Abstract
Endothelial cells store the adhesive glycoprotein von Willebrand factor (VWF) in Weibel-Palade bodies (WPBs), distinctively shaped regulated secretory organelles that undergo exocytosis in response to secretagogue. A significant proportion of newly synthesized VWF is also secreted spontaneously from nonstimulated cells, through what is thought to be the constitutive secretory pathway. To learn more about VWF trafficking, we performed kinetic analyses of the storage and nonstimulated secretion of VWF in cultured human endothelial cells. We found that most VWF was secreted through a route that was significantly delayed compared with constitutive secretion, although this pathway was responsible for secretion of a small amount of uncleaved VWF precursor. Disruption of pH-dependent sorting processes with ammonium chloride converted the secretion kinetics of mature VWF to that of its precursor. Conversely, preventing constitutive secretion of nascent protein with brefeldin A had only a modest effect on the spontaneous release of VWF, showing that most VWF secreted by nonstimulated cells was not constitutive secretion but basal release of a post-Golgi storage organelle, presumably the WPB. These data suggest that VWF is sorted to the regulated secretory pathway in endothelial cells much more efficiently than previously reported.
Introduction
Von Willebrand factor (VWF) is a large, adhesive glycoprotein synthesized and secreted by endothelial cells and platelets that plays a vital role in primary and secondary hemostasis.1,2 Within endothelial cells VWF is stored as high molecular weight, disulfide-bonded, homo-oligomers in distinctive rod-shaped, regulated secretory organelles called Weibel-Palade bodies (WPBs).3,4 WPBs originate from the trans-Golgi network (TGN) in a process driven by VWF itself and dependent on the drop in pH that occurs in the TGN.5-8 Curiously, although it is known that VWF expression is able to induce the formation of its own storage organelle,5 the only published estimate of the efficiency of VWF sorting to WPB in endothelial cells suggests it is a very inefficient process with only 5% to 10% of newly synthesized VWF being sorted, the rest (90%-95%) being secreted through the constitutive secretory pathway.9 This figure has become generally accepted, although a study based on the analysis of VWF multimers secreted from, or retained within, human umbilical vein endothelial cells (HUVECs) concluded that constitutive secretion of VWF was insignificant.10
We wanted to understand the factors influencing VWF trafficking in endothelial cells and used metabolic labeling of cultured HUVECs combined with immunoprecipitation of the 2 cleavage products of the VWF precursor proVWF, mature VWF and the proVWF pro-polypeptide (VWF:AgII, referred to in the study as AgII). The data from these experiments suggested a model in which most VWF was sorted away from the constitutive secretory pathway into a post-Golgi compartment that was subsequently secreted without a requirement for cell stimulation. The spontaneous release of regulated secretory cargo in the absence of stimulation has been termed “basal” secretion to distinguish it from constitutive secretion.11 To test this model, we made use of the known effects of ammonium chloride (NH4Cl), brefeldin A (BFA), and cycloheximide (CHX) on the mammalian secretory pathway. The importance of this finding to our understanding of VWF sorting in endothelial cells is discussed.
Methods
Materials
Unless stated otherwise, all materials were purchased from Sigma-Aldrich (Poole, United Kingdom).
Cell culture
Pooled donor HUVECs (TCS CellWorks, Botolph Claydon, United Kingdom) were cultured as described,12 except that the endothelial cell growth supplement was purchased from Upstate (Temecula, CA). Cells were maintained at 37°C, 5% CO2, and experiments were performed on low-passage-number cells grown to confluence on gelatin-coated plastic. Chinese hamster ovary (CHO) cells stably expressing full-length human pre-proVWF (CHO-VWF) were maintained at 37°C, 5% CO2 in Ham F12, 10% fetal bovine serum (FBS), 50 μg/mL gentamicin, and 1 mg/mL G418 sulfate. CHO-VWF was made by transfecting CHO-K1 with pVWF, a neomycin-selectable vector encoding full-length human pre-proVWF driven off a viral promoter. This vector was derived from pVWF-EGFP.13 Briefly, EGFP was removed, and the VWF stop codon was reintroduced using a EcoRV/NotI polymerase chain reaction fragment that was generated using the primers 5′-gagtgcaacgacatcactgcc-3′ (forward) and 5′-gagcggccgctcacttgctgcacttcc-3′ (reverse) and pMT2-ADA-VWF14 as the template.
AgII antiserum
Rabbits were immunized with a synthetic peptide corresponding to the putative carboxyl-terminus of AgII, conjugated to keyhole limpet hemocyanin. Detailed characterization of the antibody will be published elsewhere (L.J.H. and M.J.H., manuscript in preparation).
Metabolic labeling
Pulse-chase.
Cells were metabolically labeled for 30 minutes with labeling medium (cysteine and methionine-free Dulbecco modified Eagle medium [DMEM] supplemented with 10% dialyzed FBS containing 50 μCi [1.85 MBq]/mL [35S]-cysteine/methionine [PRO-MIX L-[35S]; GE Healthcare, Little Chalfont, United Kingdom]) after which they were incubated in the appropriate medium for varying periods before harvesting the culture medium and cell lysates for immunoprecipitation.
Long-term labeling.
Cells were labeled overnight with 10 μCi (0.37 MBq)/mL PRO-MIX L-[35S] in full-growth medium, after which they were treated as described in “Pulse-chase.”
Preparation of lysates and media for immunoprecipitation
Lysates.
Cell monolayers were lysed in Tris-buffered saline (TBS; 100 mM Tris, 150 mM NaCl, pH 7.5), 1% (vol/vol) Triton X-100 (TX), 0.5 mM EDTA, 1 mM iodoacetamide, and a protease inhibitor cocktail. TX-insoluble material (containing most of the mature VWF) was pelleted by centrifugation and solubilized by heating to 95°C in one-tenth volume of 1% SDS. The TX soluble and SDS soluble fractions were pooled together for immunoprecipitation.
Culture medium.
Culture media were harvested and centrifuged to remove cellular debris before immunoprecipitation.
Immunoprecipitation
VWF and AgII were immunoprecipitated from cell lysates and medium using rabbit anti-VWF (Dako UK, Ely, United Kingdom) and rabbit anti-AgII antibodies, respectively, bound to protein-A sepharose. Immunoprecipitates were eluted from the sepharose at 95°C in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 10% (vol/vol) β-mercaptoethanol, and proteins were resolved by SDS-PAGE on 6% gels.15 Coomassie stained, dried gels were exposed to storage phosphor plates (GE Healthcare) which were scanned using a Storm 860 PhosphorImager (GE Healthcare) and [35S]-labeled proteins were quantified using ImageQuant software (GE Healthcare). Preliminary experiments showed that VWF and AgII antibodies immunoprecipitated more than 80% of their respective antigens from cell lysates and media (J.P.G., unpublished data, July 2006).
Endoglycosidase treatment
VWF immunoprecipitates were incubated overnight at 37° C with 25 mU Endoglycosidase H (Roche Diagnostics, Lewes, United Kingdom) according to the manufacturer's instructions before elution and resolution by SDS-PAGE.
Measurement of VWF secretion by enzyme-linked immunosorbent assay
Cell monolayers were washed and media replaced with fresh culture media containing appropriate reagents. Media were collected at various times and centrifuged to remove cell debris, and VWF in the supernatant was analyzed in duplicate by enzyme-linked immunoabsorbent assay (ELISA) using commercial reagents and a standard protocol (Dako UK). The concentrations of VWF in international units per milliliter (IU/mL) were calculated from a standard curve prepared using human plasma calibrated against a VWF standard (WHO First International Standard; Von Willebrand Factor concentrate, 00/514; National Institute for Biological Standards and Control [NIBSC], Potters Bar, United Kingdom).
Results
Significant proportion of newly synthesized VWF is retained within HUVECs for at least 24 hours
Figure 1shows [35S]-VWF and [35S]-AgII immunoprecipitated from chase media and cell lysates harvested from pulse-labeled HUVECs at various time points after labeling. Immediately after labeling (0 hour chase time) the major radioactive species immunoprecipitated from cell lysates by anti-VWF antibodies was a protein of approximately 360-kDa apparent molecular weight, corresponding to the VWF precursor proVWF (Figure 1A top, open arrowhead). At this time point no radioactive AgII could be precipitated using an anti-AgII antibody (Figure 1A bottom), showing that the 30-minute labeling period was too brief to allow any nascent proVWF to undergo proteolytic processing to generate mature VWF and AgII. Subsequent chase of the metabolically labeled cells led to a time-dependent decrease in the amount of [35S]-labeled proVWF in the cells, concomitant with the appearance in the cells and medium, of a [35S]-labeled protein of approximately 260 kDa precipitated with the anti-VWF antibody (Figure 1A top, closed arrowhead) and a 100-kDa protein precipitated with the anti-AgII antibody (Figure 1A bottom, closed arrowhead). These 2 proteins are mature VWF and AgII, respectively. The time course of VWF processing and secretion is consistent with previously reported studies,16-18 although to our knowledge this is the first time that biosynthesis and secretion of AgII has been simultaneously followed. At the end of the 24-hour incubation a significant proportion of radiolabeled VWF (both mature VWF and AgII) that had been synthesized during the initial 30-minute labeling period was recovered from the cell lysates. Quantification of pulse chase data for 4 experiments showed that after 24 hours of incubation an average of approximately 50% of the total (cells plus medium) mature VWF and AgII were recovered in the cell lysates (44.9% ± 6.3% and 67.1% ± 6.8%, respectively).
Pulse-chase analysis of VWF and AgII processing and secretion in HUVECs. (A) Phosphor-Imager data of VWF (top) and AgII (bottom) immunoprecipitates from cell lysates (Cells) and chase media (Medium) of HUVECs metabolically labeled with [35S]-cysteine/methionine for 30 minutes and subsequently chased for the times indicated. Immunoprecipitates were separated by SDS-PAGE on 6% polyacrylamide gels and exposed to a PhosphorImager plate for 7 days (see “Immunoprecipitation”). (B) PhosphorImager data of VWF immunoprecipitates from cell lysates (C) and chase medium (M) of HUVECs metabolically labeled as in panel A and chased for 24 hours. Immunoprecipitates were treated with 25 mM Endo H (+) or buffer alone (−). (C) Quantification of pulse-chase experiments such as that shown in panel A. The left panel shows the time course for the appearance of mature VWF (▴) and AgII (●) and the disappearance of proVWF (■) in cell lysates. The right panel shows the corresponding time course for the appearance of these proteins in the media. The radiolabeled bands were quantified as described in “Immunoprecipitation.” The value for each time point was calculated from duplicate observations made in each of 2 independent experiments. Within each experiment the mean value of duplicates at each time point was calculated and expressed as a percentage of the maximum mean observation. The plotted data represent the means (± range) of these percentages over the 2 independent experiments. The experiment was repeated on 3 further occasions with slightly different time points but yielding equivalent results.
Pulse-chase analysis of VWF and AgII processing and secretion in HUVECs. (A) Phosphor-Imager data of VWF (top) and AgII (bottom) immunoprecipitates from cell lysates (Cells) and chase media (Medium) of HUVECs metabolically labeled with [35S]-cysteine/methionine for 30 minutes and subsequently chased for the times indicated. Immunoprecipitates were separated by SDS-PAGE on 6% polyacrylamide gels and exposed to a PhosphorImager plate for 7 days (see “Immunoprecipitation”). (B) PhosphorImager data of VWF immunoprecipitates from cell lysates (C) and chase medium (M) of HUVECs metabolically labeled as in panel A and chased for 24 hours. Immunoprecipitates were treated with 25 mM Endo H (+) or buffer alone (−). (C) Quantification of pulse-chase experiments such as that shown in panel A. The left panel shows the time course for the appearance of mature VWF (▴) and AgII (●) and the disappearance of proVWF (■) in cell lysates. The right panel shows the corresponding time course for the appearance of these proteins in the media. The radiolabeled bands were quantified as described in “Immunoprecipitation.” The value for each time point was calculated from duplicate observations made in each of 2 independent experiments. Within each experiment the mean value of duplicates at each time point was calculated and expressed as a percentage of the maximum mean observation. The plotted data represent the means (± range) of these percentages over the 2 independent experiments. The experiment was repeated on 3 further occasions with slightly different time points but yielding equivalent results.
Immediately after passing through the Golgi, proVWF is either cleaved or secreted
In addition to mature VWF, the anti-VWF antibodies precipitated a higher molecular weight protein that appeared in the medium during the chase (Figure 1A top, and 1B, open arrowhead with asterisk). This protein was of similar molecular weight to the 360-kDa proVWF immunoprecipitated from cell lysates (open arrowhead), but on closer inspection it was found to migrate slightly slower (see also Figures 2A, 3A). The secreted protein was Endo-β-N-acetylglucosaminidase H (Endo H)–resistant, whereas the 360-kDa protein in the cell lysates was entirely Endo H–sensitive (Figure 1B). The Endo H–resistant, secreted proVWF therefore corresponded to the complex sugar form of the precursor previously described.16-18 Endo H resistance of N-glycans occurs as a result of sugar processing events that take place in the Golgi apparatus,19 and consequently these 2 molecular weight variants of proVWF represented pre-Golgi (ie, nascent biosynthetic protein in the endoplasmic reticulum [ER]) and post-Golgi forms of the precursor. Endo H–resistant proVWF was not detected in the cell lysates (in these and >10 other pulse chase experiments; J.P.G., unpublished data, November 2007), although the acquisition of Endo H resistance is an intracellular event; therefore, these molecules must have existed within the cells for a finite length of time before secretion. These data show that once proVWF has passed through the Golgi apparatus (and in so doing acquiring Endo H resistance) it is either cleaved or rapidly secreted from the cell. The lack of accumulation of post-Golgi material is the hallmark of the constitutive secretory pathway,20 and consequently secreted proVWF behaves with the characteristics of a protein secreted through this route.
Treatment with NH4Cl diverts VWF and AgII into the constitutive secretory pathway. (A) PhosphorImager data of VWF (left) and AgII (right) immunoprecipitated from cell lysates (C) and medium (M) of HUVECs metabolically labeled for 30 minutes with [35S]-cysteine/methionine and chased in the presence (NH4Cl) or absence (Control) of 25 mM NH4Cl for 24 hours. (B) The time courses for appearance of proVWF (□, ■) and mature VWF (▵, ▴) in the media of HUVECs metabolically labeled as in panel A and chased for the times indicated in the presence (■, ▴) or absence (□, ▵) of 25 mM NH4Cl. Data are expressed as a percentage of the maximum value for each protein species secreted. Data shown are the means (± range) of 2 observations. The experiment was repeated with similar results. (C) A representative time course for the appearance of VWF immunoreactivity, determined by ELISA, in fresh growth medium (□) and growth medium containing 25 mM NH4Cl (▴) or 30 μM histamine (●). Data points represent means (± range) of duplicate observations. The experiment was repeated on at least 3 occasions with similar results. The amount of VWF immunoreactivity secreted is expressed as IU/mL after calibration against a known standard (see “Measurement of VWF secretion by enzyme-linked immunosorbent assay”).
Treatment with NH4Cl diverts VWF and AgII into the constitutive secretory pathway. (A) PhosphorImager data of VWF (left) and AgII (right) immunoprecipitated from cell lysates (C) and medium (M) of HUVECs metabolically labeled for 30 minutes with [35S]-cysteine/methionine and chased in the presence (NH4Cl) or absence (Control) of 25 mM NH4Cl for 24 hours. (B) The time courses for appearance of proVWF (□, ■) and mature VWF (▵, ▴) in the media of HUVECs metabolically labeled as in panel A and chased for the times indicated in the presence (■, ▴) or absence (□, ▵) of 25 mM NH4Cl. Data are expressed as a percentage of the maximum value for each protein species secreted. Data shown are the means (± range) of 2 observations. The experiment was repeated with similar results. (C) A representative time course for the appearance of VWF immunoreactivity, determined by ELISA, in fresh growth medium (□) and growth medium containing 25 mM NH4Cl (▴) or 30 μM histamine (●). Data points represent means (± range) of duplicate observations. The experiment was repeated on at least 3 occasions with similar results. The amount of VWF immunoreactivity secreted is expressed as IU/mL after calibration against a known standard (see “Measurement of VWF secretion by enzyme-linked immunosorbent assay”).
Brefeldin A inhibits constitutive secretion of VWF but has a modest effect on the nonstimulated release of VWF from HUVECs. (A) PhosphorImager data of VWF (left) and AgII (right) immunoprecipitated from cell lysates (C) and medium (M) of HUVECs metabolically labeled with [35S]-cysteine/methionine for 30 minutes and chased in the presence (BFA) or absence (Control) of 5 μM BFA for 6 hours. (B,C) A time course of appearance of VWF immunoreactivity, determined by ELISA, in fresh growth medium (□) and fresh growth medium containing 5 μM BFA (▴) from HUVECs (B) and CHO cells stably expressing VWF (C). The amount of VWF immunoreactivity secreted is expressed as IU/mL after calibration against a known standard (see “Measurement of VWF by enzyme-linked immunosorbent assay”). In each case data points represent the means (± range) of duplicate observations in a representative experiment. Experiments in CHO were repeated on a further 2 occasions with similar results. The experiment in HUVECs was repeated on many occasions (n = 17) with variable results, see discussion in “BFA blocks secretion of newly synthesized VWF but does not abolish spontaneous release of VWF.”
Brefeldin A inhibits constitutive secretion of VWF but has a modest effect on the nonstimulated release of VWF from HUVECs. (A) PhosphorImager data of VWF (left) and AgII (right) immunoprecipitated from cell lysates (C) and medium (M) of HUVECs metabolically labeled with [35S]-cysteine/methionine for 30 minutes and chased in the presence (BFA) or absence (Control) of 5 μM BFA for 6 hours. (B,C) A time course of appearance of VWF immunoreactivity, determined by ELISA, in fresh growth medium (□) and fresh growth medium containing 5 μM BFA (▴) from HUVECs (B) and CHO cells stably expressing VWF (C). The amount of VWF immunoreactivity secreted is expressed as IU/mL after calibration against a known standard (see “Measurement of VWF by enzyme-linked immunosorbent assay”). In each case data points represent the means (± range) of duplicate observations in a representative experiment. Experiments in CHO were repeated on a further 2 occasions with similar results. The experiment in HUVECs was repeated on many occasions (n = 17) with variable results, see discussion in “BFA blocks secretion of newly synthesized VWF but does not abolish spontaneous release of VWF.”
Mature VWF and AgII appear in the medium more slowly than proVWF and more slowly than they appear in the cells
Further examination of pulse-chase data, such as that depicted in Figure 1A, showed differences in the kinetics of appearance of the various proVWF-derived proteins in the cells and media (Figure 1C). The appearance of both AgII and mature VWF within cell lysates occurred with almost identical kinetics, reaching a peak approximately 5 hours after the end of labeling (Figure 1C left ● and ▴, respectively). The kinetics of disappearance of their precursor, proVWF, in the cells (Figure 1C left ■) was complementary to this increase, such that the time required to reach 50% of their maximum values was similar for all 3 proteins (2-2.5 hours). These data are consistent with AgII and mature VWF being formed within the cell at the same time, as would be expected of the 2 products of proVWF cleavage.
Strikingly, the 3 proteins (proVWF, mature VWF, and AgII) appeared in the media with significantly different kinetics (Figure 1C, right). The Endo H–resistant form of proVWF peaked in the medium rapidly, just 3 hours into the chase (Figure 1C right ■). Both mature VWF and AgII appeared in the medium with kinetics that were almost identical to each other (Figure 1C right ● and ▴) but much slower than that of proVWF (Figure 1C right ■), and also much slower than their appearance in the cells (Figure 1C left ● and ▴). The precise kinetic profile of proVWF secretion was complicated by the apparent instability of this antigen in the medium (see next paragraph), but these data strongly suggested that both mature VWF and AgII were being secreted into the medium by the same pathway, but that this was a significantly slower route than that taken by the small fraction of secreted proVWF. (Note that in Figure 1C the amounts of [35S]-labeled protein recovered at each time point are expressed as percentages of the maximum value for that protein. The actual quantity of radioactivity recovered from the conditioned media as proVWF was a small fraction of that recovered as mature VWF. See Figure 1A top, and compare axes for [35S]-labeled proVWF and mature VWF in Figure 4B,C.) The simplest explanation for the delayed secretion compared with their appearance in the cells was that, unlike secreted proVWF that appeared to be secreted constitutively (as described), the newly formed mature VWF and AgII were accumulating (albeit transiently) in a post-Golgi compartment, and it was from this compartment that they were subsequently being released into the medium. Because the only post-Golgi storage organelle known to contain mature VWF and AgII is the WPB, we postulated that AgII and mature VWF were being efficiently sorted to their storage organelle the WPB and then spontaneously secreted from this compartment, in the absence of stimulation; a process termed basal secretion.11
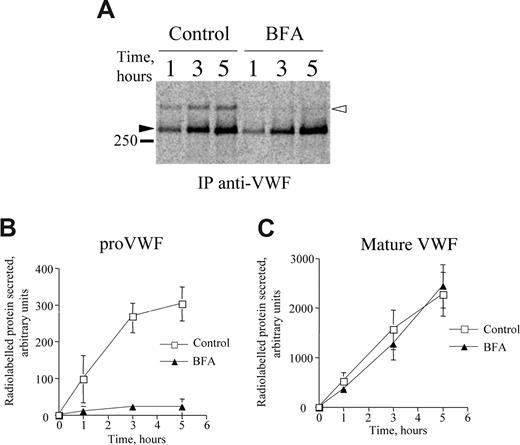
Brefeldin A preferentially inhibits secretion of proVWF from HUVECs. (A) PhosphorImager data of VWF immunoprecipitated from medium of long-term metabolically labeled HUVECs and chased in the presence (BFA) or absence (Control) of 5 μM Brefeldin A during a 5-hour period. Mature VWF and proVWF are indicated by the closed and open arrowheads, respectively. (B,C) Quantification of the time course of appearance of radiolabeled proVWF (B) and mature VWF (C) in the medium of cells chased in the presence (▴) or absence (□) of 5 μM BFA. Data points represent means (± SD) of 6 observations. Experiments were repeated on 2 further occasions with similar results.
Brefeldin A preferentially inhibits secretion of proVWF from HUVECs. (A) PhosphorImager data of VWF immunoprecipitated from medium of long-term metabolically labeled HUVECs and chased in the presence (BFA) or absence (Control) of 5 μM Brefeldin A during a 5-hour period. Mature VWF and proVWF are indicated by the closed and open arrowheads, respectively. (B,C) Quantification of the time course of appearance of radiolabeled proVWF (B) and mature VWF (C) in the medium of cells chased in the presence (▴) or absence (□) of 5 μM BFA. Data points represent means (± SD) of 6 observations. Experiments were repeated on 2 further occasions with similar results.
As shown in the preceding paragraph, secreted proVWF, but not mature VWF or AgII, appeared to be unstable in the release medium (Figure 1C right, ■). We do not know what caused this but we saw it consistently (see Figure 2B), and it has also been noted in at least one other published study.18 Proteolysis was the most likely explanation for the disappearance of extracellular proVWF, and, although inclusion of a broad-spectrum protease inhibitor cocktail (effective against most lysosomal hydrolases) in the culture medium had no effect (J.P.G., unpublished data, November 2007), it is possible that the endoproteinase furin was responsible. Furin would probably not have been inhibited by the inhibitor cocktail used, and, although generally thought to be an enzyme of the TGN (where it is almost certainly the enzyme that normally cleaves intracellular proVWF21 ), it is also known to be able to act on extracellular substrates as it cycles between the TGN and the cell surface.22 Work is currently ongoing to investigate this phenomenon and the possible confounding effects of this putative extracellular processing on our kinetic analysis.
NH4Cl prevents sorting of newly synthesized VWF to WPB and converts the secretion kinetics of mature VWF to that of proVWF
Treatment of HUVECs with membrane-permeable weak bases such as chloroquine or NH4Cl has been shown to divert the trafficking of newly synthesized VWF away from storage in WPBs and leads to it being secreted from the cell in an apparently constitutive manner.23 This is thought to be due to perturbation of a pH-dependent sorting process at the level of the TGN,23 although it has also been shown that NH4Cl can influence protein sorting in post-TGN compartments.24 If the secretion kinetics of mature VWF observed above (Figure 1) were due to basal release after pH-dependent sorting to the WPBs, they should be sensitive to NH4Cl. Conversely, the secretion kinetics of proteins released by the constitutive secretory pathway should be relatively unaffected. We first confirmed that NH4Cl ablated VWF storage in our cells using pulse-labeled HUVECs chased for 24 hours in the presence or absence of 25 mM NH4Cl. Under control conditions 40% and 56% of the immunoprecipitated radiolabeled mature VWF and AgII, respectively, were found associated with the cell lysates at the end of the 24-hour chase period (Figure 2A control lanes, C expressed as percentage of C + M). This was consistent with our previous observations (Figure 1). In contrast, after a 24-hour chase in the presence of 25 mM NH4Cl, metabolically labeled mature VWF and AgII were found exclusively in the media (Figure 2A NH4Cl lanes).
Having confirmed that VWF storage in our cells was sensitive to NH4Cl, we looked at the effects of the drug on the rate of appearance of newly synthesized mature VWF and proVWF in the medium. The experiment was performed as for Figure 1 except that the metabolically labeled cells were chased in the presence or absence of 25 mM NH4Cl. Under control conditions, as seen previously, we observed a striking contrast in the rate of appearance of proVWF and mature VWF in the medium (Figure 2B □ compared with ▵, respectively) with mature VWF being secreted at a reduced rate. The addition of 25 mM NH4Cl to the chase medium caused the initial rate of secretion of mature VWF to increase significantly (Figure 2B, ▵ compared with ▴) so that it closely resembled that of the control value for proVWF (Figure 2B □). The drug had almost no effect on the initial rate of appearance of proVWF itself (Figure 2B □ compared with ■). The sensitivity of the kinetics of mature VWF secretion to NH4Cl was entirely consistent with perturbation of pH-dependent sorting into the regulated secretory pathway at the level of the TGN or very soon after. In addition, the similarity between the initial rate of secretion of proVWF in the presence and absence of NH4Cl supported our initial conclusion that proVWF was constitutively secreted.
NH4Cl leads to a many-fold increase in spontaneous VWF secretion
After confirming that NH4Cl abolished the storage of newly synthesized (metabolically labeled) VWF, we investigated the effect of NH4Cl on the secretion of cellular VWF immunoreactivity measured with ELISA. Under control conditions VWF was secreted from the cells in a linear fashion during the 5-hour period (1.1 U/mL per hour; Figure 2C □). The addition of NH4Cl to the incubation medium caused a 4-fold increase in the rate of VWF release (4.5 U/mL per hour; Figure 2C ▴). For comparison the effects of stimulation with a known WPB secretagogue, histamine, are shown (Figure 2C ●). Histamine caused a nonlinear increase in VWF release with its major effect occurring in the first hour. These data were entirely consistent with NH4Cl diverting biosynthetic VWF into the constitutive secretory pathway at the TGN, although they could not rule out a post-Golgi site of action.24
BFA blocks secretion of newly synthesized VWF but does not abolish spontaneous release of VWF
To estimate the contribution of constitutive secretion to the VWF spontaneously secreted by endothelial cells, we made use of BFA, a fungal metabolite, widely used in membrane trafficking studies to block anterograde trafficking through the secretory pathway at the level of the Golgi apparatus,25-27 without affecting the release of preformed regulated secretory organelles.26 The efficacy of BFA can vary according to cell type; therefore, we first established that it was effective at preventing secretion of newly synthesized VWF from HUVECs using pulse-labeled cells chased for 6 hours in fresh growth medium in the presence or absence of 5 μM BFA (Figure 3A). Under control conditions a significant amount of metabolically labeled VWF (Figure 3A left, control) and AgII (Figure 3A right, control) were recovered in the medium after 5 hours of chase (note that the shorter chase time, compared with Figure 2, leads to a decrease in the proportion of antigens recovered in the medium). However, in the presence of BFA there was no detectable VWF or AgII in the medium and virtually no conversion of the proVWF to its lower molecular weight products.
We next asked what effect BFA would have on the VWF immunoreactivity spontaneously secreted from nonstimulated HUVECs over a similar time course measured by VWF ELISA. For comparison we also performed this experiment on CHO fibroblasts stably transfected with a plasmid encoding full-length human proVWF. These cells do not have a regulated secretory pathway and are known to secrete exogenous VWF constitutively.5,28 Representative examples of such experiments are shown (Figure 3B,C). Under control conditions, there was a continuous release of VWF from the HUVECs at a fairly constant rate of approximately 2 U/mL per hour (Figure 3B □). The addition of 5 μM BFA to the medium produced only a modest inhibition of VWF secretion (Figure 3B ▴). Secretion still appeared to be linear but at a rate of approximately 1.7 U/mL per hour (representing a 15% inhibition). In contrast, BFA completely abolished the secretion of VWF from the transfected CHO cells (Figure 3C ▴).
The extent of BFA-induced inhibition in the HUVECs varied considerably between experiments, but over the course of 17 independent experiments the mean BFA inhibition during a 3-hour period was less than 20% (18.2% ± 15.7%; the maximum inhibition in any single experiment was 41%). Work is ongoing to try to explain this interexperimental variability, and, although we were careful in these experiments to maintain uniform culturing conditions, it may be related to the known influence of environmental factors on VWF trafficking in HUVECs.29
These data show that constitutive secretion did not constitute the major route for nonstimulated release of VWF from HUVECs. On average, less than 20% of the spontaneously secreted VWF was leaving the cells by this pathway.
BFA preferentially inhibits secretion of proVWF
We examined the effect of BFA on VWF secretion in molecular detail using long-term metabolically labeled HUVECs. Because this method relies on PhosphorImager analysis of radiolabeled, immunoprecipitated VWF resolved by SDS-PAGE, it allowed the discrimination of proVWF and mature VWF, something that the VWF ELISA was not able to do. The PhosphorImager data and quantification of a representative experiment are shown (Figure 4A-C). Under control conditions the amount of mature VWF in the medium increased in a linear fashion during the 5-hour incubation (Figure 4C □). The amount of proVWF appearing in the medium also increased during this period, but as already noted (see Figures 1, 2) the rate of appearance started to plateau between the 3- and 5-hour time points (Figure 4B □). As described above, the proVWF appearing in the medium represented constitutive secretion of metabolically labeled proVWF that was in the ER at the end of the 16-hour labeling period, and, consistent with this, the BFA treatment completely abolished its secretion during the subsequent 5-hour chase (Figure 4B ▴).
In contrast, spontaneous secretion of metabolically labeled, mature VWF from the same cells was almost completely unaffected by the BFA treatment (Figure 4C ▴), showing that it was not being constitutively released but secreted from a pool that had passed beyond the brefeldin block during the 16-hour labeling period. This mature VWF almost certainly represented VWF that had been sorted to WPBs and subsequently basally secreted from WPBs during the 5-hour chase period.
CHX inhibits basal VWF release through a mechanism independent of VWF biosynthesis
Spontaneous secretion of VWF from nonstimulated HUVECs is generally interpreted as arising from constitutive secretion.9 One of the reasons for this was the observation that VWF secretion from cultured endothelial cells was completely inhibited by the protein synthesis inhibitor CHX (whereas secretagogue-stimulated secretion was not).30 We were interested to determine whether the basal secretion of VWF that we had characterized was equivalent to the CHX-sensitive secretion reported previously and therefore looked at the effect of overnight treatment with 5 μM CHX on basal and 30 μM histamine–stimulated secretion of VWF immunoreactivity. A representative example of such an experiment is shown (Figure 5A). Consistent with the previously published reports, overnight preincubation of HUVECs with 5 μM CHX almost completely inhibited nonstimulated secretion of VWF into the culture medium during a 5-hour period (Figure 5A compare □ with ●). The CHX treatment had only a minor influence on the histamine-stimulated release of VWF immunoreactivity (Figure 5A compare ▵ and ▴). As well as showing secretion from a storage pool, the histamine-stimulated release of VWF from CHX-treated cells showed that the inhibitory effect of CHX on the appearance of VWF immunoreactivity in the medium under nonstimulated conditions was due to inhibition of a secretory process rather than general toxicity of the drug or loss of cells during the overnight incubation.
Cycloheximide inhibits basal secretion of VWF. (A) HUVECs were incubated overnight (∼16 hours) with full-growth medium alone (□, ▵) or growth medium containing 5 μM cycloheximide (●, ▴). Medium was replaced with fresh full-growth medium with no additions (Control; □) or supplemented with 30 μM histamine (▵), 5 μM cycloheximide (CHX; ●), or both histamine and cycloheximide (▴) and secretion of VWF immunoreactivity into the media during a 5-hour period was measured by ELISA. Data points represent the means (± range) of duplicate observations. (B) HUVECs were metabolically labeled with [35S]-cysteine/methionine for 30 minutes, chased for 5 hours in fresh full-growth medium before a further 24-hour incubation in growth medium with (CHX) or without (Control) 5 μM cycloheximide. Radiolabeled VWF secreted into fresh growth medium alone (Control) or fresh growth medium supplemented with 5 μM cycloheximide (CHX) during a further 5-hour period was immunoprecipitated, separated by SDS-PAGE, and quantified (Med), along with the VWF remaining in the cell lysates (Cells) at the end of the experiment. Representative PhosphorImager data are shown. Mature VWF is indicated by ▶. (C) Amount of VWF recovered from cells and medium expressed relative to Control values. Values represent means (± SD), n = 9.
Cycloheximide inhibits basal secretion of VWF. (A) HUVECs were incubated overnight (∼16 hours) with full-growth medium alone (□, ▵) or growth medium containing 5 μM cycloheximide (●, ▴). Medium was replaced with fresh full-growth medium with no additions (Control; □) or supplemented with 30 μM histamine (▵), 5 μM cycloheximide (CHX; ●), or both histamine and cycloheximide (▴) and secretion of VWF immunoreactivity into the media during a 5-hour period was measured by ELISA. Data points represent the means (± range) of duplicate observations. (B) HUVECs were metabolically labeled with [35S]-cysteine/methionine for 30 minutes, chased for 5 hours in fresh full-growth medium before a further 24-hour incubation in growth medium with (CHX) or without (Control) 5 μM cycloheximide. Radiolabeled VWF secreted into fresh growth medium alone (Control) or fresh growth medium supplemented with 5 μM cycloheximide (CHX) during a further 5-hour period was immunoprecipitated, separated by SDS-PAGE, and quantified (Med), along with the VWF remaining in the cell lysates (Cells) at the end of the experiment. Representative PhosphorImager data are shown. Mature VWF is indicated by ▶. (C) Amount of VWF recovered from cells and medium expressed relative to Control values. Values represent means (± SD), n = 9.
These data showed that the spontaneous VWF secretion we had characterized was equivalent to the CHX-sensitive secretion previously reported.30 Inhibiting de novo synthesis of VWF with CHX would obviously inhibit its constitutive secretion, but our model of a basal rather than constitutive secretory process predicted that the inhibitory effect of CHX on the nonstimulated release of VWF was due to inhibition of synthesis of a protein other than the secreted VWF. To test this, we metabolically labeled HUVECs for 30 minutes, followed by a 5-hour incubation in full-growth medium to ensure that most of the [35S]-labeled VWF had been chased into the regulated secretory pathway (see Figure 1B). The cells were then incubated overnight in either full-growth medium or growth medium containing 5 μM CHX, followed by a 5-hour release period in the presence or absence of CHX. VWF was immunoprecipitated from cells and medium and the amounts were quantified. We found that CHX treatment inhibited secretion of the metabolically labeled VWF (Figure 5B,C) showing that the inhibitory effect of CHX on the spontaneous secretion of VWF was independent of the synthesis of VWF itself, which is entirely consistent with our model of basal secretion.
Discussion
It has been proposed that 90% to 95% of VWF synthesized by endothelial cells in culture is secreted constitutively with only 5% to 10% being sorted to the regulated secretory pathway.9 Conversely, other work has suggested that constitutive secretion of VWF from cultured endothelial cells is insignificant.10 Our data are consistent with the latter proposal. We have shown that most mature VWF (and AgII) spontaneously released into the medium by nonstimulated HUVECs is not secreted by the constitutive secretory pathway but by a route that is kinetically delayed, involves a pH-dependent sorting process, is insensitive to BFA-induced blockade of the anterograde secretory pathway, and does not require de novo synthesis of VWF itself. This secretory profile has all the characteristics of basal secretion, which is defined as the nonstimulated secretion of regulated secretory cargo.11 Basal secretion can be due to nonstimulated release of fully formed regulated secretory granules, release of newly formed “immature” secretory granules, or budding of vesicles from maturing secretory granules (a process also referred to as constitutive-like secretion31,32 ). The crucial point is that all this material had been sorted into the regulated secretory pathway before being released.
The possibility of 2 modes of unstimulated secretion (constitutive and basal) from regulated secretory cells had not been recognized when the original estimates of VWF sorting in HUVECs were reported. Our demonstration that the majority (on average 80%) of VWF spontaneously secreted from cultured HUVECs is actually by basal secretion of mature VWF that had been sorted to the regulated secretory pathway has a significant effect on our understanding of VWF trafficking, and it requires a reevaluation of the only published estimate of the sorting efficiency of newly synthesized VWF to WPBs.9 The method used to provide these data were based on VWF secreted from metabolically labeled HUVECs that had been chased for 6 to 7 hours before a 10-minute challenge with a WPB secretagogue. The VWF secreted during the entire chase period and during the stimulation was immunoprecipitated and the radiolabeled VWF was quantified. The VWF released during the 6- to 7-hour chase period was defined as having been constitutively secreted, whereas the VWF released during the secretagogue treatment was defined as the VWF that was sorted. Although the data themselves were not published, the 5% to 10% sorting estimate was presumably calculated by dividing the latter value from the sum of both values.9
The 2 main limitations of this type of approach that would lead to underestimates of sorting efficiencies have been pointed out in the context of the sorting of regulated secretory proteins in neuroendocrine cells.11 First, comparing the ratio of stimulated to unstimulated secretion is not a measure of sorting itself, but more a measure of “secretagogue responsiveness”: the fraction of the material that has been sorted and that can be released by stimulation with secretagogue. With the possible exception of mast cells, stimulation rarely leads to complete depletion of the regulated secretory compartment, and this is certainly the case with HUVECs.33 Second, as we have shown here, most VWF collected during the 6- to 7-hour chase period will not have been constitutively secreted but basally secreted material that had been sorted into WPBs, but not stored in WPBs for very long. Basal secretion therefore leads to a double underestimate of sorting efficiency when calculated by this method. It increases the apparent rate of constitutive secretion while simultaneously depleting material from a potentially secretagogue-responsive pool.
Although basal secretion of regulated secretory organelles is known to occur in other cell types, until now no attempts were made to quantify the phenomenon in endothelial cells. Our data show that approximately 50% of newly synthesized mature VWF is secreted from nonstimulated HUVECs during a 24-hour period (see Figure 1). The majority of this is basal secretion of post-Golgi secretory organelles, probably WPBs. If the basally secreted organelles are representative of the WPB population as a whole, it would give a half-life for WPBs of about a day, which is consistent with a published estimate.34 This is also reasonable in light of the time it takes to deplete cellular WPB populations with NH4Cl.23 However, it is known that newly formed WPBs exist for a time in an immature state with respect to both membrane composition35 and VWF content,36 and it is therefore possible that basal secretion of WPBs varies with maturation state of the organelle.
There are still many questions that remain to be answered with regard to the trafficking of VWF in endothelial cells. However, this work raises the possibility that circulating VWF (most of which is thought to be derived from nonregulated secretion from endothelial cells) is more the result of unstimulated exocytosis of WPBs rather than inefficient sorting of VWF into that organelle. Therefore, as well as changing our understanding of the cell biology of VWF, these data also emphasize the need to focus future studies on the mechanisms of WPB maturation and exocytosis (stimulated and nonstimulated) in the hope that we may learn enough to be able to perturb these processes in vivo to effect beneficial change.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Iain Robinson and Tom Carter for useful discussion during the work and Tom Carter for critical comments on the manuscript.
This work was supported by the Medical Research Council. J.P.G. is an MRC Career Development Fellow. L.J.H. is an MRC Staff Scientist. M.J.H. is an MRC Career-Track Scientist.
Authorship
Contribution: J.P.G. performed the research, analyzed the data, and wrote the paper; L.J.H. contributed vital new reagents; and M.J.H. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew James Hannah, Department of Molecular Neuroendocrinology, Medical Research Council National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, United Kingdom; e-mail: mhannah@nimr.mrc.ac.uk.

![Figure 1. Pulse-chase analysis of VWF and AgII processing and secretion in HUVECs. (A) Phosphor-Imager data of VWF (top) and AgII (bottom) immunoprecipitates from cell lysates (Cells) and chase media (Medium) of HUVECs metabolically labeled with [35S]-cysteine/methionine for 30 minutes and subsequently chased for the times indicated. Immunoprecipitates were separated by SDS-PAGE on 6% polyacrylamide gels and exposed to a PhosphorImager plate for 7 days (see “Immunoprecipitation”). (B) PhosphorImager data of VWF immunoprecipitates from cell lysates (C) and chase medium (M) of HUVECs metabolically labeled as in panel A and chased for 24 hours. Immunoprecipitates were treated with 25 mM Endo H (+) or buffer alone (−). (C) Quantification of pulse-chase experiments such as that shown in panel A. The left panel shows the time course for the appearance of mature VWF (▴) and AgII (●) and the disappearance of proVWF (■) in cell lysates. The right panel shows the corresponding time course for the appearance of these proteins in the media. The radiolabeled bands were quantified as described in “Immunoprecipitation.” The value for each time point was calculated from duplicate observations made in each of 2 independent experiments. Within each experiment the mean value of duplicates at each time point was calculated and expressed as a percentage of the maximum mean observation. The plotted data represent the means (± range) of these percentages over the 2 independent experiments. The experiment was repeated on 3 further occasions with slightly different time points but yielding equivalent results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-12-130740/6/m_zh80110819830001.jpeg?Expires=1769110563&Signature=gKz8DvkPjOIIG54gYkiOk~wLCRdb2rMC4euFwzy0iLnJGoRBO3kaDzs25IrCoJDGwuqlprd040IHv04vvlAa9BZBvs3OZJdgInjxENXlNTpzL662tnzupQiL8ZFh6lsS2pILEx50K9~VyKpT8dctS4gUP8P5Q6xBbhHVXRQVdLVR8n2t8OMhXvAi-PooGFyL6S4Lckn3wxuCnjp~BRbFEtzDEpQ~v8wrPTaz~fAorekICM7wF5OxOQheFrAhFsqKjL2ntGdYmpcnV1w0QXTBcZ3gQZ0bggG13yGC3pZzUnJzjGyIGDWhiI9a6kVUmQLkecvm0HKpxK3DTr1ztJIckQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Treatment with NH4Cl diverts VWF and AgII into the constitutive secretory pathway. (A) PhosphorImager data of VWF (left) and AgII (right) immunoprecipitated from cell lysates (C) and medium (M) of HUVECs metabolically labeled for 30 minutes with [35S]-cysteine/methionine and chased in the presence (NH4Cl) or absence (Control) of 25 mM NH4Cl for 24 hours. (B) The time courses for appearance of proVWF (□, ■) and mature VWF (▵, ▴) in the media of HUVECs metabolically labeled as in panel A and chased for the times indicated in the presence (■, ▴) or absence (□, ▵) of 25 mM NH4Cl. Data are expressed as a percentage of the maximum value for each protein species secreted. Data shown are the means (± range) of 2 observations. The experiment was repeated with similar results. (C) A representative time course for the appearance of VWF immunoreactivity, determined by ELISA, in fresh growth medium (□) and growth medium containing 25 mM NH4Cl (▴) or 30 μM histamine (●). Data points represent means (± range) of duplicate observations. The experiment was repeated on at least 3 occasions with similar results. The amount of VWF immunoreactivity secreted is expressed as IU/mL after calibration against a known standard (see “Measurement of VWF secretion by enzyme-linked immunosorbent assay”).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-12-130740/6/m_zh80110819830002.jpeg?Expires=1769110563&Signature=Le8yH9WHPZdVqyHxiUX9NMS3wGCTxNKLsznDu64fpUt7T5gJ9qAumBh1ZKbmW~1bw4Acxh0CFbGrUUWhiwWVcnxP8~3uJyTML2FP8HLUc~vBU201mJiVCHMzONGVYfnjAhD8sDwAK6oDuqbq12SukSoymaW4Wox~8YSlJ1WZnIV1pJKSkv5fN8B5rL6IUL2Yl4uT7e68WxrkYDVWkDt4feIuN8MIMHwwqymQRC3hezgkNjZbQ33DfEpy4CUcjyuP8WUiN2NuR5tizror1ZgCkCX0wl94kz-oe7sUhGJTXXQGkGUg8rgCbc5MWSY7O8PG20MUtkUea1WqVCiHQ5qPMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Brefeldin A inhibits constitutive secretion of VWF but has a modest effect on the nonstimulated release of VWF from HUVECs. (A) PhosphorImager data of VWF (left) and AgII (right) immunoprecipitated from cell lysates (C) and medium (M) of HUVECs metabolically labeled with [35S]-cysteine/methionine for 30 minutes and chased in the presence (BFA) or absence (Control) of 5 μM BFA for 6 hours. (B,C) A time course of appearance of VWF immunoreactivity, determined by ELISA, in fresh growth medium (□) and fresh growth medium containing 5 μM BFA (▴) from HUVECs (B) and CHO cells stably expressing VWF (C). The amount of VWF immunoreactivity secreted is expressed as IU/mL after calibration against a known standard (see “Measurement of VWF by enzyme-linked immunosorbent assay”). In each case data points represent the means (± range) of duplicate observations in a representative experiment. Experiments in CHO were repeated on a further 2 occasions with similar results. The experiment in HUVECs was repeated on many occasions (n = 17) with variable results, see discussion in “BFA blocks secretion of newly synthesized VWF but does not abolish spontaneous release of VWF.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-12-130740/6/m_zh80110819830003.jpeg?Expires=1769110563&Signature=PcMvwIiPMhcPWrvaz86uYSjuudFZSFBsky5K15qKgKv0lV-DF6KXpFcReEryY9SYAGSUh0PNdSLxhcJl10HaPmW~kqfWDS9MXic~hwmRx6DNae~WfLZjb3KLqiiJu8Y~MsFeJmbsBpgyex64UEiVtM1faG8I0BM3S4SFIfWTkKQ6BBg476xoOJwRLGZ3s5tDOxiDBMeAftwFvZiGuQgW7H328Ej~7jZuxH-4rm6wPWiYtyddQWDUgD3QW9Hwhum~2s7DwKZbOawAa6VYe0Ylb5MC6UOuuFr9uv2Afy8ByJb5sJ686HiynCgV8l1d1BL8CLjl2sfwgONKlGjvSj2cUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Cycloheximide inhibits basal secretion of VWF. (A) HUVECs were incubated overnight (∼16 hours) with full-growth medium alone (□, ▵) or growth medium containing 5 μM cycloheximide (●, ▴). Medium was replaced with fresh full-growth medium with no additions (Control; □) or supplemented with 30 μM histamine (▵), 5 μM cycloheximide (CHX; ●), or both histamine and cycloheximide (▴) and secretion of VWF immunoreactivity into the media during a 5-hour period was measured by ELISA. Data points represent the means (± range) of duplicate observations. (B) HUVECs were metabolically labeled with [35S]-cysteine/methionine for 30 minutes, chased for 5 hours in fresh full-growth medium before a further 24-hour incubation in growth medium with (CHX) or without (Control) 5 μM cycloheximide. Radiolabeled VWF secreted into fresh growth medium alone (Control) or fresh growth medium supplemented with 5 μM cycloheximide (CHX) during a further 5-hour period was immunoprecipitated, separated by SDS-PAGE, and quantified (Med), along with the VWF remaining in the cell lysates (Cells) at the end of the experiment. Representative PhosphorImager data are shown. Mature VWF is indicated by ▶. (C) Amount of VWF recovered from cells and medium expressed relative to Control values. Values represent means (± SD), n = 9.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-12-130740/6/m_zh80110819830005.jpeg?Expires=1769110563&Signature=AXRJ~sOkIwPFIZuHtpriXKXBMKQ4bIrfZgyyXKJn2r3GTdgDkboXWKZPpJZzN7YVB6f4VexaCWaBhYQj3BS-x3U8MtwEMhAGWjBkIpUzsNp1yPQKLuGWxdSshoKwuC6erJhyN9VdezBCFeWSxBf7SbgPkTUtojLKzOG~rUxW5J5wteEhDHTdyLnnL1ICjcf56ChWzWVg4XS3kG9nhbKq9fNGtmonV0N4RqfGE9KiDZR8AAAy3bQeo8KRB-Snqs2BgEmtp7N5nmTmULIDPip~ypPvUA-a4gLkhtp8k2zlU05zt2gRA~pToZNOt5vNAX7lL3NClWqYt4muom5e-23T0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal