Abstract
Central nervous system (CNS) involvement by Hodgkin lymphoma (HL) is rare. As a result, there is limited guidance for clinicians on how to manage these patients. Detailed information was collected on 16 patients, the largest number to date, with meningeal or parenchymal CNS-HL confirmed by histopathology (15) or CSF (1). Eight patients presented with CNS-HL at diagnosis, 2 of whom had isolated CNS disease, while 8 patients developed CNS-HL at relapse. Patients received a range of treatments including surgery or radiation alone, radiation with chemotherapy, or chemotherapy alone. Median overall survival for all 16 patients was 60.9 months from first diagnosis of HL (systemic or CNS) and 43.8 months from diagnosis of CNS-HL. Although a majority of patients have died, long-term survival is possible in patients who achieve a complete response to treatment, particularly those who present with CNS involvement or involvement of the CNS is the sole site of relapsed disease.
Introduction
Hodgkin lymphoma (HL), unlike non-Hodgkin lymphoma, rarely involves the central nervous system (CNS). The incidence has been reported as 0.2% to 0.5% of all HL cases, but a recent review of 14 868 patients identified only 2 cases of HL involving the CNS.1-3 Most reports consist of single cases or small case series highlighting our limited knowledge about the clinical characteristics, prognosis, and management of this neurologic complication of HL.4-8 Consequently, there is no consensus to guide therapeutic decision-making. We gathered data on CNS-HL cases from member sites of the International Primary CNS Lymphoma Collaborative Group (IPCG) in order to better understand the presentation, prognosis, and therapeutic options for patients with CNS-HL.
Methods
We collected data on 16 adult patients diagnosed with CNS-HL from 1972 to 2008 at IPCG member sites. Patients were included if there was either brain parenchymal or meningeal HL confirmed by histology or cerebrospinal fluid (CSF) analysis. Pathology reports were reviewed for each case that underwent tissue sampling. Detailed information was collected by chart review after approval was received from appropriate institutional review boards. Institutional review board approval was obtained from all participating institutions except where approval was not required as data were sent anonymously. The Kaplan-Meier method was used to estimate median overall survival.
Results and discussion
We identified 16 adult patients with CNS involvement by HL from 13 centers in 6 countries (Table 1). All 16 patients had classical HL histology based on the World Health Organization classification system: 7 classical HL not otherwise specified (NOS), 7 nodular sclerosis, and 2 mixed cellularity (Figure 1). Fifteen of 16 patients had histologic confirmation of CNS-HL by biopsy, resection, or autopsy and 1 patient had cells in the CSF that stained strongly for CD30 in the setting of concurrent systemic HL. Two patients had pathologically confirmed primary CNS-HL without evidence of systemic HL. Four patients had Epstein-Barr virus (EBV) associated disease, 5 patients were negative when tested, and the remaining patients either did not have testing or the results were unknown. Six patients had a history of immunosuppression/autoimmune disease not related to HIV infection. The median age at onset of CNS-HL was 45 years. CNS-HL was discovered simultaneously with or prior to systemic HL in 6 patients. Eight patients developed CNS-HL a median of 11.7 months (range 6.9-189 months) after discovery of systemic HL. Five of these 8 had no evidence of systemic HL when their CNS disease was discovered. The most common presenting symptoms included pain/sensory symptoms (5/16), weakness (3/16), altered mental status (3/16), headache (3/16), and seizure (3/16). Five patients had B symptoms at the time of CNS-HL diagnosis, all of whom had concurrent systemic disease.
Patient characteristics
| . | Timing of CNS-HL diagnosis* . | Sex . | Year of CNS-HL Diagnosis . | Age at CNS-HL diagnosis, y . | Type of HL . | Stage at first HL diagnosis¶ . | Stage at CNS-HL diagnosis¶ . | Confirmation of CNS-HL diagnosis . | Chemotherapy for CNS-HL . | Radiation for CNS-HL (dose) . | Response to first-line CNS treatment . | Status at last follow-up (survival since HL diagnosis) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Primary‡ | F | 2000 | 58 | CHL NOS | IE | IE | Resection | None | WBRT (35 Gy) | CR | Alive (90.3+ mo) in CR |
| 2 | Primary‡ | F | 2008 | 60 | NS | IE | IE | Resection | None | WBRT (to begin) | NA | Alive (1+ mo) |
| 3 | Initial§ | M | 2004 | 46 | CHL NOS | IE | IE | Biopsy | Unknown | Unknown | Unknown | Alive (2.1+ mo) |
| 4 | Initial†§ | M | 2007 | 72 | CHL NOS | IE | IE | Resection | None (surgery alone) | None (surgery alone) | NA | Alive (5.4+ mo) in CR |
| 5 | Initial§ | F | 1980 | 37 | MC | IE | IE B | Biopsy | MVPP | WBRT | PR | Dead from systemic PD (15.5 mo) |
| 6 | Initial† | M | 1997 | 19 | NS | IV | IV | Biopsy | Stamford V + PBSCT + it MTX | PBRT (36 Gy) | CR | Alive (128.5+ mo) in CR |
| 7 | Initial† | M | 2007 | 44 | CHL NOS | IV | IV | CSF | ABVD | Radiosurgery | PR | Dead from CNS PD (4.5 mo) |
| 9 | Initial† | F | 2003 | 23 | NS | IV | IV B | Resection | ABVD + it thiotepa | PBRT (36 Gy) | CR | Alive (53.8+ mo) in CR |
| 10 | Relapse | M | 2003 | 61 | CHL NOS | IV | IE | Resection | BVAM, BEAM + PBSCT + it MTX | None | CR | Dead in CR (58.8 mo) |
| 11 | Relapse§ | F | 2004 | 41 | CHL NOS | IV | IE | Biopsy | ifosfamide | WBRT (36 Gy) | CR | Alive (35.5 mo) in CR |
| 12 | Relapse§ | M | 1998 | 70 | NS | III | IE | Biopsy | None | Radiosurgery | PR | Dead from systemic PD (23.3 mo) |
| 13 | Relapse† | F | 1988 | 29 | NS | II | IE | Biopsy | MOPP | WBRT (45 Gy) | CR | Alive (273+ mo) in CR |
| 19 | Relapse§ | M | 2005 | 32 | CHL NOS | III | IE | Biopsy | rituximab, IVAC, high-dose methotrexate | None | CR | Dead from systemic PD (27.8 mo) |
| 14 | Relapse | M | 2000 | 65 | NS | Unknown | IV | Resection | None | WBRT (10.8 Gy with 23.4 Gy boost) | CR | Dead in CR (235.3 mo) |
| 15 | Relapse | F | 1986 | 29 | NS | II | IV B | Autopsy | lomustine, procarbazine, prednisone, cytoxan + it cytarabine | None | PD | Dead from PE with systemic and CNS PD (60.9 mo) |
| 16 | Relapse† | M | 2004 | 59 | MC | IV | IV B | Biopsy | Bonner Protocol | None | CR | Dead from systemic PD (18.4 mo) |
| . | Timing of CNS-HL diagnosis* . | Sex . | Year of CNS-HL Diagnosis . | Age at CNS-HL diagnosis, y . | Type of HL . | Stage at first HL diagnosis¶ . | Stage at CNS-HL diagnosis¶ . | Confirmation of CNS-HL diagnosis . | Chemotherapy for CNS-HL . | Radiation for CNS-HL (dose) . | Response to first-line CNS treatment . | Status at last follow-up (survival since HL diagnosis) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Primary‡ | F | 2000 | 58 | CHL NOS | IE | IE | Resection | None | WBRT (35 Gy) | CR | Alive (90.3+ mo) in CR |
| 2 | Primary‡ | F | 2008 | 60 | NS | IE | IE | Resection | None | WBRT (to begin) | NA | Alive (1+ mo) |
| 3 | Initial§ | M | 2004 | 46 | CHL NOS | IE | IE | Biopsy | Unknown | Unknown | Unknown | Alive (2.1+ mo) |
| 4 | Initial†§ | M | 2007 | 72 | CHL NOS | IE | IE | Resection | None (surgery alone) | None (surgery alone) | NA | Alive (5.4+ mo) in CR |
| 5 | Initial§ | F | 1980 | 37 | MC | IE | IE B | Biopsy | MVPP | WBRT | PR | Dead from systemic PD (15.5 mo) |
| 6 | Initial† | M | 1997 | 19 | NS | IV | IV | Biopsy | Stamford V + PBSCT + it MTX | PBRT (36 Gy) | CR | Alive (128.5+ mo) in CR |
| 7 | Initial† | M | 2007 | 44 | CHL NOS | IV | IV | CSF | ABVD | Radiosurgery | PR | Dead from CNS PD (4.5 mo) |
| 9 | Initial† | F | 2003 | 23 | NS | IV | IV B | Resection | ABVD + it thiotepa | PBRT (36 Gy) | CR | Alive (53.8+ mo) in CR |
| 10 | Relapse | M | 2003 | 61 | CHL NOS | IV | IE | Resection | BVAM, BEAM + PBSCT + it MTX | None | CR | Dead in CR (58.8 mo) |
| 11 | Relapse§ | F | 2004 | 41 | CHL NOS | IV | IE | Biopsy | ifosfamide | WBRT (36 Gy) | CR | Alive (35.5 mo) in CR |
| 12 | Relapse§ | M | 1998 | 70 | NS | III | IE | Biopsy | None | Radiosurgery | PR | Dead from systemic PD (23.3 mo) |
| 13 | Relapse† | F | 1988 | 29 | NS | II | IE | Biopsy | MOPP | WBRT (45 Gy) | CR | Alive (273+ mo) in CR |
| 19 | Relapse§ | M | 2005 | 32 | CHL NOS | III | IE | Biopsy | rituximab, IVAC, high-dose methotrexate | None | CR | Dead from systemic PD (27.8 mo) |
| 14 | Relapse | M | 2000 | 65 | NS | Unknown | IV | Resection | None | WBRT (10.8 Gy with 23.4 Gy boost) | CR | Dead in CR (235.3 mo) |
| 15 | Relapse | F | 1986 | 29 | NS | II | IV B | Autopsy | lomustine, procarbazine, prednisone, cytoxan + it cytarabine | None | PD | Dead from PE with systemic and CNS PD (60.9 mo) |
| 16 | Relapse† | M | 2004 | 59 | MC | IV | IV B | Biopsy | Bonner Protocol | None | CR | Dead from systemic PD (18.4 mo) |
CHL-NOS indicates classical Hodgkin's lymphoma not otherwise specified; MC, mixed cellularity; NS, nodular sclerosis; IE, isolated brain disease (“Initial” patients subsequently developed systemic HL, “Relapse” patients had no evidence of systemic disease when the brain relapse was discovered); it, intrathecal; PBSCT, peripheral blood stem cell transplantation; PBRT, partial brain radiation therapy; WBRT, whole brain radiation therapy; PE, pulmonary embolism; CR, complete response; PR, partial response; PD, progressive disease; MVPP, mustine, vinblastine, procarbazine, prednisone; BVAM, carmustine, vincristine, cytarabine, and methotrexate; BEAM, carmustine, etoposide, cytarabine, and melphalan; IVAC, ifosfamide, etoposide, and high-dose cytarabine; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; CEP, lomustine, etoposide, prednisone; and Bonner Protocol, methotrexate, vincristine, ifosfamide, dexamethasone, procarbazine, vindesine, and cytarabine
Initial indicates CNS disease diagnosed prior to or concomitantly with systemic disease.
Dural based disease.
No evidence of systemic HL.
Had immune dysregulation.
Staging determined by Cotswolds Staging Classification.
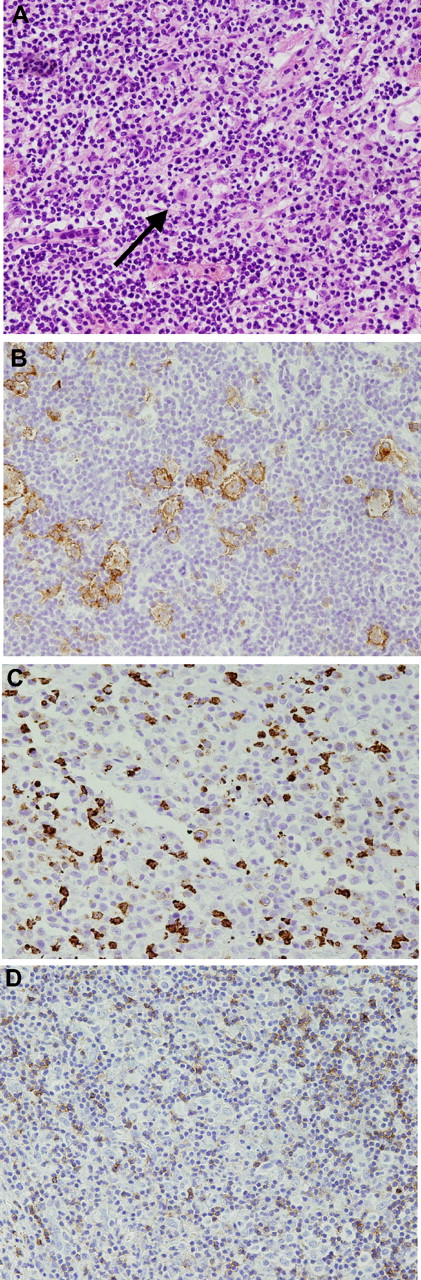
Histology from a patient with nodular sclerosis Hodgkin lymphoma involving the cerebellum. A Reed-Sternberg cell ( ) is seen in the center of the panel (40×, hematoxylin-eosin stain) (A). The large cells stained for CD30 (B). The cells are focally CD15 positive (C) and CD45 negative (D). All images were acquired at 40× magnification using an Olympus CX411 microscope and an Olympus DP70 camera with medium acquisition software (Melville, NY).
) is seen in the center of the panel (40×, hematoxylin-eosin stain) (A). The large cells stained for CD30 (B). The cells are focally CD15 positive (C) and CD45 negative (D). All images were acquired at 40× magnification using an Olympus CX411 microscope and an Olympus DP70 camera with medium acquisition software (Melville, NY).
Histology from a patient with nodular sclerosis Hodgkin lymphoma involving the cerebellum. A Reed-Sternberg cell ( ) is seen in the center of the panel (40×, hematoxylin-eosin stain) (A). The large cells stained for CD30 (B). The cells are focally CD15 positive (C) and CD45 negative (D). All images were acquired at 40× magnification using an Olympus CX411 microscope and an Olympus DP70 camera with medium acquisition software (Melville, NY).
) is seen in the center of the panel (40×, hematoxylin-eosin stain) (A). The large cells stained for CD30 (B). The cells are focally CD15 positive (C) and CD45 negative (D). All images were acquired at 40× magnification using an Olympus CX411 microscope and an Olympus DP70 camera with medium acquisition software (Melville, NY).
Ten patients (63%) had brain parenchymal disease and 5 (31%) had dural/meningeal-based lesions without parenchymal disease. Single parenchymal lesions involved the frontal lobe (2), parietal lobe (2), temporal lobe (2), and cerebellum (1). Multifocal disease was found in 3 patients, 1 of whom had brain, spinal cord, and leptomeningeal disease. All cases represented noncontiguous spread of HL except for one case that also involved the orbit where the origin of the lesion (ie, orbit or intracranial) was unclear. Lumbar puncture was performed in 9 patients. Two patients had atypical cells in cerebrospinal fluid (CSF; CD30+ in 1 case). Five of 9 patients had elevated protein.
Treatment for CNS-HL varied: surgery, radiation, or 1 of 8 different chemotherapy regimens was used. Radiographic response to any type of treatment was observed in 11 of 13 evaluable patients, including a complete response (CR) in 9. Six patients received chemotherapy and radiation resulting in 2 partial responses (PRs) and 4 CRs; 4 patients received chemotherapy alone resulting in 3 CRs and 1 patient with progressive disease (PD); 3 patients received radiation alone (2 whole brain radiation, 1 stereotactic radiosurgery), resulting in 2 CRs and 1 PRs; 1 patient underwent gross total resection (and reduction of chronic low-dose methotrexate for rheumatoid arthritis); and 1 patient had an unknown treatment history. Four patients received intrathecal chemotherapy with methotrexate, thiotepa, or cytarabine, and 2 patients received high-dose chemotherapy and peripheral blood stem cell transplantation.
Eight patients have died and the median overall survival for all 16 patients was 60.9 months (range 4.5-273 months) from the first diagnosis of HL (systemic or CNS). Median overall survival from the diagnosis of CNS-HL was 43.8 months (range 4.5-46.3 months). Five patients died from progressive HL (4 systemic, 1 CNS), 1 patient died of a pulmonary embolism with evidence of systemic and CNS-HL at autopsy, and 2 died from other causes in CR. Five of the 8 patients with CNS involvement at diagnosis are alive without evidence of disease progression at a median follow-up of 10 months (range 1-128 months), while only 2 of the 8 patients with CNS involvement at relapse are alive at 35.5 and 273 months of follow-up, perhaps suggesting a worse prognosis for these patients. However, median follow-up for patients with CNS-HL at initial diagnosis was 10 months versus 42.3 months in patients who presented at relapse. Therefore, longer follow-up is needed to confirm whether or not patients with cerebral disease at relapse fare worse. All evaluable patients alive at last follow up achieved a CR in the CNS to treatment.
The median age of our patients at CNS-HL diagnosis (45 years) was slightly older than earlier reports.2 Also unlike previous series in which mixed cellularity was the most commonly observed histology, we found no association with a particular HL histology.2,9
In most reported cases, CNS involvement occurred in the setting of relapsed systemic disease with up to 61% of cases having extraneural evidence of HL synchronous with CNS disease.1,2,9 However, 5 patients in our series were in systemic CR when CNS-HL was discovered and 8 patients presented with CNS-HL, including 2 with isolated CNS disease.
There are no known risk factors for the development of CNS spread in patients with HL. A role for immunosuppression and EBV infection in systemic HL as well as in primary CNS-HL has been suggested.10,11 Five of the 6 patients with immunosuppression/autoimmune disease had disease limited to the brain when CNS-HL was diagnosed, perhaps suggesting a predilection in these patients for the CNS. These patients had a variety of diagnoses and no one diagnosis stood out as being strongly associated with CNS-HL. One primary CNS-HL patient in this study was EBV positive but the second was negative. The lack of EBV testing in the remaining patients makes firm conclusions difficult regarding the role of EBV in promoting CNS disease. It is reasonable to consider EBV testing for patients with CNS-HL in order to clarify the role of EBV in disease pathogenesis.
The rarity of CNS-HL makes prospective studies difficult if not impossible. Although retrospective, our report is the largest series to date of CNS-HL cases using the most systematic method possible of collecting data on this rare complication. We were unable to perform central review of all CNS pathology specimens and immunophenotyping and molecular diagnostic testing have evolved over time so we cannot exclude misclassification of early cases. Nevertheless, all the CNS histology samples were reviewed by experienced hematopathologists at academic cancer centers and were determined to be HL.
The durable survival in several CNS-HL patients in our series is encouraging. All 7 evaluable surviving patients achieved a CR in the CNS to treatment, suggesting treatment with radiation and/or chemotherapy is warranted in appropriate cases and may be associated with a durable response. Patients with relapsed disease limited to the brain or with CNS involvement at initial diagnosis of HL may have a better prognosis but longer follow-up is needed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ji-Yeon Kim, MD (Department of Pathology, Massachusetts General Hospital) for help with Figure 1.
Authorship
Contribution: L.E.A., D.S., A.J.M.F., A.L., S.M., R.T., E.T., F.G., D.B., G.I., S.W., P.W., A.V., N.L.H., and T.T.B. all contributed patients to this series and reviewed the final manuscript. E.R.G. analyzed the information and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth R. Gerstner, MD, Stephen E. and Catherine Pappas Center for Neuro-Oncology, Yawkey 9E, Massachusetts General Hospital, 55 Fruit Street, Boston, Massachusetts 02114; e-mail: egerstner@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal