments, analyzed results, and wrote the paper; all authors commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart G. Tangye, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst 2010, NSW, Australia; e-mail: s.tangye@garvan.org.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal