Conventionally, complement is considered mostly a component of innate immune system; however, emerging data suggest that complement has pleiotropic effects impacting many aspects of the immune system. The modulation of T-cell responses and shaping of the overall immune response by locally produced/activated complement has far-reaching implications for therapeutic targeting of the complement system for the treatment of a variety of pathologic conditions.
The complement system consists of a set of soluble and cell surface proteins including components, receptors, and regulators. Complement activation is usually initiated by the interaction of several pattern-recognition receptors with foreign surface structures. Depending on the activation trigger, the complement cascade follows one of 3 pathways: the classical, lectin, or alternative pathway.1 Recently, however, additional activation pathways have been identified. For example, mannose-binding lectin (MBL)–associated protease-2 (MASP-2; of the lectin pathway) or thrombin (of the coagulation pathway) seems to directly cleave C3 or C5, respectively.2
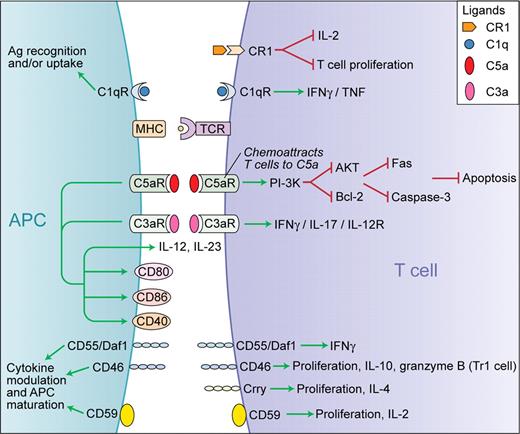
Traditionally, the complement system was thought to be mostly important in host defense functioning as a major component of the innate immune system. In recent decades, however, a considerable body of research has revealed that complement is also able to activate cells involved in both the innate and adaptive immune response, positioning it as an important link between the innate and the adaptive immunity.1 For example, complement provides a second signal to B lymphocytes that have recognized a complement-opsonized antigen.1 More recent work indicates that complement can also modulate T-cell responses during the induction, effector, and contraction phases of the immune response.3 Emerging data suggest complex and pleomorphic effects of complement activity on the production of key cytokines, T-cell effector differentiation, and function, as summarized in the figure. 3 Medof, Heeger, and colleagues have previously demonstrated that, upon cognate interactions between antigen-presenting cells and T cells, locally produced complement not only functions integrally in costimulation leading to T-cell proliferation and cytokine production, but also operates constitutively in naive T cells to sustain their viability.4 The complement system has a sophisticated regulation mechanism that generally prevents the complement cascade from attacking host tissue. Such regulators, including factor H, decay accelerating factor (DAF/CD55), membrane cofactor protein (MCP/CD46), C4-binding protein (C4BP), and complement receptor 1 (CR1/CD35) either induce an accelerated decay of the convertases or degrade C3b to its inactive form. By reducing the complement activity, these complement regulators also, indirectly, regulate T-cell activation. A recently appreciated direct mechanism of regulation by complement of T-cell immune responses is the induction of T cells exhibiting characteristics of T regulatory 1–type (Tr1) cells. These cells secrete large amounts of IL-10, granzyme B, and perforin, inhibit proliferation of CD4+ T cells, and kill autologous targets when there is simultaneous ligation of CD3 and CD46 (a complement regulatory protein) on T cells.3 Furthermore, during the termination of an immune response, T cells enter a contraction phase where most of the cells undergo apoptosis, leaving a small number of viable T cells to constitute the memory pool.
Schematic representation of the functions of complement in the modulation of T-cell responses. Green arrows indicate stimulation and red lines indicate inhibition. Professional illustration by Kenneth X. Probst.
Schematic representation of the functions of complement in the modulation of T-cell responses. Green arrows indicate stimulation and red lines indicate inhibition. Professional illustration by Kenneth X. Probst.
Lalli and colleagues in this issue of Blood show that locally produced and activated complement enhances T-cell expansion by inhibiting apoptosis mediated through PI-3K–dependent enhanced Bcl-2 expression and prevention of Fas up-regulation. It is known that C5a inhibits apoptosis of neutrophils via PI-3K/AKT and increased Bcl-xL, extending their lifespan after recruitment to sites of inflammation in the caecal ligation and puncture model of sepsis.5 However, in-triguingly in the same model, T cells undergo intense apoptosis in a caspase-dependent manner and this effect can be blocked by anti-C5a antibody therapy.6 These data are at odds with those by Lalli et al and require further investigation. Guo and colleagues speculated that the contrasting effects on neutrophil and T-cell apoptosis in the sepsis model may be related to differences in C5aR density on neutrophils versus T cells, where T cells rapidly up-regulate C5aR.5 On the other hand, the differences may be due to variances in strength of the T-cell receptor (TCR) stimulation in these models, cytokine milieu, or due to utilization of distinct signaling pathways by neutrophils and T cells. Perhaps as a result, glucocorticoids have differential effects and inhibit apoptosis in neutrophils but promote thymocyte apoptosis.6
Many inflammatory, autoimmune, neurodegenerative, and infectious diseases are thought to be caused, or at least perpetuated, by excessive complement activity. Therefore, inhibition or modulation of complement activity appears to be a promising novel strategy for their treatment and a number of clinical trials are already underway.7 Complement has also been shown to mediate graft rejection through increase in proinflammatory cytokine secretion by vessel walls and recruitment of effector T cells.2 Importantly, this immune-cell intrinsic phenomenon is independent of serum complement, indicating that local production and activation of complement makes a significant contribution to local tissue inflammatory injury and potentiation of adaptive immune response in many pathological conditions. For instance, in transplantation, C3 produced by a donor kidney allograft is a key mediator of experimental transplant rejection and markedly different kidney transplant outcomes are observed in humans based on kidney donor C3 polymorphisms.8 These findings and recent advances in understanding the novel role of complement in modulation of adaptive immunity and control of T-cell immune responses further support the need for continued efforts at better understanding the complex functions of complement. We can hope for the development of new therapies targeting specific pathways of the complement system in many immune-mediated diseases.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal