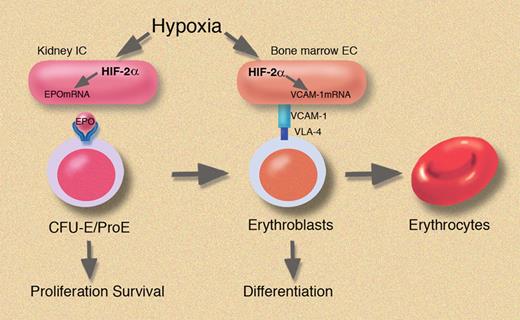
In this issue of Blood, Yamashita and colleagues provide evidence for additional oxygen-dependent regulation of erythropoiesis through HIF-2α–controlled expression of vascular adhesion molecule-1 in endothelial cells of the bone marrow microenvironment.
Oxygen-dependent regulation of erythropoiesis by hypoxia-inducible production of erythropoietin (EPO) has stimulated research for decades. It has ultimately led to the identification of cellular oxygen sensors in control of EPO production.1 Hypoxia-inducible transcription factor-1 (HIF-1) was identified by Wang et al from hypoxia-induced binding of a transcription factor complex to the EPO enhancer.2 Dissecting the regulatory pathways back to the cellular O2 sensors revealed a HIF-1 dimer consisting of an oxygen-labile α-subunit (HIF-1α, -2α, and -3α) and a constitutive β-subunit. Although HIF-1α was initially thought to control EPO gene expression, recent studies indicate a dominant role for HIF-2α in regulating EPO synthesis in mice3,4 and also in humans.5
Hypoxia stimulates EPO production mainly in the adult kidneys and cell-cell contacts of erythroblasts and endothelial cells (ECs) in the bone marrow through hypoxia-induced accumulation of HIF-2α. Professional illustration by Marie Dauenheimer.
Hypoxia stimulates EPO production mainly in the adult kidneys and cell-cell contacts of erythroblasts and endothelial cells (ECs) in the bone marrow through hypoxia-induced accumulation of HIF-2α. Professional illustration by Marie Dauenheimer.
In this issue of Blood, Yamashita and colleagues report on their use of mice with a knock-down mutation in HIF-2α that presented with normocytic anemia. Interestingly, EPO protein levels in HIF-2α knockdown mice were unaffected and obviously not responsible for causing the anemia. Instead, the authors identified a major defect in the hematopoietic microenvironment of HIF-2α knockdown mice that was due to reduced endothelial-specific expression of vascular adhesion molecule-1 (VCAM-1). VCAM-1 supports the interaction of hematopoietic and endothelial cells in the bone marrow microenvironment and is required for maturation of erythroid cells. Hypoxia-inducible VCAM expression was selectively regulated by HIF-2α, and defective erythropoiesis was rescued in HIF-2α knock-down mice with selectively restored HIF-2α expression in endothelial cells. As such, the study by Yamashita and colleagues underscores the importance of HIF-2α in the O2-dependent regulation of erythropoiesis. This work adds a further piece to the puzzle of how low oxygen tension in the bone marrow supports hematopoiesis by facilitating the interaction of stromal and hematopoietic cells.
Unexpected and puzzling, however, is the fact that HIF-2α knock-down did not reduce EPO synthesis; on the contrary, hepatic EPO mRNA levels in the knock-down mice tended to be higher than in wild-type controls. This finding deserves further study. However, the effect of reduced HIF-2α expression on the hematopoietic microenvironment, but not EPO synthesis, may indicate that remaining levels of HIF-2α in knock-down animals with incomplete deletion of the transcription factor subunit are sufficient for EPO expression. On the other hand, a recent report by Percy et al5 indicates that EPO synthesis is very sensitive to increased HIF-2α. In a family with a genetic mutation that reduces the degradation of HIF-2α under high oxygen tension, constitutively high levels of HIF-2α were made responsible for familial erythrocytosis.5 Although erythroid progenitor maturation was not specifically studied, increased HIF-2α levels may also have had an effect on the bone marrow microenvironment.
The HIF pathway is a potential therapeutic target, as evidenced by recent clinical trials in which attempts at pharmacologic inhibition of HIF degradation have been undertaken to increase EPO synthesis in patients.6 From the widespread importance of HIF-dependent regulation of gene expression, multiple effects would have been expected. So far, preliminary data indicate that erythropoiesis was preferentially induced through increased EPO synthesis and effects on enzymes involved in iron metabolism that are also under the control of HIF.6 The present study by Yamashita et al may explain why erythropoiesis is particularly sensitive to modulation of HIF-αs: because increased EPO falls on “fertile soil,” a bone marrow microenvironment that has been optimized by endothelial-specific, HIF-2α–driven expression of VCAM-1 to provide cell-cell contacts between stromal and hematopoietic progenitors.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■