Abstract
The metalloprotease ADAMTS13 efficiently cleaves only the Tyr1605-Met1606 bond in the central A2 domain of multimeric von Willebrand factor (VWF), even though VWF constitutes only 0.02% of plasma proteins. This remarkable specificity depends in part on binding of the noncatalytic ADAMTS13 spacer domain to the C-terminal α-helix of VWF domain A2. By kinetic analysis of recombinant ADAMTS13 constructs, we show that the first thrombospondin-1, Cys-rich, and spacer domains of ADAMTS13 interact with segments of VWF domain A2 between Gln1624 and Arg1668, and together these exosite interactions increase the rate of substrate cleavage by at least approximately 300-fold. Internal deletion of Gln1624-Arg1641 minimally affected the rate of cleavage, indicating that ADAMTS13 does not require a specific distance between the scissile bond and auxiliary substrate binding sites. Smaller deletions of the P2-P9 or the P4′-P18′ residues on either side of the Tyr1605-Met1606 bond abolished cleavage, indicating that the metalloprotease domain interacts with additional residues flanking the cleavage site. Thus, specific recognition of VWF depends on cooperative, modular contacts between several ADAMTS13 domains and discrete segments of VWF domain A2.
Introduction
ADAMTS13 is a member of the “a disintegrin-like and metalloprotease with thrombospondin repeats” family, and it consists of a metalloprotease domain (M), a disintegrin-like domain (D), a thrombospondin type 1 repeat (TSP1, T), a Cys-rich domain (Cys, C), a spacer domain (Spacer, S), 7 additional TSP1 repeats, and 2 CUB domains (Figure 1A).3-5 The only known ADAMTS13 substrate in vivo is von Willebrand factor (VWF), a multimeric plasma protein that mediates platelet adhesion at sites of vascular injury. Excessive cleavage of VWF causes von Willebrand disease type 2A, an inherited bleeding disorder.6 Conversely, failure of ADAMTS13 to cleave VWF causes thrombotic thrombocytopenic purpura,3,7,8 which is usually lethal unless ADAMTS13 activity can be restored.
ADAMTS13 and its substrate, VWF, are trace components in the blood, present at concentrations at least 10 000-fold lower than the total plasma protein concentration. Even though ADAMTS13 is constitutively active9 and has no known inhibitors in vivo, it cleaves only VWF. This remarkable specificity is critical for hemostasis and depends on several mechanisms. Tensile force on VWF unfolds the A2 domain and exposes the Tyr1605-Met1606 scissile bond,10 which appears to be buried within the native, folded structure of the protein.1 Cofactors that bind VWF facilitate recognition by ADAMTS13.11 Finally, interactions between VWF and several ADAMTS13 exosites—substrate binding sites distant from the active site—enhance protease activity. ADAMTS13 domains distal to the spacer are required to recognize and cleave VWF multimers under conditions of high fluid shear stress.12-14 In addition, the proximal MDTCS domains (Figure 1A) are necessary under all conditions, and they are sufficient for many substrates that do not depend on fluid shear stress to expose the scissile bond.12,15,16
The ADAMTS13 metalloprotease domain recognizes the Tyr1605-Met1606 bond of VWF, and an exosite in the spacer domain binds a C-terminal segment of the A2 domain that is approximately 60 residues distant. Consequently, the intervening “DTC” domains (Figure 1) are candidates to bind intervening segments of VWF domain A2. In fact, C-terminal truncations of ADAMTS13 after the S, C, T, D, and M domains cause progressive decreases in protease activity.12,13,17 Similarly, decreasing the length of peptides derived from the C-terminus of the VWF A2 domain causes a progressive decrease in their potency as ADAMTS13 inhibitors.18 These data indicate that the MDTCS domains of ADAMTS13 interact with an extended segment of VWF domain A2.
To characterize ADAMTS13 exosites and their corresponding binding sites on VWF, we prepared a series of ADAMTS13 and VWF variants for kinetic analysis. The results indicate that several ADAMTS13 domains interact with distinct sequences on an extended segment of VWF domain A2. Each of these interactions is relatively weak, but together they cooperate to enhance ADAMTS13 substrate specificity, which is critical for hemostasis.
Methods
ADAMTS13 substrates
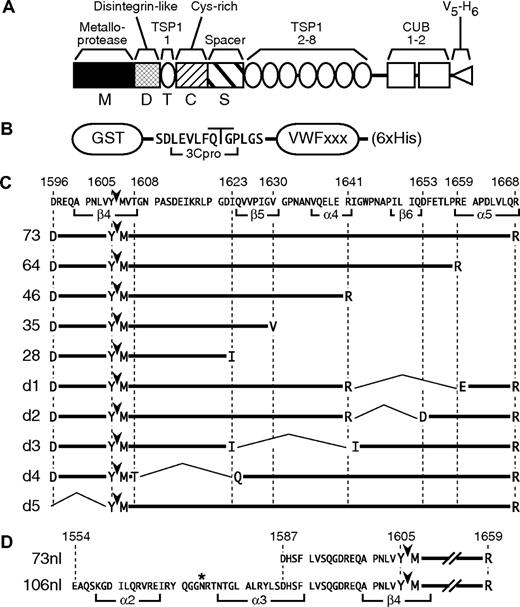
The preparation of GST-VWF73 and GST-VWF64 was described previously.17,19 Plasmids encoding GST-VWF46, GST-VWF35, GST-VWF28, GST-VWFd5, GST-VWF73nl, and GST-VWF106nl (Figure 1C,D) were constructed similarly in Schistosoma japonicum GST fusion expression vector pGEX-6P-1 (GE Healthcare, Little Chalfont, United Kingdom). Internal deletions were created with one primer for each construct in a single cloning step using a QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) and primers 5′-ACGTGCAGGAGCTGGAGAG-GGAGGCTCCTGACCTGGTGC-3′ (pGST-VWFd1), 5′-ACGTGCAGGAGCTGGAGAGGGACTTTGAGACGCTCCCCC-3′ (pGST-VWFd2), 5′-AGAGGCTGCCTGGAGACATCATTGGCTGGCCCAATGCCC-3′ (pGST-VWFd3), and 5′-ACCTGGTCTACATGGTCACCCAGGTGGTGCCCATTGGAGTGGGC-3′ (pGST-VWFd4). GST fusion proteins were expressed in Escherichia coli BL21 and purified by chromatography on Ni2+-NTA and glutathione-agarose (GE Healthcare) as described.17,19 Protein concentration was determined using a BCA total protein assay kit using a bovine serum albumin standard (Pierce, Rockford, IL).
To obtain peptide substrates, GST fusion proteins were cleaved with a modified recombinant rhinovirus 3C protease (PreScission protease; GE Healthcare) and the C-terminal peptide products were purified by high-performance liquid chromatography (HPLC) as described.17 Peptide concentrations were determined by amino acid analysis and absorbance at 280 nm. Substrates were stored at −80°C.
Recombinant human ADAMTS13 variants
Human ADAMTS13 (FL) and variants truncated after the spacer domain (MDTCS), Cys-rich domain (MDTC), first TSP1 repeat (MDT), disintegrin domain (MD), and metalloprotease domain (M) with a C-terminal V5 tag and 6 × His tag were expressed in stably transfected T-REx 293 cell lines and quantitated in conditioned medium by Western blotting with anti–V5 antibody, standardized with V5-tagged Positope reference protein (Invitrogen, Carlsbad, CA) as described.15,17,20,21
GST fusion substrate cleavage by ADAMTS13
The cleavage reaction included 40 μL reaction buffer containing 50 mM Hepes, pH 7.4, 150 mM NaCl, 5 mM CaCl2, 0.1 μM ZnCl2, 0.9 μL BSA (5 mg/mL), 0.1 μL AEBSF (40 mM), 0.1 μg of each GST fusion substrate, and 5 μL citrated normal human plasma or 45 nM recombinant enzyme to make a total volume of 45 μL. After incubation at 37°C for various times, reactions were stopped by adding an equal volume of 2 × SDS sample buffer containing 30 mM EDTA. Products were quantitated by SDS–polyacrylamide gel electrophoresis (PAGE) on 10% to 20% gradient gels (Invitrogen), Western blotting with HRP-conjugated rabbit anti-GST (GE Healthcare), and chemifluorescence (ECL Plus; GE Healthcare).17
Alternatively, reactions were stopped with an equal volume of 30 mM EDTA and products analyzed by an enzyme-linked immunosorbent assay (ELISA) method,22 with modifications to accommodate substrates digested in solution. Samples of stopped reactions and controls were incubated for 1 hour at room temperature in microtiter plates coated with anti-GST antibodies (Pierce). After washing, uncleaved substrates were detected by incubation with HRP-conjugated anti-His antibodies (Invitrogen) for 1 hour at room temperature. Plates were developed with an Illumination kit (Pierce) and read at 450 nm. The time course of product generation was analyzed by fitting to the integrated Michaelis-Menten equation to obtain kinetic constants.
Kinetics studies by MALDI-TOF MS
Reactions were performed at room temperature (25°C) in reaction buffer containing various concentrations of peptide substrates, and ADAMTS13 or a variant (1 nM enzyme for substrates VWF46, VWF35, and VWF28; 0.25 nM enzyme for substrates VWFd1, VWFd2, VWFd3, and VWFd4). Reactions were stopped with EDTA, and then desalted by adsorption on C18 micropipette tips (Glygen, Columbia, MD) and elution with 60% acetonitrile/0.1% formic acid. The N-terminal 1615-Da cleavage product was analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and quantitated by reference to an isotopically labeled 1622-Da internal standard peptide with the identical sequence.17 Cleavage of VWF73nl gave a 2587-Da N-terminal product that was quantitated using the same 1622-Da internal standard peptide.
When feasible, initial reaction rates were fitted by nonlinear least-squares regression to the Michaelis-Menten equation, v/[E] = [S]kcat/(Km + [S]), where v is rate of product formation, [E] is enzyme concentration, and [S] is substrate concentration. The specificity constant kcat/Km was calculated from the values for kcat and Km. Alternatively, for values of [S] less than 0.15 × Km, initial reaction rates were fitted to the equation v/[E] = [S]kcat/Km to obtain kcat/Km directly. To confirm that pseudo–first-order conditions prevailed, data were analyzed for several time points and several substrate concentrations.
Homology model of ADAMTS13 MD domains
The metalloprotease and disintegrin domains of ADAMTS13 (residues 75-374) and ADAMTS4 (residues 213-509) were aligned with Megalign (version 7.1; DNASTAR, Madison, WI) using the Clustal W algorithm (European Bioinformatics Institute, Cambridge, United Kingdom). The structure of ADAMTS13 was modeled based on this alignment (31% identity) and the crystallographic structure of ADAMTS4 (2rjp chain A),23 using the SWISS-MODEL automated protein modeling server.24,25
Results
C-terminal deletion substrates
Efficient cleavage of VWF requires the ADAMTS13 spacer domain to interact with the Glu1660-Arg1668 segment from the C-terminal end of the VWF A2 domain.17-19 However, additional segments of the A2 domain also contribute to substrate recognition. For example, the small C-terminal peptide Glu1660-Arg1668 cannot bind and inhibit ADAMTS13, but progressive addition of N-terminal residues up to Pro1645, Arg1618, or Met1606 is associated with a progressive decrease in Ki to 12 μM, 1.2 μM, or 0.4 μM, respectively.17,18 The ADAMTS13 domains that interact with these larger segments of VWF have not been identified.
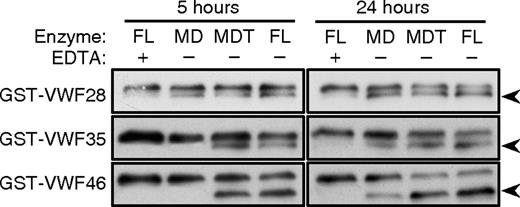
We investigated these interactions by enzymatic methods, using truncated variants of ADAMTS13 to cleave modified VWF domain A2 substrates with C-terminal deletions (Figure 1C). These substrates were screened initially using a semiquantitative Western blot assay method. Recombinant MD, MDT, and full-length ADAMTS13 all cleaved GST-VWF28 at approximately the same rate (Figure 2), suggesting that ADAMTS13 domains distal to MD do not interact with VWF residues Asp1596-Ile1623. In addition, MD cleaved GST-VWF28, GST-VWF35, and GST-VWF46 similarly, suggesting that VWF sequences distal to Ile1623 do not interact with MD. In contrast, MDT and full-length ADAMTS13 cleaved GST-VWF35 or GST-VWF46 more rapidly than GST-VWF28. This difference is particularly evident for the 5-hour time points. Therefore, the first TSP1 domain of ADAMTS13 may interact with VWF residues Gln1624-Val1630, which are present in GST-VWF35 and absent from GST-VWF28.
ADAMTS13 and VWF substrates. (A) Active ADAMTS13 consists of a metalloprotease domain (M), a disintegrin-like domain (D), a thrombospondin type 1 repeat (TSP1, T), a Cys-rich domain (Cys, C), a spacer domain (Spacer, S), 7 additional TSP1 repeats (2-8), and 2 CUB domains. In addition to full-length ADAMTS13 (FL), truncated enzymes were constructed with stop codons after Gln289 (M), Gly385 (MD), Glu439 (MDT), Cys555 (MDTC), and Ala685 (MDTCS). All constructs contain a C-terminal V5 epitope and 6 × His tag. (B) GST fusion substrates consist of a GST moiety followed by the linker SDLEVLFQGPLGS, a segment of VWF domain A2 (VWFxxx), and 6 His residues (6 × His). Rhinovirus 3C protease cleaves the indicated Gln-Gly bond in the linker sequence to produce substrates for MALDI MS analysis. (C) The amino acid sequence of VWF from Asp1596-Arg1668 is annotated to show the extent of num-bered α-helices and β-strands from a homology model of the A2 domain.1 The Tyr1605-Met1606 bond cleaved by ADAMTS13 is marked by arrowheads. Segments of VWF contained in GST constructs are indicated. (D) Substrates VWF73nl and VWF106nl represent N-terminal elongations of VWF64 and contain the sequence shown, annotated to indicate the extent of predicted α-helices and β-strands.1 The asterisk (*) indicates N-glycosylated residue Asn1574.2
ADAMTS13 and VWF substrates. (A) Active ADAMTS13 consists of a metalloprotease domain (M), a disintegrin-like domain (D), a thrombospondin type 1 repeat (TSP1, T), a Cys-rich domain (Cys, C), a spacer domain (Spacer, S), 7 additional TSP1 repeats (2-8), and 2 CUB domains. In addition to full-length ADAMTS13 (FL), truncated enzymes were constructed with stop codons after Gln289 (M), Gly385 (MD), Glu439 (MDT), Cys555 (MDTC), and Ala685 (MDTCS). All constructs contain a C-terminal V5 epitope and 6 × His tag. (B) GST fusion substrates consist of a GST moiety followed by the linker SDLEVLFQGPLGS, a segment of VWF domain A2 (VWFxxx), and 6 His residues (6 × His). Rhinovirus 3C protease cleaves the indicated Gln-Gly bond in the linker sequence to produce substrates for MALDI MS analysis. (C) The amino acid sequence of VWF from Asp1596-Arg1668 is annotated to show the extent of num-bered α-helices and β-strands from a homology model of the A2 domain.1 The Tyr1605-Met1606 bond cleaved by ADAMTS13 is marked by arrowheads. Segments of VWF contained in GST constructs are indicated. (D) Substrates VWF73nl and VWF106nl represent N-terminal elongations of VWF64 and contain the sequence shown, annotated to indicate the extent of predicted α-helices and β-strands.1 The asterisk (*) indicates N-glycosylated residue Asn1574.2
Cleavage of C-terminal deletion substrates. GST-VWF46, GST-VWF35, and GST-VWF28 (60-100 nM) were incubated with the indicated proteases (1 nM) for 5 hours or 24 hours without (−) or with (+) 10 mM EDTA. Substrates and 28-kDa cleavage products ( ) were detected by gel electrophoresis and Western blotting with anti-GST antibody. The blots for GST-VWF28 were exposed to film for 35 seconds; other blots were exposed to film for 15 seconds. Results are representative of 3 independent experiments.
) were detected by gel electrophoresis and Western blotting with anti-GST antibody. The blots for GST-VWF28 were exposed to film for 35 seconds; other blots were exposed to film for 15 seconds. Results are representative of 3 independent experiments.
Cleavage of C-terminal deletion substrates. GST-VWF46, GST-VWF35, and GST-VWF28 (60-100 nM) were incubated with the indicated proteases (1 nM) for 5 hours or 24 hours without (−) or with (+) 10 mM EDTA. Substrates and 28-kDa cleavage products ( ) were detected by gel electrophoresis and Western blotting with anti-GST antibody. The blots for GST-VWF28 were exposed to film for 35 seconds; other blots were exposed to film for 15 seconds. Results are representative of 3 independent experiments.
) were detected by gel electrophoresis and Western blotting with anti-GST antibody. The blots for GST-VWF28 were exposed to film for 35 seconds; other blots were exposed to film for 15 seconds. Results are representative of 3 independent experiments.
Internal deletion substrates
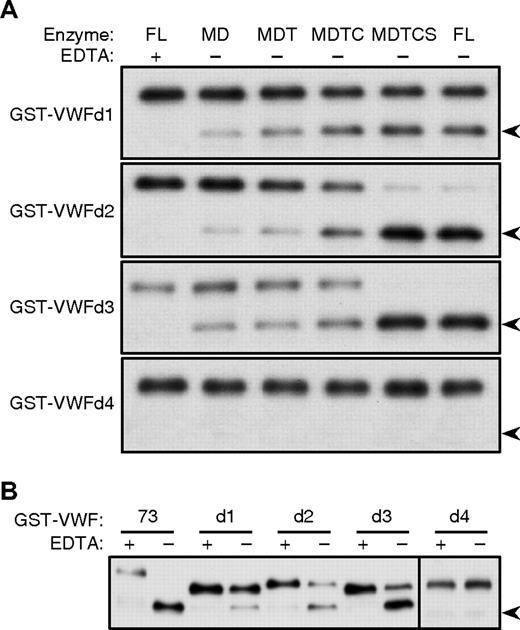
To validate the results obtained with C-terminal truncations, substrates based on GST-VWF73 were prepared with internal deletions (Figure 1C). GST-VWFd1 lacks residues Ile1642-Arg1659, which are missing from GST-VWF46, but preserves the C-terminal Glu1660-Arg1668 segment that is required although not sufficient for binding to the ADAMTS13 spacer domain.17-19 GST-VWFd1 was cleaved similarly by all ADAMTS13 variants tested (Figure 3A). The slightly faster cleavage by MDTC and longer enzymes suggests that GST-VWFd1 does not interact strongly with ADAMTS13 domains distal to MD. GST-VWFd2 lacks residues Ile1642-Gln1652 and compared with GST-VWFd1 contains a longer C-terminal segment of VWF domain A2, Asp1653-Arg1668. GST-VWFd2 was cleaved rapidly by MDTCS and full-length ADAMTS13, but was cleaved more slowly by enzymes that lack the spacer domain such as MD, MDT, and MDTC. Thus, residues Asp1653-Arg1668 restore some dependence of substrate cleavage on the ADAMTS13 spacer domain. GST-VWFd3 lacks residues Gln1624-Arg1641, and the pattern of cleavage by variants of ADAMTS13 was similar to that observed for GST-VWFd2. GST-VWFd4 lacks residues Gly1609-Ile1623 and was not cleaved by any variant of ADAMTS13.
Cleavage of internal deletion substrates. Substrates (65 nM) were incubated (A) with the indicated recombinant proteases (2 nM) for 1 hour, or (B) with plasma ADAMTS13 (0.3 nM) for 2 hours, without (−) or with (+) 10 mM EDTA. Substrates and 28-kDa cleavage products ( ) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Results are representative of 3 independent experiments.
) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Results are representative of 3 independent experiments.
Cleavage of internal deletion substrates. Substrates (65 nM) were incubated (A) with the indicated recombinant proteases (2 nM) for 1 hour, or (B) with plasma ADAMTS13 (0.3 nM) for 2 hours, without (−) or with (+) 10 mM EDTA. Substrates and 28-kDa cleavage products ( ) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Results are representative of 3 independent experiments.
) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Results are representative of 3 independent experiments.
Qualitatively similar results were obtained when these substrates were incubated with ADAMTS13 in human plasma. Under conditions such that GST-VWF73 was cleaved completely, the extent to which other substrates were cleaved decreased in the following order: GST-VWFd3 (∼ 80%), GST-VWFd2 (∼ 50%), GST-VWFd1 (∼ 20%), and GST-VWFd4 (0%; Figure 3B).
Kinetic analysis of VWF-ADAMTS13 interactions
The Western blotting data provide some insight into how ADAMTS13 recognizes its substrates, but the method is inherently imprecise and cannot give precise information about reaction rates. Therefore, we used a MALDI-MS assay for quantitative kinetic analysis. GST fusion substrates were incubated with a recombinant rhinovirus 3C protease to remove the GST moiety (Figure 1B). Cleavage of the resultant peptides by ADAMTS13 generates an N-terminal 1615-Da product that was detected and quantitated by MALDI-TOF MS.17 Using this assay, specificity constants (kcat/Km) were determined from initial reaction rates. In general, the relative cleavage rates based on comparisons of specificity constants (Table 1) are consistent with the qualitative results obtained by Western blotting for the corresponding GST fusion substrates (Figures 2,3).
Specificity constants for ADAMTS13 variants and VWF substrates
| Substrate . | ADAMTS13 construct, kcat/Km × 104 M−1s−1 . | ||||
|---|---|---|---|---|---|
| MD . | MDT . | MDTC . | MDTCS . | FL . | |
| VWF73 | 0.61 ± 0.02 | 0.80 ± 0.05 | 8.1 ± 0.5 | 205 ± 66* | 75 ± 20* |
| VWF64 | 0.61 ± 0.04 | 0.95 ± 0.02 | 6.4 ± 0.2 | 10 ± 3* | 5 ± 3* |
| VWF46 | 0.022 ± 0.001 | 0.29 ± 0.02 | 0.90 ± 0.04 | 0.98 ± 0.02 | 1.02 ± 0.06 |
| VWF35 | 0.044 ± 0.004 | 0.34 ± 0.03 | 0.90 ± 0.05 | 1.12 ± 0.02 | 1.2 ± 0.1 |
| VWF28 | 0.047 ± 0.002 | 0.06 ± 0.004 | 0.17 ± 0.09 | 0.21 ± 0.01 | 0.2 ± 0.01 |
| VWFd1 | 0.58 ± 0.02 | 0.7 ± 0.1 | 1.1 ± 0.1 | 2.4 ± 0.7 | |
| VWFd2 | 0.46 ± 0.03 | 0.9 ± 0.1 | 2.6 ± 0.2 | 8.4 ± 1.7 | |
| VWFd3 | 0.81 ± 0.04 | 0.76 ± 0.04 | 10 ± 0.3 | 25 ± 5.5 | |
| Substrate . | ADAMTS13 construct, kcat/Km × 104 M−1s−1 . | ||||
|---|---|---|---|---|---|
| MD . | MDT . | MDTC . | MDTCS . | FL . | |
| VWF73 | 0.61 ± 0.02 | 0.80 ± 0.05 | 8.1 ± 0.5 | 205 ± 66* | 75 ± 20* |
| VWF64 | 0.61 ± 0.04 | 0.95 ± 0.02 | 6.4 ± 0.2 | 10 ± 3* | 5 ± 3* |
| VWF46 | 0.022 ± 0.001 | 0.29 ± 0.02 | 0.90 ± 0.04 | 0.98 ± 0.02 | 1.02 ± 0.06 |
| VWF35 | 0.044 ± 0.004 | 0.34 ± 0.03 | 0.90 ± 0.05 | 1.12 ± 0.02 | 1.2 ± 0.1 |
| VWF28 | 0.047 ± 0.002 | 0.06 ± 0.004 | 0.17 ± 0.09 | 0.21 ± 0.01 | 0.2 ± 0.01 |
| VWFd1 | 0.58 ± 0.02 | 0.7 ± 0.1 | 1.1 ± 0.1 | 2.4 ± 0.7 | |
| VWFd2 | 0.46 ± 0.03 | 0.9 ± 0.1 | 2.6 ± 0.2 | 8.4 ± 1.7 | |
| VWFd3 | 0.81 ± 0.04 | 0.76 ± 0.04 | 10 ± 0.3 | 25 ± 5.5 | |
Reactions were performed at room temperature and products were analyzed by MALDI-TOF MS. ADAMTS13 construct M did not cleave any substrate, and no other ADAMTS13 construct cleaved substrate VWFd4 or VWFd5. Values (± SD) are based on 4 to 12 independent rate determinations.
Data from Gao et al are provided for comparison.17
The results show that several ADAMTS13 domains contribute to substrate recognition (Table 1). For example, full-length ADAMTS13 and MDTCS cleaved VWF73 rapidly, and the rate of cleavage decreased at least 20-fold for MDTC and another approximately 10-fold for MDT, indicating that both the spacer and Cys-rich domains interact with substrate. MDTC cleaved VWF64 approximately 7-fold more rapidly than VWF46, suggesting that the Cys-rich domain interacts with VWF residues Ile1642-Arg1659. This segment is also missing from substrate VWFd1. Enzymes MDTC and MDT cleaved VWFd1 at a similar rate, confirming the importance of Ile1642-Arg1659 for interacting with the Cys-rich domain.
VWFd2 lacks residues Ile1642-Gln1652, a slightly smaller deletion than in VWFd1. MDTC cleaved VWFd2 approximately 3-fold faster than MDT, and full-length ADAMTS13 cleaved VWFd2 approximately 3-fold than MDTC. These differences are relatively small, but suggest that the VWF segment present in VWFd2 and missing from VWFd1, Asp1653-Arg1659, may interact with both the Cys-rich and spacer domains.
The results with the smaller substrates VWF28 and VWF35 indicate that the first TSP1 domain of ADAMTS13 may contribute to substrate recognition. MD cleaved these substrates at a similar rate, but all enzymes containing the first TSP1 domain (MDT, MDTC, MDTCS, FL) cleaved VWF35 approximately 5-fold faster than VWF28. Therefore the segment present in VWF35 and missing from VWF28, Gln1624-Val1630, may interact with the TSP1 domain of ADAMTS13. A similar dependence of cleavage rate on the first TSP1 domain was evident for substrate VWF46. In addition, the deletion in substrate VWFd3, Gln1624-Arg1641, covers the segment implicated in binding to the TSP1 domain, and VWFd3 was cleaved similarly by MD and MDT.
The results with substrates VWF35, VWF46, and VWFd3 are consistent with the proposed interaction between the ADAMTS13 TSP1 domain and the VWF A2 domain. However, substrates VWF64, VWF73, VWFd1, and VWFd2 all contain Gln1624-Arg1641 but were cleaved almost equally well by MD and MDT (Table 1). It is possible that engagement of distal binding sites in these longer substrates obscures the role of relatively weak binding to the ADAMTS13 TSP1 domain.
Modularity of ADAMTS13 binding sites
None of the active ADAMTS13 variants (MD, MDT, MDTC, MDTCS, FL) cleaved VWF46 more rapidly than VWF35 (Table 1), suggesting that residues Gly1631-Arg1641 are dispensable. In addition, residues Gln1624-Arg1641 are present in VWF73 and absent from VWFd3, but both substrates were cleaved at similar rates by all ADAMTS13 variants tested. Therefore, residues Gln1624-Arg1641 are not essential for enzyme recognition, at least in the context of VWF73. Deletion of Gln1624-Arg1641 from VWFd3 markedly reduces the distance between the Tyr1605-Met1606 scissile bond and auxiliary binding sites for the ADAMTS13 Cys-rich and spacer domains, but this change in spacing had little effect on the rate of substrate cleavage. Therefore, ADAMTS13 does not require a fixed spatial relationship between the scissile bond and auxiliary sites on the substrate that engage exosites on the enzyme.
Fidelity of substrate cleavage
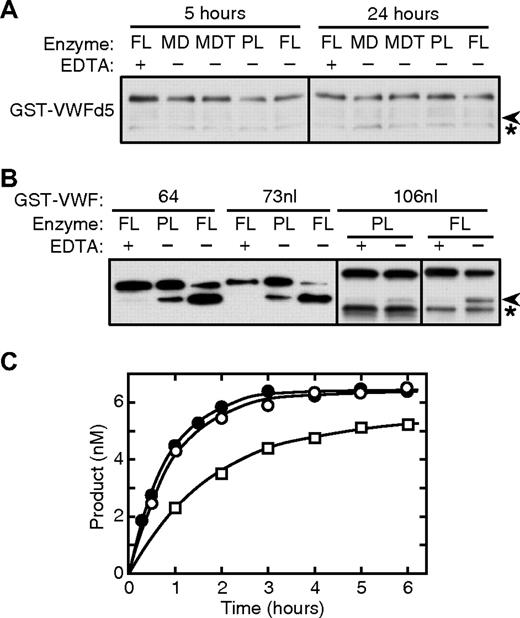
Amino acid residues of peptidyl substrates are conventionally numbered P1, P2, P3, and so on, from the scissile bond toward the N-terminus, and P1′, P2′, on the C-terminal side of the scissile bond.26 All substrates analyzed by mass spectrometry that were cleaved detectably by ADAMTS13 gave rise to the same 1615-Da product, demonstrating that all were cleaved at the expected P1-P1′ bond (Tyr1605-Met1606). Deletion of either residues P4′-P18′ (substrate VWFd4; Figure 3A) or P9-P2 (substrate VWFd5; Figure 4A) prevented cleavage. In addition, ADAMTS13 substrates beginning with Arg1597 are cleaved correctly.27 Therefore, specification of the cleavage site depends on sequences flanking the scissile bond between positions P9 (Arg1597) and P18′ (Ile1623; Figure 1).
Cleavage of N-terminal deletion and elongation substrates. (A) GST-VWFd5 (65 nM) was incubated at 37°C for the indicated time with 2 nM recombinant full-length ADAMTS13 (FL), truncated constructs MD or MDT, or 0.3 nM plasma ADAMTS13 (PL). No cleavage product ( ) was detected by gel electrophoresis and Western blotting with anti-GST antibody. (B) GST-VWF64, GST-VWF73nl, and GST-VWF106nl (65 nM) were incubated for 2 hours with 2 nM recombinant ADAMTS13 (FL), or for 6 hours with 0.3 nM plasma ADAMTS13 (PL). Substrates and cleavage products (
) was detected by gel electrophoresis and Western blotting with anti-GST antibody. (B) GST-VWF64, GST-VWF73nl, and GST-VWF106nl (65 nM) were incubated for 2 hours with 2 nM recombinant ADAMTS13 (FL), or for 6 hours with 0.3 nM plasma ADAMTS13 (PL). Substrates and cleavage products ( ) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Asterisks (*) indicates nonspecific bands observed reproducibly for GST-VWFd5 and GST-VWF106nl. Results are representative of 3 independent experiments. (C) Time course for cleavage of 6.8 nM GST-VWF64 (○), GST-VWF73nl (●), and GST-VWF106nl (□) by 1 nM ADAMTS13 at 37°C. Cleavage products were quantitated by an ELISA method. Data points represent the mean values for 2 independent experiments; the range was 2% to 9% of the mean.
) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Asterisks (*) indicates nonspecific bands observed reproducibly for GST-VWFd5 and GST-VWF106nl. Results are representative of 3 independent experiments. (C) Time course for cleavage of 6.8 nM GST-VWF64 (○), GST-VWF73nl (●), and GST-VWF106nl (□) by 1 nM ADAMTS13 at 37°C. Cleavage products were quantitated by an ELISA method. Data points represent the mean values for 2 independent experiments; the range was 2% to 9% of the mean.
Cleavage of N-terminal deletion and elongation substrates. (A) GST-VWFd5 (65 nM) was incubated at 37°C for the indicated time with 2 nM recombinant full-length ADAMTS13 (FL), truncated constructs MD or MDT, or 0.3 nM plasma ADAMTS13 (PL). No cleavage product ( ) was detected by gel electrophoresis and Western blotting with anti-GST antibody. (B) GST-VWF64, GST-VWF73nl, and GST-VWF106nl (65 nM) were incubated for 2 hours with 2 nM recombinant ADAMTS13 (FL), or for 6 hours with 0.3 nM plasma ADAMTS13 (PL). Substrates and cleavage products (
) was detected by gel electrophoresis and Western blotting with anti-GST antibody. (B) GST-VWF64, GST-VWF73nl, and GST-VWF106nl (65 nM) were incubated for 2 hours with 2 nM recombinant ADAMTS13 (FL), or for 6 hours with 0.3 nM plasma ADAMTS13 (PL). Substrates and cleavage products ( ) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Asterisks (*) indicates nonspecific bands observed reproducibly for GST-VWFd5 and GST-VWF106nl. Results are representative of 3 independent experiments. (C) Time course for cleavage of 6.8 nM GST-VWF64 (○), GST-VWF73nl (●), and GST-VWF106nl (□) by 1 nM ADAMTS13 at 37°C. Cleavage products were quantitated by an ELISA method. Data points represent the mean values for 2 independent experiments; the range was 2% to 9% of the mean.
) were detected by gel electrophoresis and Western blotting with anti-GST antibody. Asterisks (*) indicates nonspecific bands observed reproducibly for GST-VWFd5 and GST-VWF106nl. Results are representative of 3 independent experiments. (C) Time course for cleavage of 6.8 nM GST-VWF64 (○), GST-VWF73nl (●), and GST-VWF106nl (□) by 1 nM ADAMTS13 at 37°C. Cleavage products were quantitated by an ELISA method. Data points represent the mean values for 2 independent experiments; the range was 2% to 9% of the mean.
Role of N-terminal sequences
VWF sequences on the N-terminal side of Asp1596 have been reported to slow the rate of substrate cleavage by ADAMTS13 or to have no significant effect. For example, compared with GST-VWF73, which contains residues Asp1596-Arg1668 (Figure 1C), inserting more N-terminal VWF sequence up to Asp1587, Glu1554, or Asp1459 caused a progressive decrease in the rate of cleavage.19 On the other hand, VWF substrates containing residues Ser1593-Arg1668 (VWF76), Glu1554-Arg1668 (VWF115), and Glu1554-Glu1874 (VWF115-A3) were cleaved with similar specificity constants of 6.4 to 7.8 × 104 M−1s−1.28 The variation in these results may reflect other differences in assay conditions.
To examine the role of N-terminal sequences in VWF cleavage, we constructed 2 GST fusion substrates with N-terminal extensions to Asp1587 (GST-VWF73nl) or Glu1554 (GST-VWF106nl) but lacking the C-terminal Glu1660-Arg1668 segment (Figure 1D). Eliminating the distal segment reduces the dependence of cleavage on high-affinity binding to the ADAMTS13 spacer, which could facilitate the detection of effects caused by N-terminal sequences.
When assessed by Western blotting, recombinant or plasma ADAMTS13 cleaved GST-VWF64 and GST-VWF73nl at comparable rates, but cleaved GST-VWF106nl more slowly (Figure 4B). After removal of the GST moiety, these substrates were incubated with recombinant ADAMTS13 and the products analyzed by MALDI-TOF MS.17 VWF73nl was cleaved at the expected Tyr1605-Met1606 bond with kcat/Km of approximately 5.3 × 104 M−1s−1. This value is comparable with the kcat/Km of approximately 5 × 104 M−1s−1 for VWF64 (Table 1), indicating that adding Asp1587-Gly1595 does not alter substrate recognition significantly.
Although VWF106nl was cleaved by ADAMTS13, the N-terminal 6333-Da product was not detected by MALDI-TOF MS, apparently because this large peptide did not ionize under the conditions used. Therefore, the cleavage of GST-VWF106nl was analyzed by an ELISA method. In preliminary experiments, GST fusion substrates bound to microtiter plates were cleaved much more slowly than the same substrates in solution. Therefore, assays were performed in solution and the products analyzed subsequently by ELISA (Figure 4C). Reaction rates by this ELISA method were 6-fold faster than by the MALDI method, reflecting the different reaction temperatures of 37°C (ELISA) versus 25°C (MALDI). Using the ELISA, the values of kcat/Km were 32.7 ± 1.5 × 104 M−1s−1 for GST-VWF64, 29.0 ± 1.7 for GST-VWF73nl, and 14.8 ± 0.7 × 104 M−1s−1 for GST-VWF106nl. These results confirm that substrates beginning with Asp1587 or Asp1596 are cleaved similarly, and indicate that adding N-terminal residues to Glu1554 reduces the rate of cleavage approximately 2-fold.
Discussion
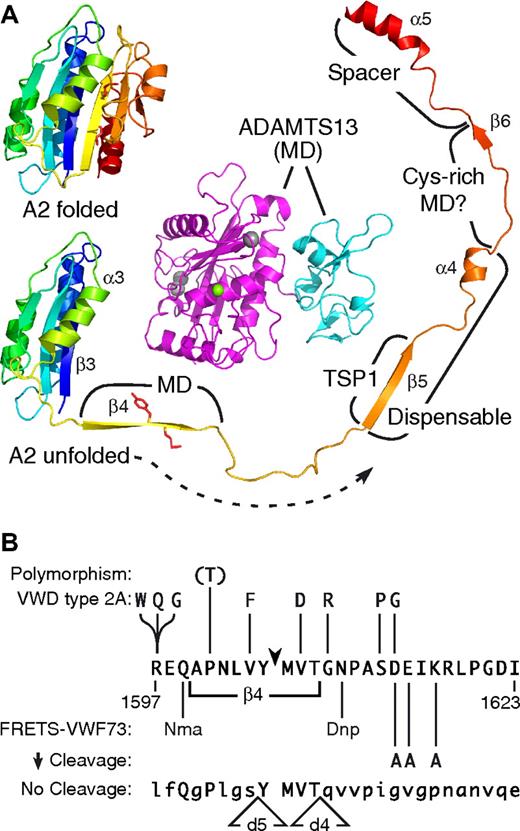
Our results support a linear correspondence between domains of ADAMTS13 and their interaction sites in VWF domain A2 (Figure 5A). The best characterized of these interactions involves the C-terminal end of the VWF A2 domain, particularly helix α5,19 and an exosite in the ADAMTS13 spacer domain.17 Kinetic analysis suggests that the spacer domain interacts with VWF segment Asp1653-Arg1668, increasing the rate of substrate cleavage approximately 10-fold to approximately 25-fold (Table 1). This conclusion is consistent with the effects of charge-to-alanine substitutions in this segment: mutation of Asp1653 or Asp1663 reduced the rate of substrate cleavage, whereas mutation of Glu1655 slightly increased cleavage.18 Ala substitutions at Arg1659, Glu1660, and Arg1668 had no significant effect, suggesting that these residues contribute little to substrate-exosite interactions.18
ADAMTS13-VWF interactions. (A) A homology model of the ADAMTS13 metalloprotease (magenta) and disintegrin-like domains (cyan) is shown (MD) to provide a sense of scale, with active site Zn2+ ion (green) and 3 structural Ca2+ ions (gray) as spheres. The VWF A2 domain is predicted to consist of a 6-stranded β-sheet surrounded by 5 α-helices. Residues Tyr1605-Met1606 (side chains in red) are buried in strand β4. Exposure of this bond to ADAMTS13 requires substantial unfolding of domain A2; more distal segments that interact with specific domains of ADAMTS13 are labeled. The locations of these ADAMTS13 domains relative to the MD moiety are not known. Deletion of strand β5 through helix α4 (dispensable) has a minimal effect on the rate of substrate cleavage. Molecular graphics prepared with PyMOL (DeLano Scientific, Palo Alto, CA). (B) The minimal segment of VWF domain A2 that ADAMTS13 is known to cleave consists of residues Arg1597-Ile1623. VWD type 2A mutations that increase cleavage are shown. A VWF polymorphism that may not impair cleavage is shown in parentheses to indicate that cleavability of VWF(1601T) has not been studied directly. Synthetic substrate FRETS-VWF73 is cleaved rapidly with replacement of side chains of Gln1599 by N-methylanthranylate and Asn1610 by 2,4-dinitrophenyl.29 Alanine substitutions at 3 positions reduce the rate of VWF cleavage.28 Substrates with deletions VWFd4 and VWFd5 (Figure 1C) have the sequences shown, with altered residues in lowercase and preserved residues in uppercase, and neither substrate is cleaved detectably.
ADAMTS13-VWF interactions. (A) A homology model of the ADAMTS13 metalloprotease (magenta) and disintegrin-like domains (cyan) is shown (MD) to provide a sense of scale, with active site Zn2+ ion (green) and 3 structural Ca2+ ions (gray) as spheres. The VWF A2 domain is predicted to consist of a 6-stranded β-sheet surrounded by 5 α-helices. Residues Tyr1605-Met1606 (side chains in red) are buried in strand β4. Exposure of this bond to ADAMTS13 requires substantial unfolding of domain A2; more distal segments that interact with specific domains of ADAMTS13 are labeled. The locations of these ADAMTS13 domains relative to the MD moiety are not known. Deletion of strand β5 through helix α4 (dispensable) has a minimal effect on the rate of substrate cleavage. Molecular graphics prepared with PyMOL (DeLano Scientific, Palo Alto, CA). (B) The minimal segment of VWF domain A2 that ADAMTS13 is known to cleave consists of residues Arg1597-Ile1623. VWD type 2A mutations that increase cleavage are shown. A VWF polymorphism that may not impair cleavage is shown in parentheses to indicate that cleavability of VWF(1601T) has not been studied directly. Synthetic substrate FRETS-VWF73 is cleaved rapidly with replacement of side chains of Gln1599 by N-methylanthranylate and Asn1610 by 2,4-dinitrophenyl.29 Alanine substitutions at 3 positions reduce the rate of VWF cleavage.28 Substrates with deletions VWFd4 and VWFd5 (Figure 1C) have the sequences shown, with altered residues in lowercase and preserved residues in uppercase, and neither substrate is cleaved detectably.
Based on deletion mutagenesis (Table 1), the ADAMTS13 Cys-rich domain interacts with the VWF segment Ile1642-Gln1652, indicating that the Cys-rich and spacer domains bind adjacent sequences on the substrate. Similarly, the first TSP1 domain of ADAMTS13 appears to bind the more proximal VWF segment Gln1624-Val1630. Each of these exosite-substrate interactions increases the rate of cleavage approximately 3-fold to approximately 10-fold depending on context, and they cooperate to achieve a rate acceleration of approximately 300-fold for the cleavage of VWF73 by MDTCS (Table 1). These conclusions are consistent with the observation that independently expressed TSP1, Cys-rich, and spacer domains can each bind to GST-VWF73 with Kd values of approximately 0.1 μM, whereas an ADAMTS13 fragment composed of domains DTCS binds with a much lower Kd of 13 nM.12
This model needs to incorporate one additional feature of the kinetics analysis. Enzyme MD cleaves VWF64 approximately 27-fold faster than VWF46 (Table 1), suggesting that the Ile1642-Arg1659 segment of VWF interacts with MD in addition to the Cys-rich domain. Such a dual interaction could be accommodated if the Cys-rich domain were juxtaposed to MD. Alternatively, truncating ADAMTS13 after the D domain may expose a binding site for VWF64 that is normally buried by the TSP1 domain, so that the unusual properties of MD may not give reliable insight into the mechanism of substrate recognition. At present, we have no structural data for ADAMTS13 to distinguish among these possibilities.
In addition to binding at exosites in noncatalytic domains, the unfolded VWF A2 domain must make the Tyr1605-Met1606 bond accessible to the ADAMTS13 metalloprotease domain, and proper cleavage depends on structural features between substrate residues P9-P18′ (Arg1597 and Ile1623; Figure 5B). Adding residues to P19 (Asp1587) had no effect, indicating that N-terminal sequences far from the scissile bond are not recognized directly by ADAMTS13. Longer N-terminal extensions to Glu1554 (VWF106nl; Figure 4C) or beyond are inhibitory,19,28 probably because longer substrates acquire secondary structure that impedes access to the scissile bond. For example, NMR spectroscopy suggests that VWF73 has an extended conformation in solution,30 whereas circular dichroism spectroscopy indicates that the intact A2 domain contains extensive β-sheet and α-helical structure.31 However, substrate cleavage is abolished by deletion of the segments Arg1597-Val1604 (substrate VWFd5) or Gly1609-Ile1623 (substrate VWFd4) on either side of the Tyr1605-Met1606 bond.
Within or near these short segments, some residues can be varied without preventing cleavage (Figure 5B). For example, VWD type 2A mutations between P9-P9′ can increase the cleavage of mutant VWF.28,32-35 A polymorphism (Pro/Thr1601) has been described at position P5, although whether it affects ADAMTS13 cleavage is not known.36 ADAMTS13 readily cleaves the substrate FRETS-VWF73 despite the presence of N-methylanthranylate and 2,4-dinitrophenyl substituents at positions P7 and P5′, respectively.29 Finally, Ala substitutions at P9′, P10′, and P12′ slow the rate but do not alter the site of cleavage.28 Therefore, critical determinants of substrate cleavage by the ADAMTS13 MD domains probably reside between P4-P7′, but may extend as far as P18′.
Our most unexpected finding was that distinct ADAMTS13 binding sites on VWF domain A2 are, to some extent, modular and portable. In particular, efficient substrate cleavage does not depend on a fixed spacing between the scissile bond and distal sequences that bind the ADAMTS13 Cys-rich and spacer domains. The ability to accommodate a large change in substrate structure suggests that ADAMTS13 is relatively flexible and can alter the relationships among the distal domains that bind VWF. Similar modularity has been demonstrated for the related ADAMTS4 and ADAMTS5 proteases, which use their Cys-rich and spacer domains to bind glycosaminoglycans chains on aggrecan while their metalloprotease domains cleave nearby peptide bonds.37-39
The mobility of ADAMTS13 structural domains may be generally important for substrate recognition. It seems likely that fluid shear stress in vivo induces a spectrum of conformational changes in VWF rather than one well-defined substrate structure. In that case, stretched VWF multimers may expose a varying number of binding sites for ADAMTS13, arrayed in a variety of contexts and combinations. Sites within VWF domain A2 interact with proximal MDTCS domains (Table 1).17 However, the cleavage of VWF multimers also requires interactions with distal TSP1 and CUB domains,12-14 and these interactions may involve binding sites outside of VWF domain A2. Further mutagenesis of both ADAMTS13 and VWF should be able to identify and characterize these interactions, providing a framework for understanding how ADAMTS13 regulates VWF-dependent platelet adhesion to promote hemostasis and prevent thrombosis.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health (Bethesda, MD) grants HL72917 and HL89746 (J.E.S.) and by an American Heart Association (Dallas, TX) National Scientist Development Award 0530110N (P.J.A.).
National Institutes of Health
Howard Hughes Funding
Authorship
Contribution: W.G. designed and performed research, analyzed and interpreted data, and wrote the paper; P.J.A. analyzed and interpreted data; and J.E.S. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: J.E.S. is a consultant for Baxter BioSciences. The remaining authors declare no competing financial interests.
Correspondence: J. Evan Sadler, Howard Hughes Medical Institute, Washington University School of Medicine, 660 S Euclid Ave, Box 8022, St Louis, MO 63110; e-mail: esadler@wustl.edu.