Abstract
Chemokine CXCL13, also known as BCA-1 (B cell–attracting chemokine-1) or BLC (B-lymphocyte chemoattractant), is a major regulator of B-cell trafficking. Hepatitis C virus (HCV) infection may be associated with B-cell dysfunction and lymphoproliferative disorders, including mixed cryoglobulinemia (MC). This study evaluates circulating levels of CXCL13 protein and specific mRNA expression in chronically HCV-infected patients with and without MC. Compared with healthy controls and HCV-infected patients without MC, CXCL13 serum levels were significantly higher in MC patients. The highest CXCL13 levels strongly correlated with active cutaneous vasculitis. CXCL13 gene expression in portal tracts, isolated from liver biopsy tissues with laser capture microdissection, showed enhanced levels of specific mRNA in MC patients with active cutaneous vasculitis. Specific CXCL13 gene mRNA expression was also up-regulated in skin tissue of these patients. These findings paralleled specific deposits of CXCL13 protein both in the liver and in the skin. Our results indicate that up-regulation of CXCL13 gene expression is a distinctive feature of HCV-infected patients. Higher levels of this chemokine in the liver as well as in the skin of patients with active MC vasculitis suggest a possible interrelation between these biologic compartments.
Introduction
Chronic active liver disease (CALD) is an inflammatory disorder recognizing several etiologies and different pathogenetic mechanisms.1 Within inflamed liver, there is an accumulation of lymphoid and myeloid cells, including T and B cells.2 Local activation of these cells is thought to be essential in perpetuating the chronic inflammatory process and accelerating liver damage.3 T and B cells in CALD frequently accumulate in the portal tracts and organize follicle-like structures, with features of germinal centers (GCs).4 In these sites, local differentiation of follicular dendritic cells, plasma cells, and antibody production may occur.5
In hepatitis C virus (HCV) infection, distinct B-cell expansions contribute to the formation of intraportal follicle-like structures.6,7 Circulating B-cell clonotypes have been suggested to be of hepatic origin, indicating that IgH VDJ mutational activity is up-regulated in the hepatic microenvironment.8 Sequence analyses of IgH CDR-3 gene segments of intraportal B-cell clonalities have revealed a wide range of variations, possibly implying that they are the result of an antigen-driven response.9 The occurrence of B-cell clonal expansions in the liver of HCV-infected patients has been found to deeply influence the clinical picture, which appears to be strictly related to mixed cryoglobulinemia (MC) and, in general, to lymphoproliferative stigmata, including high serum levels of rheumatoid factor (RF) and monoclonal gammopathy of undetermined significance.8 The relationship between emergence and persistence of intrahepatic or circulating B-cell clonotypes and HCV infection is still unknown. Accumulating evidence indicates that certain chemokines play a critical role in providing the appropriate environment for activation and expansion of naive lymphocytes in response to signals delivered by antigen-presenting cells.10
CXC ligand13 (CXCL13), also known as B cell–attracting chemokine 1 or B-lymphocyte chemoattractant, is a member of CXC subtype of chemokine superfamily.11 It is critical for secondary lymphoid tissue development and distribution of lymphocytes within microenvironments.12 The gene mapped to chromosome segment 4q21 encodes a putative protein of 109 amino acids.11,12 The primary CXCL13 receptor is CXCR5, a 7-transmembrane G-protein expressed by B lymphocytes,13 follicular B helper T cells,14 osteoblasts,15 podocytes,16 and skin-derived dendritic cells.17 Interestingly, CXCL13 and CXCR5 knockout mice exhibit similar abnormalities, including impaired development of peripheral lymphoid organs, reduced B-cell translocation to the B-cell/T-cell boundary, and a lower antibody response.18 CXCL13 is constitutively expressed in the B-cell follicles of secondary lymphoid organs,11 pleural and peritoneal cavities,18 and in ectopic lymphoid follicles within the synovial membrane of patients with rheumatoid arthritis.19
We sought to determine the plasma levels of CXCL13 in patients with chronic HCV infection, with or without MC, a chronic B-cell lymphoproliferative disorder characterized by systemic vasculitis, which can potentially progress to frank B-cell non-Hodgkin lymphoma.20 Our data indicate that the high serum levels of CXCL13 protein in MC patients parallel those of specific mRNA expression in the liver and in the skin and suggest that this chemokine plays a major role in the pathogenesis of cryoglobulin-related damage, probably through B-cell deregulation and local production.
Methods
Patients and controls
During the years 2003 to 2007, 46 patients were selected at the Liver Unit of the Department of Internal Medicine and Clinical Oncology, University of Bari Medical School. Eligibility criteria were as follows: no previous administration of interferons, steroids, or immunosuppressive therapy; serum positive tests for anti-HCV antibodies and HCV-RNA; liver biopsy showing chronic active hepatitis; and negative tests for HBsAg, HIV, and antinuclear and antismooth muscle autoantibodies. Seventy-five patients were excluded because of concomitant neoplastic and cardiovascular diseases, poorly controlled diabetes, alcohol intake greater than 40 g/day, use of hepatotoxic drugs in the last 6 months, pregnancy, and absence of informed consent.
The study was approved by the University of Bari Medical School institutional ethical committee, and written, informed consent was obtained from all patients, in accordance with the Declaration of Helsinki.
Activity score and degree of fibrosis in liver biopsy were evaluated according to Ishak.21 Among the 46 selected patients, serum cryoglobulins, isolated as described elsewhere,22 were detected in 20 (16 of type II and 4 of type III) in whom skin biopsy was provided under local anesthesia. Control skin samples were derived from (1) 4 healthy patients undergoing cosmetic surgery, (2) 5 HCV-infected patients without MC who were subjected to saphenectomy, (3) 3 patients with HCV–negative hypersensitivity vasculitis, (4) 2 hepatitis B virus (HBV)–positive patients with necrotizing vasculitis, (5) 5 patients with Henoch-Schönlein purpura, and (6) 2 with HCV- and HBV-negative cryoglobulinemia. As controls, 5 liver samples from patients with nonalcoholic steatosis and 5 with near-normal liver obtained during cholecystectomy were considered.
Liver and skin biopsy specimens were in part formalin-fixed and paraffin-embedded for routine histologic examination and in part embedded in optimal cutting temperature (Sakura Finetek USA, Torrance, CA), snap-frozen, and stored at −80°C until sectioning for use in immunofluorescence studies. Part of the biopsy samples was prepared for molecular analyses; the specimens were put in a RNase-free microtube and immediately frozen in liquid nitrogen until RNA extraction. Results of liver histology, epidemiology, virology, and laboratory parameters are reported in Table 1.
Epidemiologic, virologic, and histologic data of 46 chronically HCV-infected patients
| Parameter . | Without MC . | With MC . |
|---|---|---|
| No. of patients | 26 | 20 |
| Age, y* | 56 ± 15 | 60 ± 14 |
| Sex (M/F) | 18/8 | 6/14† |
| HCV RNA positives, no. (%) | 26 (100) | 20 (100) |
| HCV genotypes, no. (%) | ||
| 1 | 13 (50) | 8 (40) |
| 2 | 11 (42) | 10 (50) |
| 3 | 2 (8) | 2 (10) |
| Liver histology, no. (%) | ||
| Chronic active hepatitis | 24 (92) | 18 (90) |
| Cirrhosis | 2 (8) | 2 (10) |
| Source of infection, no. (%) | ||
| Blood/blood product transfusions | 3 (12) | 2 (10) |
| Unknown | 23 (88) | 18 (90) |
| Durations of infection, y* | 28 ± 17 | 26 ± 12 |
| Parameter . | Without MC . | With MC . |
|---|---|---|
| No. of patients | 26 | 20 |
| Age, y* | 56 ± 15 | 60 ± 14 |
| Sex (M/F) | 18/8 | 6/14† |
| HCV RNA positives, no. (%) | 26 (100) | 20 (100) |
| HCV genotypes, no. (%) | ||
| 1 | 13 (50) | 8 (40) |
| 2 | 11 (42) | 10 (50) |
| 3 | 2 (8) | 2 (10) |
| Liver histology, no. (%) | ||
| Chronic active hepatitis | 24 (92) | 18 (90) |
| Cirrhosis | 2 (8) | 2 (10) |
| Source of infection, no. (%) | ||
| Blood/blood product transfusions | 3 (12) | 2 (10) |
| Unknown | 23 (88) | 18 (90) |
| Durations of infection, y* | 28 ± 17 | 26 ± 12 |
HCV indicates hepatitis C virus.
indicates means plus or minus SD.
P < .05.
HCV-infected patients, with or without MC, were treated with pegylated interferons (PEG-IFNs) and ribavirin (RBV).23 Those infected with genotypes 2 or 3 were treated for 6 months, whereas all other patients received 12 months of antiviral therapy. Patients were reassessed 6 months after discontinuation of therapy to determine whether they had achieved a sustained virologic response (serum HCV RNA undetectable 6 months after treatment cessation). In the MC group, therapeutic response was evaluated as previously established.24 Complete response was defined as reduction of the cryocrit level to less than 25% of the initial value, associated with the disappearance of at least 2 of the following signs and symptoms: purpura, arthralgias, and weakness.
Patients unresponsive to PEG-IFN/RBV combination therapy underwent B-cell depletion with an anti-CD20 monoclonal antibody (rituximab; F. Hoffman-La Roche, Basel, Switzerland). They received 4 intravenous infusions of 375 mg/m2 once a week over a period of a month.25 After rituximab treatment, patients were evaluated monthly for a period of 6 months. Those who were shown to be refractory also to anti-CD20 antibody therapy were given corticosteroids (6-methylprednisolone, 16 mg/day).
Reverse-transcribed polymerase chain reaction for CXCL13 mRNA expression
RNA was extracted from skin biopsy samples using TRIzol (Invitrogen, Carlsbad, CA) and reverse-transcribed (RT) with oligo(dT) and random hexamer primers (Invitrogen). Polymerase chain reaction (PCR) was carried out with Taq DNA polymerase (AmpliTaq Gold; Applied Biosystems, Foster City, CA). Primer pair for CXCL13 was: CAG AAT CCT CTG GAA CTT GAG (5′) and CTT CCA GAC ATT CGG AGA CC (3′).19 As control, transcripts of the “housekeeping” gene β-actin were amplified (GTC CTC TCC CAA GTC CAC ACA (5′) and CTG GTC TCA AGT CAG TGT ACA GGT AA (3′). Annealing temperatures were 58°C for CXCL13 and 56°C for β-actin. PCR products resolved on 3% agarose gel were 368 bp (the CXCL13 cDNA size) and 241 bp (β-actin). Negative controls lacking template RNA or RT were included in each experiment. The identity of the PCR-amplified fragments was defined by direct sequencing on ABI PRISM 377 (Applied Biosystems).
Quantitative real-time RT-PCR on portal tracts isolated from liver biopsy sections by laser capture microdissection
Quantitative real-time RT-PCR was performed to define CXCL13 gene expression in portal tract structures, isolated from liver biopsies by a laser capture microdissection (LCM) technique, as described elsewhere.26 Frozen liver tissue specimens were cut as a series of 5-μm sections and mounted on slides coated with thermoplastic membrane (PEN foil slides, Leica Microsystems, Wetzlar, Germany). Each section was used for tissue isolation by LCM with the Leica SVS LMD System (Leica Microsystems). Portal tracts were selectively dissected by focal melting of the membrane with an ultraviolet laser beam. Dissected microsamples were dropped into cap tubes under microscopy inspection. To minimize degradation of nucleic acids, slides were fixed in ethanol (70%) and washed in diethylpyrocarbonate-treated deionized water. Isolated portal tracts were lysed in 50 μL lysis buffer. After cooling at room temperature, 20 μL precipitation solution was assessed. Samples were then centrifuged, and supernatants were transferred into tubes containing 60 μL 100% isopropanol and 1.0 μL glycogen (20 mg/mL). After centrifugation, pellets were washed with 70% ethanol and air dried. mRNA was isolated via QIAshredder columns and RNeasy kit from Qiagen (Hilden, Germany). As controls, portal tracts were recovered from 5 patients with nonalcoholic steatosis and 5 with near-normal liver obtained during cholecystectomy.
A total of 100 ng of RNA primed with oligo (dt) and reverse transcribed was used for 20-μL reactions. Specific mRNA was quantified by real-time RT-PCR Light Cycler Systems (Roche Diagnostics, Basel, Switzerland). The expression level of CXCL13 gene was relative to the β-actin gene used as internal control. The final expression was formulated by the amplification coefficient, calculated by determining the crossing point (number of cycles required to reach a set threshold) for a series of 2-fold dilutions of cDNA template. The calculated crossing point was used as a measure of gene specific RNA quantity, and fold expression was calculated by the following equation: KgeneΔCp, where Kgene is the amplification coefficient, and ΔCp is (crossing point for RT-PCR patients) − (crossing point for the RT-PCR from normal controls) for the gene. An increase of RNA levels lower than 2-fold was not considered significant.
Immunoassay for serum human CXCL13 protein
Quantitative determination of serum CXCL13 was performed with Quantikine kit (R&D Systems, Minneapolis, MN), a quantitative sandwich enzyme immunoassay that makes use of a mouse monoclonal antibody against human CXCL13 precoated onto a microplate. Human recombinant CXCL13 protein at serial concentrations and 2-fold diluted serum samples were pipetted into the wells to allow any CXCL13 to be bound by immobilized antibody. After washing away unbound substances, a mouse monoclonal antibody conjugated with horseradish peroxidase specifically directed against CXCL13 protein was added to the wells. After further washings, stabilized tetramethylbenzidine was added to the wells and color developed in proportion to the amounts of bound CXCL13. Quantikine kit standards were used for construction of standard curves. The sensitivity threshold of the test was 2 pg/mL.
Immunofluorescence for CXCL13 protein
Indirect immunofluorescence to detect CXCL13 in liver and skin biopsy samples was performed with goat anti–human CXCL13 antibody (R&D Systems) at a working concentration of 15 μg/mL. Tissues were cut into 6-μm sections, dried at room temperature for 1 hour, and fixed in acetone at −20°C. Human tonsil sections were treated in the same manner as positive control. Samples were incubated with primary antibody for 3 hours at room temperature. Isotype-matched antibodies were used for control staining. Thereafter, fluorescein isothiocyanate–conjugated rabbit antigoat antibody, F(ab)′2 fragment (Dako Denmark, Glostrup, Denmark), was used as the secondary reagent. It was incubated for 2 hours at room temperature. Slides were then cover-slipped with aqueous medium and evaluated under fluorescence microscopy. To block positive reaction on selected samples, the primary antibody was preincubated with human recombinant CXCL13 protein (5 ng/mL; R&D Systems). Omission of primary antibody and use of irrelevant antibody (anti–human chorionic gonadotropin) were also performed. Slides were viewed with a Leica DMBL research microscope (Leica Microsystems) using N-PLAN lens. Images were acquired using a Leica camera model DFC 490 (Leica Microsystems) and were processed with Leica application suite version 2.4.0R1.
Statistical analysis
Significance of differences in the distribution of quantitative variables was assessed with one-way analysis of variance. All P values were 2-tailed, and a level of .05 was considered statistically significant. The probability of response to therapy was assessed by the Kaplan-Meier method. Cox regression model was used for univariate analysis to measure degree of association between serum levels of CXCL13 and virologic and immunologic parameters.
Results
Study population
Twenty patients with MC (16 of type II and 4 of type III) and 26 without extrahepatic disorders were considered in this study. As reported in Table 1, all were chronically infected with HCV. The number of females was significantly higher in MC patients (P < .05). CALD was histologically defined in the patients of the 2 groups, in which cirrhosis was almost equally distributed. Except for a moderate prevalence of HCV genotype 2 in MC patients, no distinct profile of their distribution was detected.
Skin histology from the MC group showed leukocytoclastic vasculitis in 5 patients, and lymphocytic vasculitis in 4, whose clinical presentation was characterized by palpable purpura at lower extremities, leg ulcers, and blistering erythematous urticarial plaques. Pandermal vasculitis included thrombosis and severe endothelial cell alterations. Eleven patients belonging to the asymptomatic group showed pauci-inflammatory vasculopathy, focal perivascular infiltrates, and sparse, hyalinizing vascular changes.
Serum CXCL13 protein
Compared with 30 healthy blood donors (19 men and 11 women, with a mean age of 55 ± 11 years), serum CXCL13 levels showed progressively higher mean levels when patients without and with MC were considered (mean ± SD, 48.2 ± 11.0 vs 113.9 ± 40.2 pg/mL, P < .01; vs 273.6 ± 98 pg/mL, P < .005, respectively). Cross-sectional analysis showed that CXCL13 serum levels in MC patients were significantly higher than those found in patients without MC (P < .04).
The relationships between CXCL13 serum levels and virologic, histologic, and immunologic parameters are depicted in Table 2. In each group, no correlation was observed between CXCL13 concentration and circulating viral load, liver histology activity index, or grade of liver fibrosis and alanine aminotransferase levels. In addition, in MC patients no correlation was seen between CXCL13 levels and cryocrit percentages, serum IgM, RF activity, and C4 concentration.
Mean virologic, histologic, and immunologic parameters, and pair-wise correlation with serum CXCL13 concentrations in HCV-infected patients without and with mixed cryoglobulinemia
| Parameter . | Mixed cryoglobulinemia . | |||||
|---|---|---|---|---|---|---|
| Without . | With . | |||||
| Mean plus orminus SD . | r . | P . | Mean plus or minus SD . | r . | P . | |
| Circulating HCV-RNA (IU/mL) | 1 100 238 ± 1 203 834 | 0.06 | .87 | 491 248 ± 655 695 | 0.17 | .54 |
| Severity of liver disease | ||||||
| Activity index | 5.7 ± 1.8 | 0.07 | .84 | 4.2 ± 2.2 | 0.08 | .83 |
| Stage | 2.9 ± 0.8 | 0.14 | .69 | 2.1 ± 0.78 | 0.39 | .29 |
| ALT (normal values, 30-65 IU/L) | 93 ± 50 | 0.54 | .09 | 75 ± 34 | 0.24 | .39 |
| Immunologic features | ||||||
| Cryocrit, % | — | — | — | 7.0 ± 7.8 | 0.11 | .68 |
| Serum IgM (normal values, 40-230 mg/dL) | 120 ± 56 | 0.49 | .71 | 545 ± 818 | 0.37 | .16 |
| Rheumatoid factor activity (normal values, ≤ 20 IU/mL) | 16 ± 9 | 0.7 | .88 | 1850 ± 2456 | 0.3 | .26 |
| Complement C4 (normal values, 20-40 mg/dL) | 22.9 | 0.78 | .67 | 7.0 ± 8.2 | 0.06 | .81 |
| Parameter . | Mixed cryoglobulinemia . | |||||
|---|---|---|---|---|---|---|
| Without . | With . | |||||
| Mean plus orminus SD . | r . | P . | Mean plus or minus SD . | r . | P . | |
| Circulating HCV-RNA (IU/mL) | 1 100 238 ± 1 203 834 | 0.06 | .87 | 491 248 ± 655 695 | 0.17 | .54 |
| Severity of liver disease | ||||||
| Activity index | 5.7 ± 1.8 | 0.07 | .84 | 4.2 ± 2.2 | 0.08 | .83 |
| Stage | 2.9 ± 0.8 | 0.14 | .69 | 2.1 ± 0.78 | 0.39 | .29 |
| ALT (normal values, 30-65 IU/L) | 93 ± 50 | 0.54 | .09 | 75 ± 34 | 0.24 | .39 |
| Immunologic features | ||||||
| Cryocrit, % | — | — | — | 7.0 ± 7.8 | 0.11 | .68 |
| Serum IgM (normal values, 40-230 mg/dL) | 120 ± 56 | 0.49 | .71 | 545 ± 818 | 0.37 | .16 |
| Rheumatoid factor activity (normal values, ≤ 20 IU/mL) | 16 ± 9 | 0.7 | .88 | 1850 ± 2456 | 0.3 | .26 |
| Complement C4 (normal values, 20-40 mg/dL) | 22.9 | 0.78 | .67 | 7.0 ± 8.2 | 0.06 | .81 |
HCV indicates hepatitis C virus; ALT, alanine aminotransferase; —, not applicable; and IgM, immunoglobulin M.
Patients of the MC group were stratified according to the occurrence of active or nonactive phase of cutaneous vasculitis. Laboratory findings and clinical features of the 2 subgroups are summarized in Table 3. Although not significant, the average cryocrit value was lower in patients with active vasculitis, whereas type III MC occurred more frequently in those with nonactive vasculitis. Serum γ-globulin and IgM levels, RF activity, and complement C4 concentration were almost similar in the 2 subgroups, and not dissimilar was the frequency of nephropathy and peripheral neuropathy. Conversely, a significantly higher value (P < .001) of CXCL13 serum concentration was demonstrated in patients with active cryoglobulinemic vasculitis, whereas the mean serum concentration of CXCL13 in MC patients without active vasculitis was comparable with that found in non-MC patients.
Clinical and immunologic parameters of MC patients with active and nonactive cutaneous vasculitis
| Parameter . | Cutaneous vasculitis . | |
|---|---|---|
| Active (n = 9) . | Nonactive (n = 11) . | |
| Purpura, % | 9 (100) | 0 |
| Urticarial lesions, % | 3 (33) | 0 |
| Livedo reticularis, % | 2 (22) | 0 |
| Leg ulcers, % | 6 (66) | 0 |
| Cryocrit, % (mean ± SD) | 3.76 ± 1.2 | 8.14 ± 2.8 |
| Cryoglobulin immunochemical types, % | ||
| Type II | 8 | 8 |
| Type III | 1 | 3 |
| γ -Globulin levels (mean ± SD; normal values, 0.7-1.8 g/dL) | 1.16 ± 0.7 | 1.13 ± 0.9 |
| Serum IgM (mean ± SD; normal values, 40-230 mg/dL) | 400 ± 114 | 413 ± 190 |
| RF activity (mean ± SD; normal values, ≤ 20 IU/mL) | 390 ± 110 | 415 ± 167 |
| Complement C4 (mean ± SD; normal values, 20-40 mg/dL) | 4.1 ± 2.1 | 5.3 ± 1.9 |
| CXCL13 serum concentration, pg/mL (mean ± SD) | 325 ± 160* | 112 ± 32 |
| Peripheral neuropathy, % | 4 (44) | 3 (27) |
| Nephropathy, % | 2 (22) | 2 (18) |
| Parameter . | Cutaneous vasculitis . | |
|---|---|---|
| Active (n = 9) . | Nonactive (n = 11) . | |
| Purpura, % | 9 (100) | 0 |
| Urticarial lesions, % | 3 (33) | 0 |
| Livedo reticularis, % | 2 (22) | 0 |
| Leg ulcers, % | 6 (66) | 0 |
| Cryocrit, % (mean ± SD) | 3.76 ± 1.2 | 8.14 ± 2.8 |
| Cryoglobulin immunochemical types, % | ||
| Type II | 8 | 8 |
| Type III | 1 | 3 |
| γ -Globulin levels (mean ± SD; normal values, 0.7-1.8 g/dL) | 1.16 ± 0.7 | 1.13 ± 0.9 |
| Serum IgM (mean ± SD; normal values, 40-230 mg/dL) | 400 ± 114 | 413 ± 190 |
| RF activity (mean ± SD; normal values, ≤ 20 IU/mL) | 390 ± 110 | 415 ± 167 |
| Complement C4 (mean ± SD; normal values, 20-40 mg/dL) | 4.1 ± 2.1 | 5.3 ± 1.9 |
| CXCL13 serum concentration, pg/mL (mean ± SD) | 325 ± 160* | 112 ± 32 |
| Peripheral neuropathy, % | 4 (44) | 3 (27) |
| Nephropathy, % | 2 (22) | 2 (18) |
MC indicates mixed cryoglobulinemia; IgM, immunoglobulin M; and RF, rheumatoid factor.
P < .001.
In situ CXCL13 immunodetection and gene quantitation
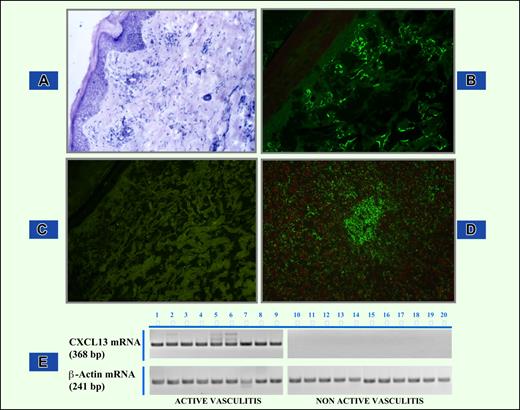
CXCL13 protein, studied by indirect immunofluorescence on skin biopsy samples of cryoglobulinemic patients, was mostly expressed along interstitial array between collagen bundles and involved the superficial dermis with variable mild/deep dermal extension in patients with active vasculitis (Figure 1B). No CXCL13-protein deposition was detected in skin biopsy tissues from controls that included healthy subjects and HCV-infected patients without MC (Figure 1C).
Demonstration of CXCL13 protein and CXCL13 mRNA in skin biopsy samples. (A) Hematoxylin and eosin staining of skin sample from a patient with cryoglobulinemic active vasculitis. Magnification, 10×/0.25. (B) CXCL13 protein immunofluorescence staining in skin biopsy showing feature of active vasculitis. CXCL13- specific signal appears as linear green deposits along collagen bundles. Magnification, 10×/0.25. (C) Complete absence of CXCL13 immunoreactant in skin tissue of an MC patient without active phase of vasculitis. Magnification, 10×/0.25. (D) Positive control staining in human tonsil. Magnification, 20×/0.40. (E) RT-PCR analysis of CXCL13 mRNA extracted from MC patients with and without active cryoglobulinemic vasculitis.
Demonstration of CXCL13 protein and CXCL13 mRNA in skin biopsy samples. (A) Hematoxylin and eosin staining of skin sample from a patient with cryoglobulinemic active vasculitis. Magnification, 10×/0.25. (B) CXCL13 protein immunofluorescence staining in skin biopsy showing feature of active vasculitis. CXCL13- specific signal appears as linear green deposits along collagen bundles. Magnification, 10×/0.25. (C) Complete absence of CXCL13 immunoreactant in skin tissue of an MC patient without active phase of vasculitis. Magnification, 10×/0.25. (D) Positive control staining in human tonsil. Magnification, 20×/0.40. (E) RT-PCR analysis of CXCL13 mRNA extracted from MC patients with and without active cryoglobulinemic vasculitis.
Consistent with immunofluorescence results, we demonstrated a definite expression of CXCL13 mRNA, amplified with RT-PCR, extracted from frozen skin samples of patients with cryoglobulinemic vasculitis. No amplifiable product was shown in patients with nonactive vasculitis (Figure 1E) and in controls (data not shown). CXCL13 amplicons fulfilled criteria of specificity under electrophoresis and direct sequencing of both forward and reverse DNA strands.
CXCL13 protein and specific mRNA expression were also explored in 12 control HCV-negative patients. Both CXCL13 protein and specific RNA were demonstrated in skin biopsy samples in 1 of 3 HCV-negative patients with hypersensitivity vasculitis, in the 2 patients with HBV-associated necrotizing vasculitis, in 3 of 5 patients with Henoch-Schönlein purpura, and in 1 of 2 HBV- and HCV-negative cryoglobulinemic patients. In these patients, CXCL13 protein was expressed to a variable extent: whereas expression was detected in restricted areas of the sections, other sections displayed expression within or around focal inflammatory infiltrates (not shown). In any case, protein expression paralleled that of specific mRNA.
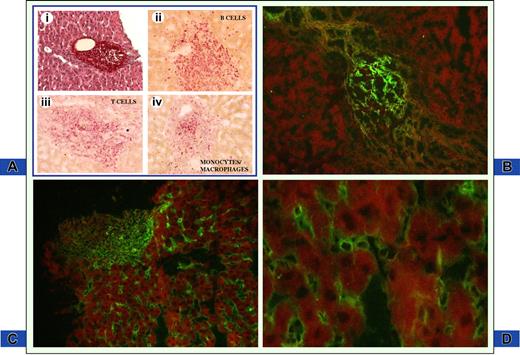
CXCL13 immunoreactive deposits were found in 18 of 20 (80%) and in 16 of 26 (61%) liver biopsy samples from patients with and without MC, respectively. Details regarding immunodetection of CXCL13 protein are shown in Figure 2. Hepatocytes and bile duct epithelium were not stained. On the contrary, intense expression of CXCL13 protein was evident in inflammatory cells within portal tracts and, less frequently, into the sinusoids. The following 2 main immunofluorescence patterns of CXCL13 protein expression were established: (1) granular bead-like deposits mimicking follicle-like structures within portal tracts (Figure 2B); and (2) fine as well as coarse dot-like immunofluorescence diffusely extending to the entire region of portal tract or confined to some defined areas (Figure 2C). Furthermore, CXCL13-containing cells were found in sinusoidal inflammatory cells (Figure 2C,D). Indeed, CXCL13 follicle-like labeling pattern and intrasinusoidal positivity were features more frequently found in patients with MC (83%) than in those without (31%). Sometimes, the 2 features of CXCL13 immunodetection coexisted in the same section.
CXCL13 immune deposits in the liver of HCV-infected patients. (A) Hematoxylin and eosin staining of liver biopsy from an MC patient. Note enlarged portal tract containing infiltrating inflammatory cells with follicle-like structure (Ai; magnification, 10×/0.25). Phenotypic characterization of inflammatory cells is depicted in panels Aii to Aiv. Magnification, 20×/0.40. B cells, T cells, and monocytes/macrophages were studied by indirect immunohistochemistry using mouse anti-CD3, anti-CD20, and anti-CD68 antibodies and revealed with antimouse immunoglobulins conjugated with alkaline phosphatase in sections adjacent to panel Ai. All 3 cell populations are abundantly present within inflamed portal tract. (B) Follicle-like appearance of CXCL13 protein restricted to a portal tract structure on a section adjacent to panel A. Magnification, 20×/0.40. (C) CXCL13 deposits are homogeneously distributed in portal tract and in hepatic sinusoids. Magnification, 20×/0.40. (D) Higher magnification (63×/0.75) demonstrates that CXCL13 is present on intrasinusoidal inflammatory cells and along sinusoidal walls.
CXCL13 immune deposits in the liver of HCV-infected patients. (A) Hematoxylin and eosin staining of liver biopsy from an MC patient. Note enlarged portal tract containing infiltrating inflammatory cells with follicle-like structure (Ai; magnification, 10×/0.25). Phenotypic characterization of inflammatory cells is depicted in panels Aii to Aiv. Magnification, 20×/0.40. B cells, T cells, and monocytes/macrophages were studied by indirect immunohistochemistry using mouse anti-CD3, anti-CD20, and anti-CD68 antibodies and revealed with antimouse immunoglobulins conjugated with alkaline phosphatase in sections adjacent to panel Ai. All 3 cell populations are abundantly present within inflamed portal tract. (B) Follicle-like appearance of CXCL13 protein restricted to a portal tract structure on a section adjacent to panel A. Magnification, 20×/0.40. (C) CXCL13 deposits are homogeneously distributed in portal tract and in hepatic sinusoids. Magnification, 20×/0.40. (D) Higher magnification (63×/0.75) demonstrates that CXCL13 is present on intrasinusoidal inflammatory cells and along sinusoidal walls.
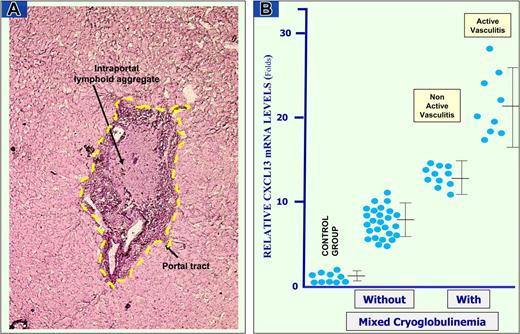
CXCL13 mRNA expression in LCM-based portal tracts. Example of LCM is reported in panel A. An enlarged portal tract with heavy inflammatory infiltrate and lymphoid aggregate with the appearance of lymphoid follicle was dissected by laser beam along the yellow dotted line from cryostat section of liver biopsy. (B) Quantitative real-time RT-PCR performed on nucleic acids extracted from microdissected portal tracts of HCV-infected patients without and with MC and controls. Data are means plus or minus SD for each group.
CXCL13 mRNA expression in LCM-based portal tracts. Example of LCM is reported in panel A. An enlarged portal tract with heavy inflammatory infiltrate and lymphoid aggregate with the appearance of lymphoid follicle was dissected by laser beam along the yellow dotted line from cryostat section of liver biopsy. (B) Quantitative real-time RT-PCR performed on nucleic acids extracted from microdissected portal tracts of HCV-infected patients without and with MC and controls. Data are means plus or minus SD for each group.
Assessment of CXCL13 specificity was carried out on both liver- and skin-positive samples before and after the absorption of the probe with recombinant CXCL13 protein. Preincubation with CXCL13 protein completely abolished the positive signal, whereas the signal was unchanged when an irrelevant protein (human chorionic gonadotropin) was used (data not shown).
Quantitative real-time RT-PCR assay for specific CXCL13 mRNA expression was performed on nucleic acids recovered from LCM-based microsamples. Several laser pulses were used to obtain microdissected portal tracts of comparable size (mean ± SD, 460 078 ± 65 780 μm2) from both groups of patients. After microscopic control of tissue preservation and demonstration of RNA integrity, liver biopsy sections were used for LCM (Figure 3).
Quantitative differences in specific CXCL13 mRNA expression in the liver of MC patients were defined and compared with CXCL13 quantity normalized with β-actin levels in portal tracts (Figure 3B). MC patients with active vasculitis were shown to express 4- to 15-fold more CXCL13 than MC patients with nonactive vasculitis and 5- to 30-fold more than patients without MC.
Therapy-induced modifications of serum CXCL13 levels
Because of the potential key role of B cells in the pathophysiology of MC, changes in CXCL13 levels were investigated in patients who underwent different therapeutic protocols. The results showed that CXCL13 levels were not affected by the PEG-IFN-α/RBV combination or rituximab but were significantly reduced after corticosteroid therapy. Indeed, in 12 MC patients, treatment with IFN-α/RBV resulted in a dramatic improvement of cryoglobulin-related signs and symptoms, a remarkable decrement of the cryocrit, and a drastic lowering in the viral load, but CXCL13 levels remained largely unchanged. Similar features were detected in 5 patients treated with rituximab, in whom the reduction of the cryoprecipitate occurred in step with a progressive increase of HCV viremia. Conversely, in 3 patients treated with corticosteroids, a significant decrease of the serum concentration of CXCL13 was noticed, whereas levels of cryoprecipitates and circulating viral load remained mostly unchanged.
Discussion
Many viruses, including HCV, play a critical role in a wide variety of inflammatory processes by regulating the expression of transcriptional factors and proinflammatory genes, including tumor necrosis factor and members of its superfamily, interleukins, and chemokines.27 Chemokines have been shown to orchestrate migration to and preferential sequestration of B and T cells in HCV-infected compartments.28 Indeed, an increased number of circulating B cells has been demonstrated in these patients, probably reflecting deregulation of B-cell traffic.29,30 The homeostatic trafficking of B cells is mainly regulated by the chemokine CXCL13 through interaction with CXCR5, its only known receptor, expressed on all mature circulating B cells and on a subset of memory CD4 T cells in healthy people.13,14
In the present study, serum CXCL13 levels were found to be elevated in chronic HCV infection compared with healthy controls. The highest levels occurred in HCV-infected cryoglobulinemic patients. Usually, chemokines associate with endothelial cells and the extracellular matrix near the site of their production, but elevated levels of specific chemokines have been reported in the serum of HCV-infected patients, including CXCR3-binding chemokines (CXCL9, CXCL10, CXCL11).31 It can be inferred that the high serum levels of these chemokines may be a consequence of high local production. In particular, CXCL13 expression may be induced by the ongoing hepatic inflammation, which maintains the pathologic process in the tissue by attracting additional lymphocytes and leading to chronic damage. We have been unable to demonstrate any direct relation between serum concentrations of CXCL13 and either fibrosis score or inflammatory index of liver histology. This strongly emphasizes the major role of the nature and composition of inflammatory cells in the portal tracts, which seem to be essential in the production of large amounts of CXCL13 chemokine.
The mechanism(s) underlying the different production between HCV-infected patients with cryoglobulins and those without remains to be elucidated. In this context, the formation of follicle-like structures in portal tracts of the liver of patients with chronic HCV infection may be considered the morphologic counterpart of ectopic lymphoid tissue, which includes naive B cells in the central zone surrounded by mature B and T cells.32 A more frequent formation of intraportal lymphoid follicles in MC patients compared with patients without MC has been reported.33 It is assumed that these structures contribute to antigen presentation in situ, to clonal expansion of antigen-specific B and T cells, and to switching of an acute inflammation into a chronic one.34
In this study, CXCL13 expression was recognized in the liver of MC patients with morphologic pictures of follicle-like structures, suggesting its major role in the organization and maintenance of ectopic lymphoid tissue. CXCL13 chemokine may attract B cells and initiate the formation of germinal centers, thus contributing to the development of chronic inflammation.35,36
A genetic polymorphism of CXCL13 could explain the different levels of CXCL13 in MC patients compared with those without MC. Although there are no data describing such polymorphism, several high-producer alleles of proinflammatory cytokines have been associated with HCV infection.37,38 Whether a CXCL13 high-producer allele is associated with MC patients remains to be explored. Another possibility is that T-regulatory CD4+CD25+ cells that are defective in MC patients39 could influence the production of chemokine by stromal cells. This hypothesis also deserves further investigation.
Of note, no direct correlation between CXCL13 serum levels and circulating viral load was noticed. Although these findings may indicate the lack of a direct effect of the virus on the sources of CXCL13 production, they do not highlight the in situ relation between HCV and CXCL13 producer cells.
HCV minus strand RNA, the viral replicative intermediary, was frequently found in lymph nodes of HCV-infected patients.40 Recently, evidence of productive HCV infection was unequivocally achieved in lymphomonocytes of MC patients, but not in those without MC, implying lymphoid compartmentalization of active viral replication.41 Thus, it can be speculated that lymphoid productive infection of HCV particles in MC patients can be effective on CXCL13-producing cells.
The demonstration of CXCL13 in the skin of cryoglobulinemic patients with active cutaneous vasculitis indicates its involvement in the pathogenesis of tissue damage. No previous information is available concerning the expression of this molecule in the skin of these patients. At variance from cutaneous lymphoproliferative B-cell disorders, in which CXCL13 was detected as a cell-associated molecule constantly expressed by neoplastic B cells and follicular dendritic cells within lymphoid infiltrates,42 we established a staining pattern consisting of a diffuse expression of CXCL13 protein in the dermis along collagen bundles. This feature, with variable expression in the different areas of the dermis, paralleled the expression of specific mRNA signal, suggesting that CXCL13 is dependent on up-regulation of CXCL13-producing cells. Lack of CXCL13-positive cells in the skin may be the result of interference of reacting CXCL13 molecules on the cells through steric hindrance or else, as recently suggested, skin-derived dendritic cells, under favorable pressure of chemokines and chemokine receptors, may rapidly migrate to lymph nodes.43 A similar feature of CXCL13 immunoreactant deposits was recently described in normal and aberrant gut-associated lymphoid tissue.44
CXCL13 was mainly associated with extracellular fibrils and to a much lower extent with cells displaying a follicular dendritic cell phenotype. Notably, monocytes/macrophages are potent inducible producers of CXCL1345 in inflammatory sites. It has been suggested that extravasated monocytes in inflammatory lesions may give rise to cells capable of producing this chemokine,45 as it probably occurs in the skin of cryoglobulinemic patients with active vasculitis. Although monocytes/macrophages do not constitutively express CXCL13, they need a certain level of activation to secrete it.
To establish whether CXCL13 changes contributed to the success of therapy, serum levels of this chemokine were monitored throughout the administration of different treatments, including IFN-α/RBV combination, rituximab, and corticosteroids. Chemokine levels did not change after both a successful response to antiviral therapy and B-cell depletion induced by rituximab. On the contrary, CXCL13 significantly declined during and after corticosteroid treatment. Although additional studies on a larger number of patients are needed to strengthen these findings, our results indicate that neither the cryoglobulin concentration nor the viral load plays an effective role in determining vascular damage in HCV-associated cryoglobulinemic vasculitis. It has been assumed that lymphoid cells in inflammatory sites predominantly interact with antigen-presenting cells, including monocytes/macrophages and dendritic cells.46 Chronic inflamed liver contains lymphoid aggregates, sharing many of the structural and functional features of secondary lymphoid tissue.4,5 Stromal cells within inflamed tissue overproduce chemokines that have been shown to play an important role in lymphoid organogenesis.47 Accumulation of lymphocytes in germinal center-like structures is associated with ectopic expression of CXCL13 chemokine.48 In this context, unchanged levels of CXCL13 in cryoglobulinemic patients responsive to PEG-IFN-α/RBV and to rituximab may be the result of inappropriate spatial and temporal expression of CXCL13 by stromal cells. Conversely, effective control of CXCL13 levels by corticosteroids may be largely mediated by inhibition of the transcriptional activity of several genes encoding proinflammatory cytokines and chemokines.49
In conclusion, in this study, we have observed that patients with HCV infection have increased plasma levels of CXCL13, possibly as the result of overproduction in the liver and the skin. In patients with cryoglobulinemia, CXCL13 contributes to lymphoid homing in the liver by creating a local microenvironment supportive of focal B-cell aggregation with structural features remarkably similar to ectopic lymphoid follicles. In these patients, CXCL13 production seems to be involved in the exacerbation of cryoglobulinemic vasculitis, probably through aberrant dissemination of “antigen-priming” information from the liver to extrahepatic sites. Thus, HCV-related vasculitic damage is the result of multifaceted pathogenetic mechanisms, in which immune reactants are differently regulated, and explains why tailored therapeutic manipulations may be required to treat cryoglobulinemic vasculitis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants from the Italian Ministry of University and Research, Associazione Italiana per la Ricerca sul Cancro, and University of Bari.
Authorship
Contribution: D.S. and F.D. designed research, analyzed data, and wrote the paper; F.A.T., L.T., M.M., V.C., and L.S. performed research; and G.L. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Domenico Sansonno, Department of Internal Medicine and Clinical Oncology, University of Bari Medical School, Policlinico, Piazza Giulio Cesare 11, 70124 Bari, Italy; e-mail: d.sansonno@dimo.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal