In this issue of Blood, Ny and colleagues disclose a modifier role for VEGF-D and a critical function for VEGFR-3 in embryonic lymphangiogenesis in Xenopus tadpoles.
VEGF-D potently stimulates lymphangiogenesis in adults and promotes metastasis of both human and experimental mouse tumors.1-4 However, studies in gene-deficient mice have suggested that it is dispensable for embryonic lymphatic vascular development.5 Ny et al now show that VEGF-D functions as a modifier of VEGF-C–driven early sprouting and migration of lymphatic endothelial cells in developing frog embryos and demonstrate the critical role of their receptor VEGFR-3 in this process.
Stimulation or inhibition of lymphangiogenesis, especially via the VEGF-C/VEGF-D/VEGFR-3 pathway, has proven beneficial in preclinical models of lymphedema and tumor metastasis, respectively, but their application to the treatment of human disease requires more detailed understanding of the molecular basis of lymphangiogenesis. Genetic studies in mice have revealed the importance of several key players in this process but many important issues remain to be resolved. Recently, the characterization of a lymphatic vascular system in zebrafish and the analysis of lymphatic vessel development in frog embryos have made these small organisms models of choice for genetic and chemical screens for regulators of lymphatic vascular development as well as for in vivo imaging of lymphangiogenic processes. Ny et al employ genetic down regulation in Xenopus tadpoles to demonstrate the subtle transient role of VEGF-D and its cooperation with VEGF-C in embryonic lymphangiogenesis.
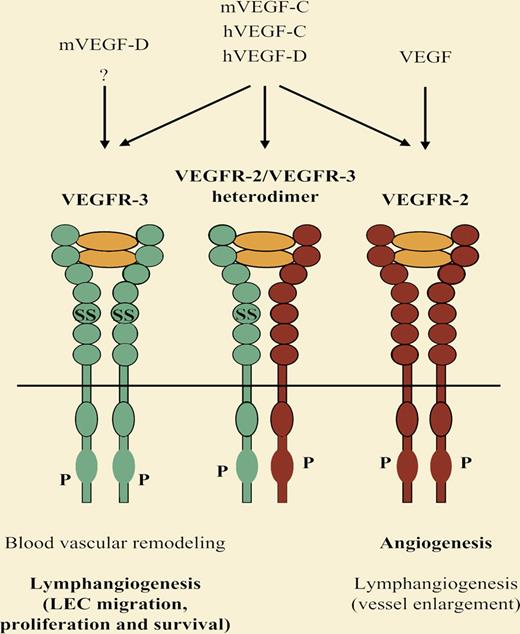
VEGF-C is essential for embryonic lymphatic vascular development whereas VEGF-D has a dispensable modifier role in this process. Nevertheless, both growth factors potently stimulate lymphangiogenesis in adults. VEGFR-3 is required for the remodeling of the primary blood vascular plexus in midgestation mouse embryos, apparently in a VEGF-C/VEGF-D independent manner.7 Later in embryogenesis, VEGFR-3 becomes restricted to lymphatic vessels and is critical for embryonic lymphangiogenesis and postnatal lymphatic vessel survival. At least in adult lymphangiogenesis, VEGFR-3 can cooperate with the major angiogenic receptor, VEGFR-2. ? indicates possible additional ligand or interaction.
VEGF-C is essential for embryonic lymphatic vascular development whereas VEGF-D has a dispensable modifier role in this process. Nevertheless, both growth factors potently stimulate lymphangiogenesis in adults. VEGFR-3 is required for the remodeling of the primary blood vascular plexus in midgestation mouse embryos, apparently in a VEGF-C/VEGF-D independent manner.7 Later in embryogenesis, VEGFR-3 becomes restricted to lymphatic vessels and is critical for embryonic lymphangiogenesis and postnatal lymphatic vessel survival. At least in adult lymphangiogenesis, VEGFR-3 can cooperate with the major angiogenic receptor, VEGFR-2. ? indicates possible additional ligand or interaction.
VEGF-C and VEGF-D have similar domain structure, both undergo proteolytic processing in a similar manner and, in humans, both share the same receptor-binding specificity. Mouse VEGF-D differs from its human counterpart and from VEGF-C in that it does not bind to the major angiogenic receptor VEGFR-2, which has been shown to cooperate with VEGFR-3 in lymphatic endothelial cell migration and proliferation. Whereas VEGF-C is essential in mouse lymphatic vascular development, it remains to be resolved to what extent the lack of VEGFR-2 binding accounts for the dispensable role of VEGF-D in this process.5,6 Elucidation of the receptor-binding pattern of Xenopus VEGF-D and finding whether it explains the functional difference between VEGF-C and VEGF-D in this organism might bring us a step forward in solving this question. A hint to the lymphangiogenic modifier role of VEGF-D is provided by the fact that exogenous VEGF-D protein administration is able to partially rescue the impaired sprouting and migration of lymphatic endothelial cells in VEGF-C–deficient mouse embryos,6 although loss of endogenous VEGF-D does not aggravate the VEGF-C null phenotype.7
Death of VEGFR-3–deficient mice due to blood vascular defects prior to lymphatic vessel development has precluded studies of the role of VEGFR-3 in mouse embryonic lymphangiogenesis.8 The importance of VEGFR-3 signaling in embryonic lymphatic vascular development, postnatal lymphatic vessel survival, and stimulation of adult lymphangiogenesis has been directly or indirectly demonstrated in various experimental settings in several species. By using genetic down-regulation and chemical inhibition, Ny et al document the critical role of VEGFR-3 in developmental lymphangiogenesis in the Xenopus tadpole model and thus the functional conservation of VEGFR-3 in this species.
A profound understanding of the molecular regulation of lymphatic vascular development and the entire range of functions of its major players and modifiers should enable the development of controlled antilymphangiogenic and prolymphangiogenic therapies. Characterization of the lymphatic system in small model organisms has provided new tools to study lymphangiogenic processes and to complement the methods available in mammals. Ny et al highlight the potential of Xenopus tadpoles in lymphatic vascular research by demonstrating the subtle modifier role of VEGF-D in lymphatic vascular development.
Conflict-of-interest disclosure: T.K. declares no competing financial interests. K.A. is a minority shareholder of Lymphatix Ltd. and the Chairman of the Scientific Advisory Board of Vegenics Ltd. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal