Abstract
Leukocyte adhesion deficiency II (LAD II), also known as congenital disorder of glycosylation IIc (CDG-IIc), is a human disease in which a defective GDP-fucose transporter (SLC35C1) causes developmental defects and an immunodeficiency that is based on the lack of fucosylated selectin ligands. Since the study of in vivo leukocyte trafficking in patients with LAD II is experimentally limited, we analyzed this process in mice deficient for Slc35c1. We found that E-, L-, and P-selectin–dependent leukocyte rolling in cremaster muscle venules was virtually absent. This was accompanied by a strong but not complete decrease in firm leukocyte adhesion. Moreover, neutrophil migration to the inflamed peritoneum was strongly reduced by 89%. Previous reports showed surprisingly normal lymphocyte functions in LAD II, which indicated sufficient lymphocyte trafficking to secondary lymphoid organs. We now found that while lymphocyte homing to lymph nodes was reduced to 1% to 2% in Slc35c1−/− mice, trafficking to the spleen was completely normal. In accordance with this, we found a defect in the humoral response to a T cell–dependent antigen in lymph nodes but not in the spleen. Taken together, Slc35c1−/− mice show strongly defective leukocyte trafficking but normal lymphocyte homing to the spleen, which may explain normal lymphocyte functions in LAD II.
Introduction
Leukocyte adhesion deficiency II (LAD II), also known as congenital disorder of glycosylation IIc (CDG-IIc), is a rare inherited human disease that is characterized by the lack of fucosylated structures, including ligands for the selectin class of adhesion molecules.1-3
Several years ago we identified the genetic defect in this disease and found that a defective nucleotide sugar transporter called solute carrier family 35 member C1 (SLC35C1), which transfers GDP-fucose into the Golgi apparatus, is responsible for the hypofucosylation in LAD II.4,5 Patients with LAD II show strong mental and growth retardation as well as leukocytosis and immunodeficiency.1,2,6-8 Whereas the cause for the developmental defects in LAD II is still unknown, the immunodeficiency can readily be explained by the absence of fucosylated selectin ligands.1,9 Selectin ligands mediate leukocyte–endothelial cell interactions by binding to the 3 selectins, E-, L-, and P-selectin. These interactions are characterized by leukocyte rolling on endothelium, which is required for subsequent leukocyte activation, arrest, and extravasation into lymph nodes (LNs) and inflamed tissues.10 Of note, migration to LNs is dependent on the presence of fucosylated selectin ligands on high endothelial venules (HEVs) in LNs, whereas trafficking to sites of acute inflammation requires selectin ligand activity on leukocytes.11
A large body of data shows that in vitro binding of leukocytes of patients with LAD II to selectins is strongly reduced.1,6-9,12 However, in vivo studies are scarce due to the limitations of studies in humans. Von Andrian et al13 analyzed rolling of neutrophils from a patient with LAD II in inflamed venules of rabbit mesenteries and found a reduction of more than 80% of the number of rolling cells. In addition, it was shown that the number of neutrophils migrating to the inflamed skin in a patient with LAD II was only 2% to 6% of normal.14 Interestingly, T cell–dependent antibody responses to the bacteriophage ΦX174 and to keyhole limpet hemocyanin (KLH) were found to be normal in LAD II, implying that T-cell and B-cell functions are not perturbed.14,15 These findings led to the classification of LAD II in reviews and textbooks as a disease that mainly affects phagocytic cells.16-18 Importantly, normal T cell–dependent antibody responses suggested that sufficient lymphocyte homing to secondary lymphoid organs—where such adaptive immune responses are initiated—is possible in LAD II. However, in vivo lymphocyte homing to lymph nodes cannot be studied in humans.
Residual fucosylation-dependent lymphocyte homing to lymph nodes is theoretically possible in patients with LAD II. We have detected low residual fucosylation in leukocytes and fibroblasts of patients with LAD II by sensitive flow cytometry using the fucosylation-specific Aleuria aurantia lectin (AAL).6 This may also apply to HEVs, the lymphocyte entry sites within LNs, although this has not been tested. Residual fucosylation is obviously independent of SLC35C1 since it can be detected in cells from patients with nonfunctional and mislocalized SLC35C1 molecules6 as well as in Slc35c1−/− mice.19 Thus, residual fucosylation must be based on an alternative low-efficiency GDP-fucose transport system. This transport system may be identical to an as-yet-unknown mechanism that allows efficient refucosylation in the presence of high concentrations of exogenous L-fucose, an effect that forms the basis for the successful therapy of patients with LAD II.7,12,20 Whether such an alternative transport provides sufficient residual fucosylation in the absence of exogenous fucose and would thus allow lymph node entry of lymphocytes in LAD II is not known.
An alternative site for the initiation of T cell–dependent antibody responses is the spleen. This organ is particularly adapted to mount immune responses against blood-borne antigens and pathogens.21,22 Whether fucosylation plays a role in lymphocyte homing to the spleen is not clear. On the one hand, lymphocyte homing to the spleen appears to be independent from alpha1,3-fucosyltransferases (FucT) IV and VII, the glycosyltransferases which are required for selectin ligand formation in leukocytes and HEVs.23 On the other hand, studies with the sulphated L-fucose polymer fucoidan showed that this compound inhibits lymphocyte homing to the spleen by up to 63%,24-26 raising the question whether FucTIV/VII-independent fucosylation of the spleen is required for efficient homing to the spleen and whether lymphocyte homing to the spleen may be affected in LAD II.
Recently, a mouse knock-out model for Slc35c1-deficiency was generated.19 These mice show strong hypofucosylation, severe growth retardation, changes in lung morphology that resemble those in FucTVIII−/− mice,27 and high mortality. Hypocellularity of peripheral lymph nodes and persistent leukocytosis showed that the immune system is affected in Slc35c1−/− mice. Intravital microscopic observations of leukocyte rolling in HEVs of Peyer patches demonstrated a complete absence of L-selectin–mediated rolling. In addition, binding of E-selectin and P-selectin Fc-chimeric proteins to neutrophils of Slc35c1−/− mice was dramatically reduced in static in vitro assays. Finally we found that, like in patients with LAD II, exogenous L-fucose can correct the fucosylation defect in Slc35c1−/− cells, which points to the existence of an alternative Golgi GDP-fucose transport system.19
Here, we made use of Slc35c1−/− mice to study in vivo leukocyte trafficking under conditions similar to those in patients with LAD II. We show that leukocyte rolling and adhesion in cremaster muscle venules, neutrophil migration to inflamed peritoneum, and lymphocyte homing to LNs are strongly reduced in the LAD II model mice. In contrast, we found that lymphocyte trafficking to the splenic white pulp is normal in Slc35c1−/− mice. Accordingly, humoral immune responses of lymph nodes but not of the spleen were defective. We therefore suggest that SLC35C1-independent lymphocyte homing to the spleen partially compensates for the lack of LN accessibility, which explains why adaptive immune responses appear to be normal in patients with LAD II.
Methods
Animals
Slc35c1-deficient mice were generated as described earlier19 and kept as a heterozygous breeding colony. All mice used in these experiments (8 to 12 weeks old) were of mixed 129Sv/C57BL/6 background. Homozygous (+/+) littermates were used as control mice throughout. All animals were housed in a pathogen-free barrier facility, and experiments were approved by the Regierungspräsidium Karlsruhe, Germany (AZ 35-9185.81/G-69/05 and G35/06).
Generation of Slc35c1−/− bone marrow chimeric mice
Bone marrow chimeric mice with a Slc35c1−/− hematopoietic system (Slc35c1bm−/−) were generated by bone marrow transplantation as described.28 Briefly, recipient littermate control mice were irradiated by the linear accelerator Mevatron KD2 (Siemens, München, Germany) in 2 doses of 3.5 Gy (350 rad) each (total dose of 7 Gy [700 rad]) approximately 3 hours apart, followed by a recovery time of 1 hour before transplantation. Approximately 106 unfractionated donor Slc35c1−/− bone marrow cells in 0.5 mL media were delivered through the tail vein of each recipient mouse. More than 4 weeks after adoptive transfer, mice were used for intravital microscopic experiments. To formally test the successful adoptive transfer of Slc35c1−/− bone marrow into lethally irradiated control mice, binding of fucose-specific AAL to Gr1+ leukocytes from Slc35c1bm−/− mice was assessed by flow cytometric analysis. Similar to Slc35c1−/− Gr-1+ leukocytes, Gr-1+ cells from Slc35c1bm−/− mice showed dramatically reduced AAL binding when compared with Gr-1+ cells from control mice, indicating that the transfer of Slc35c1−/− bone marrow cells into lethally irradiated littermate control mice led to successful reconstitution of the hematopoietic system (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Antibodies and cytokines
The following mAbs were used: anti–P-selectin mAb RB40.34 (rat IgG1),29,30 anti–E-selectin mAb 9A9 (rat IgG1; gift from Dr B. Wolitzky, Mitocor, Nutley, NJ), anti–L-selectin mAb MEL-14 (rat IgG2a),29,30 anti-CD99 mAb mCD99–102.2 (rat IgG2a; own production, M.G.B.), anti–MAdCAM-1 mAb MECA 367 (rat IgG2a; BD Pharmingen, San Diego, CA), and granulocyte-specific anti–Ly-6G mAb Gr-1 (BD Pharmingen). Recombinant murine TNF-α (R&D Systems, Minneapolis, MN) was injected intrascrotally at a dose of 500 ng per mouse in a volume of 0.3 mL sterile saline 2 hours prior to the intravital microscopic experiment.
Flow cytometry for lectin binding to Gr1+ leukocytes
Flow cytometry was performed according to standard protocols12 with 10 μg/mL biotinylated AAL (Vector Laboratories, Burlingame, CA) and phycoerythrin (PE)–conjugated streptavidin (BD Pharmingen). Subsequently, cells were stained with APC-conjugated mAb Gr-1 and analyzed using the 4-decade FACS LSR II and FACSDiva and Cell Quest software package (Becton Dickinson, San Jose, CA).
Surgical preparation and intravital microscopy
Anesthesia and surgical preparation of the cremaster muscle for intravital microscopy experiments was performed as described previously.28 Intravital microscopy was conducted on an upright microscope (Model 512815/20; Leitz, Wetzlar, Germany) with a saline immersion objective (SW 40/0.75 numerical aperture). Experiments were recorded via a CCD camera system (model CF8/1; Kappa, Gleichen, Germany) on a Panasonic S-VHS recorder (Hamburg, Germany). Postcapillary venules under observation ranged from 20 to 40 μm in diameter. Systemic blood samples (10 μL) were taken before and during the experiment, stained with Tuercks solution (Merck, Darmstadt, Germany), and assessed for white blood cell count using a hemocytometer.
Data analysis of intravital experiments
Diameter and segment length of postcapillary venules were measured using a digital image processing system.31 Venular centerline erythrocyte velocity in the cremaster muscle was assessed using a dual photodiode and a digital online cross-correlation program (Circusoft Instrumentation, Hockessin, DE) as described.32 Wall shear rates (γw) were calculated as 4.9 (8vbd), where vb is the mean blood flow velocity and d the vessel diameter.33 Rolling leukocyte flux fraction was defined as the percentage of rolling leukocytes to all leukocytes passing through the same venule per unit time.30
Peritonitis assays
Frozen section binding assays
Experiments were performed as described previously36 with the following modifications. Mesenteric lymph nodes were sectioned to 10 μm thickness. Binding was assayed with 2 × 106 lymphocytes/section. Lymphocytes were obtained from wild-type spleens by centrifugation on Lympholyte M (Cedarlane Laboratories, Burlington, ON). In some experiments lymphocytes were preincubated for 20 minutes with mAb Mel-14 (2 μg/mL) or control mAb mCD99-102.2, which binds to CD99, a protein that is expressed on lymphocytes and endothelial cells and does not participate in homing to LNs.37 Subsequently, cells were washed and allowed to bind to the sections. Following fixation sections were blocked for 1.5 hours in phosphate-buffered saline (PBS)/2% bovine serum albumin (BSA)/10% normal goat serum and incubated for 1 hour with 10 μg/mL MECA 367 mAb for HEV detection before they were stained with peroxidase-conjugated secondary antibodies. The sections were photographed under an Axioskop 2 plus microscope (Zeiss, Oberkochen, Germany) at 100× magnification using a 10×/0.45 NA Plan-Apochromat objective (Zeiss). Lymphocyte adhesion was quantified using the ImageJ analysis program (National Institutes of Health, Bethesda, MD) and expressed as the number of bound lymphocytes per 1 mm2 of HEV area.
Lymphocyte homing assay
Lymphocytes from peripheral LNs (PLNs) and mesenteric LNs (MLNs) or from spleens for the spleen homing assays (Figures 6B and 7) of wild-type littermate mice were labeled with CellTracker Green CMFDA (Invitrogen–Molecular Probes, Karlsruhe, Germany) and purified by centrifugation on Lympholyte M. A total of 6 × 107 lymphocytes in Hanks balanced salt solution (PAA Laboratories, Pasching, Austria) were injected into tail veins of 10- to 12-week-old Slc35c1−/− mice and wild-type littermates. After 3 hours mice were killed, LNs and spleens were collected, and the cell number in suspensions of each organ was determined. Cells were analyzed by flow cytometry to determine the fraction of fluorescence-positive cells.
To assess homing of labeled cells to the white pulp the spleens were frozen, sectioned, and mounted as described. Sections were incubated with 10 μg/mL anti–mSIGN-R1 mAb DSR1-14.238 and secondary Alexa 568–coupled antibody (Invitrogen–Molecular Probes) to visualize marginal zones and photographed as described. Fluorescent lymphocytes in the white pulp were counted and binding was expressed as the number of fluorescent cells per 1 mm2 of white pulp area.
Induction and analysis of humoral immune responses
Mice were immmunized with 100 μg KLH (Sigma, Deisenhausen, Germany) either subcutaneously into the hind legs as described39 or into the tail vein. At the indicated times, blood was taken from the submandibular vein. Sera were tested for KLH-specific antibodies in an enzyme-linked immunosorbent assay (ELISA). ELISA plates were coated with 1 μg KLH in PBS and blocked with 1 mg/mL casein/PBS. Serial dilutions of the sera (in casein/PBS) were added and incubated for 1 hour at 4°C. The KLH-specific antibodies 12B4-G3-A8 (mIgG; Antibodies Online, Aachen, Germany) and C48–6 (mIgM; BD Pharmingen) were used as standards. Wells were washed and incubated with peroxidase-conjugated anti–mouse IgG and IgM antibodies (Dianova, Hamburg, Germany), respectively. Color reaction was induced using o-phenylenediamine dihydrochloride (Sigma). Absorption at 495 nm was determined using a Synergy 2 ELISA reader (Friedrichshall, Germany).
Statistics
The Sigma Stat 2.0 software package (SPSS Science, Chicago, IL) was used for statistical analysis. Hemodynamic parameters, leukocyte-rolling flux fractions, and leukocyte adhesion between groups and treatments were compared with the one-way analysis of variance (ANOVA) on ranks (Kruskal-Wallis) with a multiple pairwise comparison test (Dunn test). Statistical significance was set at a P level of less than .05, indicated by asterisks in Figures 1 and 2.
Results
Leukocyte rolling in TNF-α–induced inflammation
In order to analyze in vivo leukocyte migration and trafficking in Slc35c1-deficient mice we first studied leukocyte rolling under inflammatory conditions by means of intravital microscopy of cremaster muscle venules. Since breeding resulted in very few Slc35c1−/− mice, we also analyzed chimeric mice (Slc35c1bm−/−), which were obtained by transplanting bone marrow from Slc35c1−/− mice into lethally irradiated recipient animals. Slc35c1bm−/− mice showed full chimerism as confirmed by strong hypofucosylation in all blood leukocytes (Figure S1). Microvascular parameters for the groups are presented in Table 1 and show similar vessel diameters, centerline velocities, and wall shear rates, but significant leukocytosis in Slc35c1−/− and Slc35c1bm−/− mice as compared with control mice.
Hemodynamic parameters of TNF-α–stimulated cremaster muscle venules
| . | Mice, n . | Venules, n . | Diameter, μm ± SEM . | Centerline velocity, μm/s ± SEM . | Shear rate, L/s ± SEM . | WBC, × 109/L ± SEM . |
|---|---|---|---|---|---|---|
| Slc35c1−/− | 3 | 12 | 33 ± 1 | 1800 ± 200 | 1400 ± 140 | 15.7 ± 1.45 |
| Slc35c1bm−/− | 2 | 7 | 31 ± 1 | 1800 ± 190 | 1400 ± 170 | 13.6 ± 2.75 |
| Control | 6 | 20 | 31 ± 1 | 1800 ± 130 | 1500 ± 110 | 2.8 ± 0.37 |
| P | NS | NS | NS | < .05 vs control |
| . | Mice, n . | Venules, n . | Diameter, μm ± SEM . | Centerline velocity, μm/s ± SEM . | Shear rate, L/s ± SEM . | WBC, × 109/L ± SEM . |
|---|---|---|---|---|---|---|
| Slc35c1−/− | 3 | 12 | 33 ± 1 | 1800 ± 200 | 1400 ± 140 | 15.7 ± 1.45 |
| Slc35c1bm−/− | 2 | 7 | 31 ± 1 | 1800 ± 190 | 1400 ± 170 | 13.6 ± 2.75 |
| Control | 6 | 20 | 31 ± 1 | 1800 ± 130 | 1500 ± 110 | 2.8 ± 0.37 |
| P | NS | NS | NS | < .05 vs control |
WBC indicates white blood cell; and NS, nonsignificant. Significant differences are as indicated.
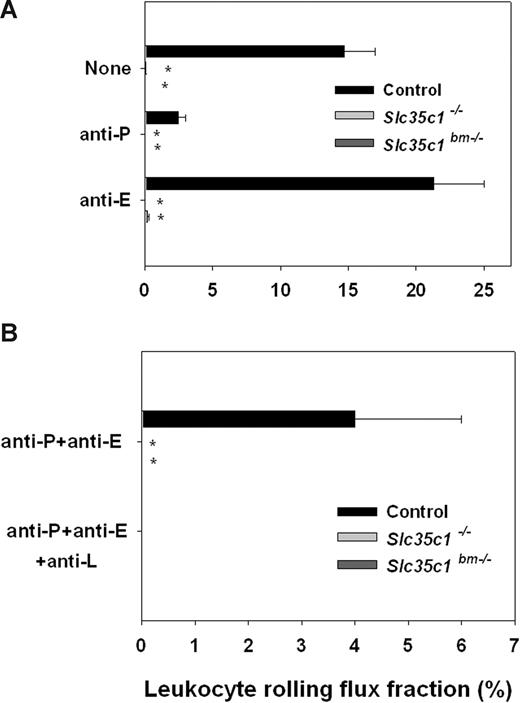
Intrascrotal injection of TNF-α leads to E- and P-selectin–dependent rolling 2 to 4 hours after TNF-α injection and to E-, P-, and L-selectin–dependent rolling more than 4 hours after TNF-α application.40 We first investigated leukocyte rolling 2 to 4 hours after TNF-α treatment in 14 venules of 3 Slc35c1−/− mice and 7 venules of 2 Slc35c1bm−/− mice and compared the results with rolling in 20 venules of 6 control animals.
We found that the leukocyte rolling flux fraction in cremaster muscle venules of control mice was 15% plus or minus 2%, which is similar to previous reports (Figure 1A).41 In line with published results, P-selectin blocking mAb RB40.34 reduced rolling dramatically, whereas blockade of E-selectin using mAb 9A9 led to an increase in rolling flux fraction, which is due to significantly higher rolling velocities and an impairment of leukocyte extravasation as described before.42 In contrast, leukocyte rolling was completely absent in Slc35c1−/− and Slc35c1bm−/− mice (Figure 1A), suggesting that both P- and E-selectin–mediated leukocyte rolling is impaired in the absence of Slc35c1.
Leukocyte rolling in TNF-α–stimulated cremaster muscle venules. Leukocyte rolling flux fraction (mean ± SEM) was assessed in cremaster muscle venules of Slc35c1−/− mice, Slc35c1bm−/− mice, and littermate control mice 2 to 4 hours (A) and more than 4 hours (B) after injection of TNF-α. *Significant differences (P < .05) in leukocyte rolling flux fraction between Slc35c1−/− and littermate control groups.
Leukocyte rolling in TNF-α–stimulated cremaster muscle venules. Leukocyte rolling flux fraction (mean ± SEM) was assessed in cremaster muscle venules of Slc35c1−/− mice, Slc35c1bm−/− mice, and littermate control mice 2 to 4 hours (A) and more than 4 hours (B) after injection of TNF-α. *Significant differences (P < .05) in leukocyte rolling flux fraction between Slc35c1−/− and littermate control groups.
Next, we investigated leukocyte rolling more than 4 hours after TNF-α injection, where rolling is mediated by P-, E-, and L-selectin. To isolate for L-selectin–mediated rolling, we injected blocking mAbs against P-selectin and E-selectin, leading to a decrease in rolling flux fraction to 4% plus or minus 2% in littermate control mice, which could be completely abolished by additional injection of L-selectin–blocking mAb Mel-14 (Figure 1B). In Slc35c1−/− and Slc35c1bm−/− mice we did not observe any rolling more than 4 hours after TNF-α injection and after application of anti–P-selectin mAb RB40.34 and anti–E-selectin mAb 9A9, suggesting that L-selectin ligand function is dependent on Slc35c1 in this setting. These results do not only indicate the importance of posttranslational fucosylation for P-, E-, and L-selectin ligand activity during inflammation in vivo, the experiments with the Slc35c1bm−/− mice also confirm that all of the relevant selectin ligand activity in the model of TNF-α–induced inflammation of the cremaster muscle is found on leukocytes.
Leukocyte adhesion upon TNF-α stimulation
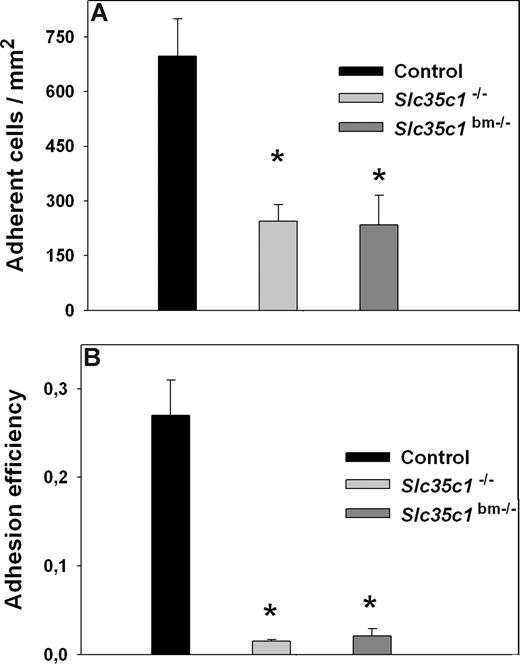
Next, we investigated the number of adherent leukocytes in cremaster muscle venules of Slc35c1−/− and Slc35c1bm−/− mice 2 to 4 hours after TNF-α stimulation. We found a significant reduction, but not a complete loss, of adherent leukocytes in Slc35c1−/− (245 ± 45 cells/mm2) and Slc35c1bm−/− mice (234 ± 81 cells/mm2) when compared with littermate control mice (697 ± 102 cells/mm2; Figure 2A), suggesting that selectin-independent mechanisms exist that allow some leukocyte adhesion during inflammation. To further assess the observed residual adhesion, we compared leukocyte adhesion efficiency between the groups (Figure 2B). Leukocyte adhesion efficiency is defined as the number of adherent cells per vessel surface area divided by systemic leukocyte count43 and takes into account differences in systemic leukocyte count between the groups. We found a dramatic (13- to 18-fold) reduction in leukocyte adhesion efficiency in Slc35c1−/− (0.015 ± 0.002) and Slc35c1bm−/− mice (0.021 ± 0.008) when compared with littermate control mice (0.27 ± 0.04; Figure 2B), demonstrating that Slc35c1−/− leukocytes are severely impaired in their ability to firmly adhere to the inflamed endothelium.
Leukocyte adhesion in TNF-α–treated cremaster muscle venules. Leukocyte adhesion (cells/mm2; mean ± SEM) was observed in TNF-α-treated (2–4 h) venules of the cremaster muscle from Slc35c1−/− mice, Slc35c1bm−/− mice, and littermate control mice (A). In addition, leukocyte adhesion efficiency (adherent leukocytes per mm2/systemic leukocyte count) is shown for the various groups (B). *Significant differences (P < .05) in leukocyte adhesion and adhesion efficiency between Slc35c1−/− mice, Slc35c1bm−/− mice and littermate control mice.
Leukocyte adhesion in TNF-α–treated cremaster muscle venules. Leukocyte adhesion (cells/mm2; mean ± SEM) was observed in TNF-α-treated (2–4 h) venules of the cremaster muscle from Slc35c1−/− mice, Slc35c1bm−/− mice, and littermate control mice (A). In addition, leukocyte adhesion efficiency (adherent leukocytes per mm2/systemic leukocyte count) is shown for the various groups (B). *Significant differences (P < .05) in leukocyte adhesion and adhesion efficiency between Slc35c1−/− mice, Slc35c1bm−/− mice and littermate control mice.
Taken together, theses results suggest that the lack of GDP-fucose transporter Slc35c1 causes absence of leukocyte rolling, which translates into a very poor endothelial adherence of each individual leukocyte. However, the strong leukocytosis that prevails in the LAD II model mice still allows a surprisingly high residual number of leukocytes to adhere to inflamed vessel endothelium.
Recruitment of granulocytes to the inflamed peritoneum
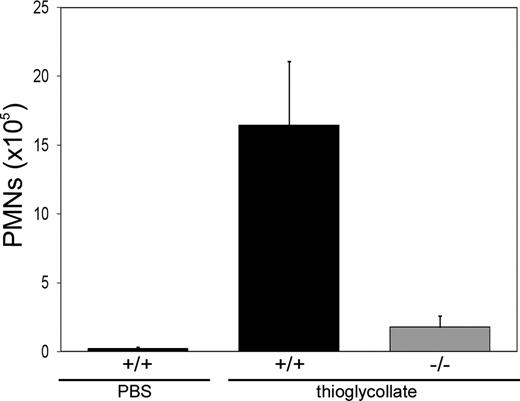
Next we were interested in how leukocytes in Slc35c1−/− mice migrate to sites of inflammation. To test this we made use of a thioglycollate-induced peritonitis model. At 4 hours after injection of thioglycollate into the peritoneum of littermate control and Slc35c1−/− mice, the number of peritoneal granulocytes was determined. Figure 3 shows that the migration of granulocytes to the inflamed peritoneum was dramatically reduced by 89% plus or minus 4% in Slc35c1−/− mice compared with control animals. The residual migration was not far above the background level which was determined by PBS treatment of control mice. These results show that, despite of the leukocytosis in Slc35c1−/− mice, the absence of the GDP-fucose transporter strongly compromises leukocyte migration to sites of inflammation.
Leukocyte trafficking to the inflamed peritoneum. Thioglycollate in PBS or PBS alone was injected intraperitoneally into littermate control (+/+) or Slc35c1−/− (−/−) mice. Four hours later, the number of peritoneal granulocytes (PMN) was determined. The data represent mean values plus or minus SD of 3 experiments with 3 or more +/+ and −/− mice, respectively, per experiment.
Leukocyte trafficking to the inflamed peritoneum. Thioglycollate in PBS or PBS alone was injected intraperitoneally into littermate control (+/+) or Slc35c1−/− (−/−) mice. Four hours later, the number of peritoneal granulocytes (PMN) was determined. The data represent mean values plus or minus SD of 3 experiments with 3 or more +/+ and −/− mice, respectively, per experiment.
Lymphocyte homing to LNs
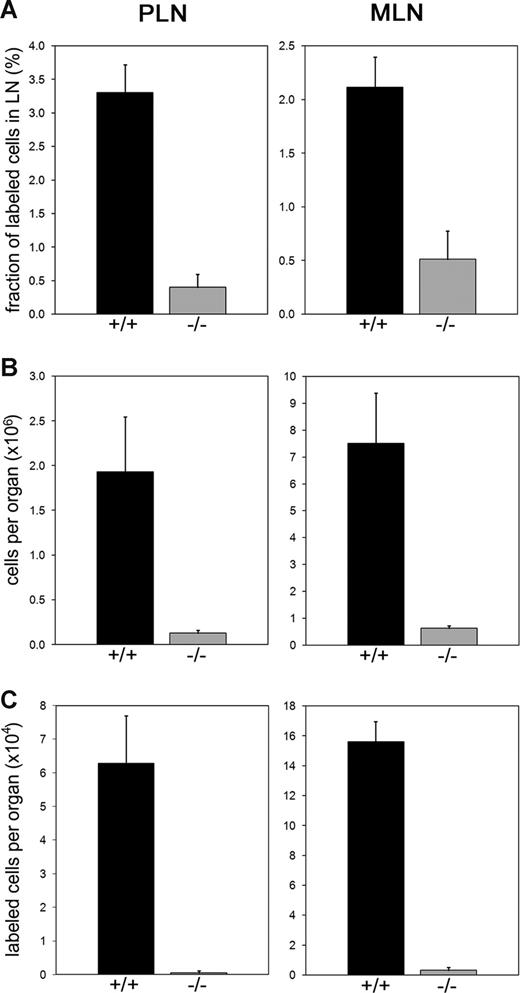
T cell–dependent antibody responses were shown to be normal in LAD II,14,15 which suggested that sufficient lymphocyte entry into LNs might be possible in this disease. This prompted us to analyze lymphocyte homing to LNs in Slc35c1−/− mice. In vivo homing was assessed with fluorescently labeled wild-type lymphocytes, which were injected intravenously into wild-type and Slc35c1−/− mice. After 3 hours, the percentages of labeled cells in PLNs and MLNs were determined by flow cytometry. Figure 4A shows that the percentages of homed lymphocytes in PLNs and MLNs of Slc35c1−/− mice were strongly decreased by 87% plus or minus 6% and 72% plus or minus 12%, respectively, when compared with control values. In addition, LNs of Slc35c1−/− mice were strongly hypocellular (Figure 4B). Consequently, the calculated absolute numbers of lymphocytes that accumulated in Slc35c1−/− PLNs and MLNs during the chosen time window were drastically reduced by 99% plus or minus 1% and 98% plus or minus 1%, respectively, as compared with control lymph nodes (Figure 4C).
Lymphocyte homing to LNs. Wild-type lymphocytes were fluorescently labeled and injected into the tail veins of Slc35c1−/− mice (−/−) or littermate controls (+/+). Three hours later, PLNs and MLNs were recovered by microdissection. The fractions of fluorescent cells in LN cell suspensions were determined by flow cytometry. Total LN cell numbers were analyzed with a cell counter. (A) Fraction of labeled cells in LN cell suspensions. (B) Total number of cells per LN. (C) Absolute number of homed lymphocytes per LN. Data are from 3 experiments, with a total of at least 42 PLNs and 18 MLNs for each group.
Lymphocyte homing to LNs. Wild-type lymphocytes were fluorescently labeled and injected into the tail veins of Slc35c1−/− mice (−/−) or littermate controls (+/+). Three hours later, PLNs and MLNs were recovered by microdissection. The fractions of fluorescent cells in LN cell suspensions were determined by flow cytometry. Total LN cell numbers were analyzed with a cell counter. (A) Fraction of labeled cells in LN cell suspensions. (B) Total number of cells per LN. (C) Absolute number of homed lymphocytes per LN. Data are from 3 experiments, with a total of at least 42 PLNs and 18 MLNs for each group.
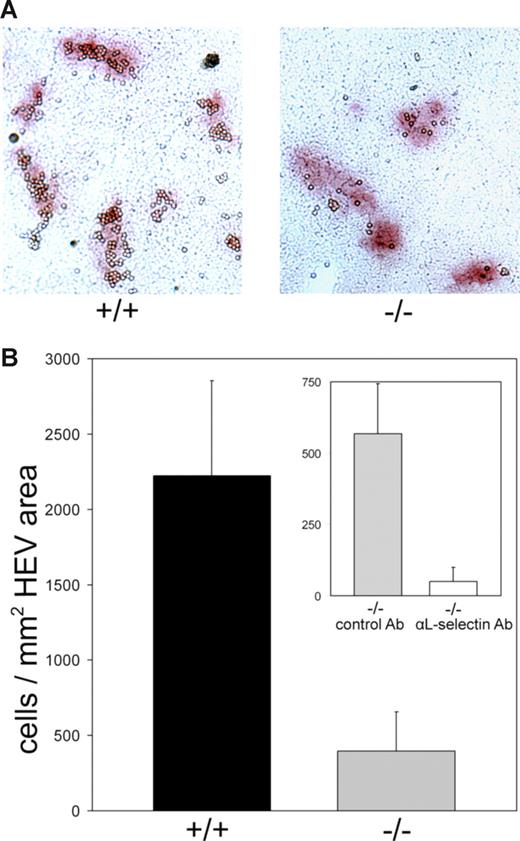
To directly analyze the ability of lymphocytes to interact with Slc35c1-deficient HEVs, we prepared frozen sections of MLNs and allowed lymphocytes to bind. Lymphocytes bound to MLN sections from wild-type mice in a HEV-specific manner with little background binding to the LN parenchyma (Figure 5A). The figure also shows that considerably fewer lymphocytes bound to HEVs of Slc35c1-deficient MLNs. Cell binding was quantified and expressed as the number of HEV-bound cells per square millimeter of HEV area on the sections. In comparison with control HEVs, the Slc35c1-deficient HEVs bound 82% plus or minus 11% less lymphocytes per square millimeter of HEV area (Figure 5B). Interestingly, we found that the residual binding to Slc35c1-deficient HEVs could be blocked by 91% with an L-selectin–specific antibody (Figure 5B; inset).
Lymphocyte binding to frozen LN sections. Wild-type lymphocytes were allowed to bind to frozen sections of MLNs of littermate control (+/+) and Slc35c1−/− (−/−) mice under mild rotation. Following fixation, HEVs were stained with anti–MAdCAM-1 mAb MECA 367. Isotype control mAb incubation gave no staining. (A) Micrograph of typical sections following lymphocyte binding and HEV staining. (B) Quantification of lymphocyte binding to HEVs. Binding is expressed as the number of HEV-bound cells per square millimeter of HEV area on the sections. Only cells bound to stained areas were considered. Background binding (cells per square millimeter of parenchyma) was subtracted. (Inset) L-selectin dependence of lymphocyte binding to sections of Slc35c1-deficient MLNs. Lymphocytes were preincubated with a nonblocking isotype control antibody directed against CD99 (“Methods”) or with the L-selectin–specific mAb Mel-14 before they were allowed to bind to the sections. For each type of mouse the data are from 4 animals and 7 MLNs with a total of 90 HEVs.
Lymphocyte binding to frozen LN sections. Wild-type lymphocytes were allowed to bind to frozen sections of MLNs of littermate control (+/+) and Slc35c1−/− (−/−) mice under mild rotation. Following fixation, HEVs were stained with anti–MAdCAM-1 mAb MECA 367. Isotype control mAb incubation gave no staining. (A) Micrograph of typical sections following lymphocyte binding and HEV staining. (B) Quantification of lymphocyte binding to HEVs. Binding is expressed as the number of HEV-bound cells per square millimeter of HEV area on the sections. Only cells bound to stained areas were considered. Background binding (cells per square millimeter of parenchyma) was subtracted. (Inset) L-selectin dependence of lymphocyte binding to sections of Slc35c1-deficient MLNs. Lymphocytes were preincubated with a nonblocking isotype control antibody directed against CD99 (“Methods”) or with the L-selectin–specific mAb Mel-14 before they were allowed to bind to the sections. For each type of mouse the data are from 4 animals and 7 MLNs with a total of 90 HEVs.
Taken together, LN homing and binding data show that HEVs of Slc35c1−/− mice have a strong defect in supporting lymphocyte binding that translates into an almost absent accumulation of lymphocytes in PLNs and MLNs from the circulation. In addition, although L-selectin mediates the low residual lymphocyte binding to Slc35c1-deficient HEVs of frozen sections, this adhesion molecule can maximally account for the 1% to 2% residual lymphocyte homing in Slc35c1−/− mice in vivo.
Lymphocyte homing to the spleen
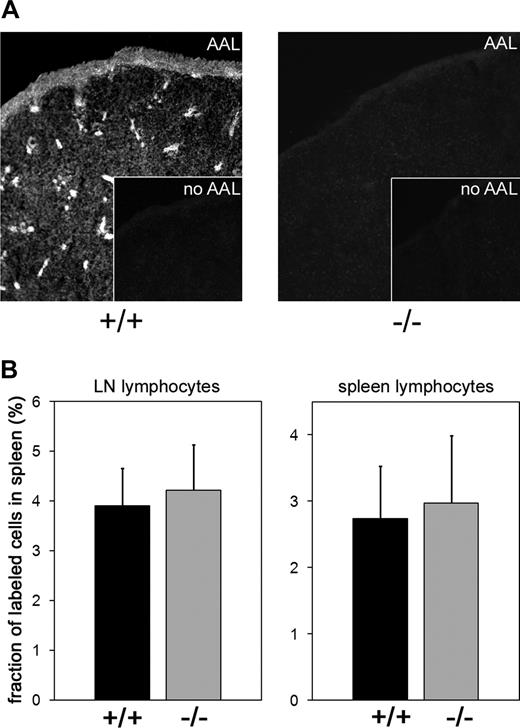
To address the role of the GDP-fucose transporter in lymphocyte homing to the spleen, we investigated spleen size and cellularity as well as lymphocyte homing to this organ in Slc35c1−/− mice. We found that spleens of Slc35c1−/− mice were devoid of detectable fucosylation (Figure 6A) but showed normal size and cellularity (Slc35c1−/−: 3.93 ± 0.60 × 107 vs wild-type: 4.04 ± 0.48 × 107 leukocytes/spleen; data from 5 spleens in each group). Importantly, spleen homing of fluorescently labeled lymphocytes obtained from lymph nodes or spleens of wild-type mice was absolutely normal in Slc35c1−/− mice (Figure 6B). Perhaps not surprisingly, homing of hypofucosylated Slc35c1-deficient lymphocytes to the spleens of wild-type mice was not reduced either (data not shown).
Lymphocyte homing to the spleen. (A) Expression of fucosylated glycans in the spleen. Frozen sections of spleens from littermate control (+/+) and Slc35c1-deficient (−/−) mice were stained with biotinylated Aleuria autantia lectin (AAL) and secondary fluorescent streptavidin reagent to detect fucosylated structures. Insets show staining with secondary reagent only. (B) Lymphocyte homing. Lymphocytes obtained from LNs or spleens of wild-type mice were fluorescently labeled and injected into the tail veins of littermate control (+/+) and Slc35c1-deficient (−/−) mice. Spleens were collected 3 hours later. Following erythrocyte lysis, splenocytes were quantified and the percentage of fluorescent cells was determined by flow cytometry. Data are from 3 experiments, with a total of 9 spleens for each type of mouse.
Lymphocyte homing to the spleen. (A) Expression of fucosylated glycans in the spleen. Frozen sections of spleens from littermate control (+/+) and Slc35c1-deficient (−/−) mice were stained with biotinylated Aleuria autantia lectin (AAL) and secondary fluorescent streptavidin reagent to detect fucosylated structures. Insets show staining with secondary reagent only. (B) Lymphocyte homing. Lymphocytes obtained from LNs or spleens of wild-type mice were fluorescently labeled and injected into the tail veins of littermate control (+/+) and Slc35c1-deficient (−/−) mice. Spleens were collected 3 hours later. Following erythrocyte lysis, splenocytes were quantified and the percentage of fluorescent cells was determined by flow cytometry. Data are from 3 experiments, with a total of 9 spleens for each type of mouse.
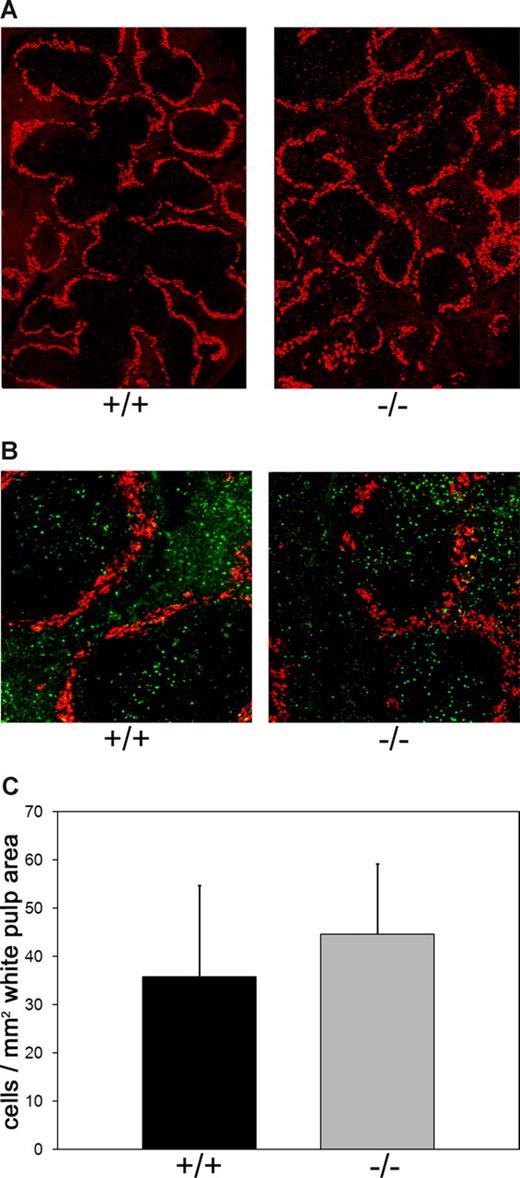
It has been shown that fucoidan specifically blocks lymphocyte entry into the white pulp, the compartment in which T cell/B cell interactions can take place.25,26 This prompted us to analyze splenic white and red pulp in Slc35c1−/− mice. We first visualized the marginal zone that surrounds the white pulp in spleen sections and found that white pulp/red pulp architecture and distribution are unperturbed in LAD II model mice (Figure 7A). Specific white pulp homing in the deficient mice was assessed using fluorescently labeled wild-type splenic lymphocytes. At 3 hours after intravenous injection of these cells, spleens were harvested, sectioned, and stained with a marginal zone–specific antibody (Figure 7B). Quantification of fluorescent cells in the white pulp of Slc35c1−/− mice disclosed that the number of homed lymphocytes in this compartment was rather slightly (but insignificantly) increased than decreased in comparison with control mice (Figure 7C). These data show that lymphocyte trafficking to the spleen is independent from Slc35c1, and that the spleen has the potential to serve as a substitute for the inaccessible lymph nodes in LAD II.
Lymphocyte homing to splenic white pulp. (A) White pulp/red pulp architecture in spleens of Slc35c1−/− mice. Frozen sections of spleens from littermate control (+/+) and Slc35c1-deficient (−/−) mice were stained with anti–mSIGN-R1 mAb DSR1–14.2, which specifically detects macrophages in the marginal zone surrounding the white pulp. (B) Distribution of homed lymphocytes in white and red pulp. Wild-type splenic lymphocytes were fluorescently labeled and injected into the tail veins of littermate control (+/+) and Slc35c1-deficient (−/−) mice. At 3 hours later, spleens were collected, sectioned, and stained with mAb DSR1-14.2. (C) Lymphocyte homing to the white pulp in Slc35c1−/− mice. Homing of lymphocytes to the white pulp is expressed as the number of fluorescent cells per white pulp area. Data are means (± SD) from 3 experiments with a total of 6 mice per group and a minimum of 1 × 103 counted cells per spleen.
Lymphocyte homing to splenic white pulp. (A) White pulp/red pulp architecture in spleens of Slc35c1−/− mice. Frozen sections of spleens from littermate control (+/+) and Slc35c1-deficient (−/−) mice were stained with anti–mSIGN-R1 mAb DSR1–14.2, which specifically detects macrophages in the marginal zone surrounding the white pulp. (B) Distribution of homed lymphocytes in white and red pulp. Wild-type splenic lymphocytes were fluorescently labeled and injected into the tail veins of littermate control (+/+) and Slc35c1-deficient (−/−) mice. At 3 hours later, spleens were collected, sectioned, and stained with mAb DSR1-14.2. (C) Lymphocyte homing to the white pulp in Slc35c1−/− mice. Homing of lymphocytes to the white pulp is expressed as the number of fluorescent cells per white pulp area. Data are means (± SD) from 3 experiments with a total of 6 mice per group and a minimum of 1 × 103 counted cells per spleen.
Humoral response to a T cell–dependent antigen
Similar to Slc35c1−/− mice, L-selectin–deficient mice show strongly reduced lymphocyte trafficking to LNs.39 Steeber et al39 subcutaneously immunized L-selectin−/− mice. This route of immunization primarily stimulates regional LNs. In accordance with the cellular trafficking defect, the same authors found that within the first week after subcutaneous immunization with KLH, a T cell–dependent antigen, KLH-specific antibody production was impaired. Following the immunization protocol of Steeber et al,39 we subcutaneously immunized Slc35c1−/− and littermate control mice with KLH. Table 2 shows that 7 days after immunization, the generation of KLH-specific IgM and IgG is reduced by 45% and 88%, respectively, in Slc35c1−/− mice. This resembles the data obtained in L-selectin−/− mice.39 In contrast to the subcutaneous route of immunization, intravenous injection of antigen directly induces immune responses in the spleen. As would be predicted from the normal lymphocyte homing to the spleen in Slc35c1−/− mice, we find that the generation of specific IgM and IgG antibodies upon intravenous immunization with KLH is as strong as in wild-type mice (Table 2). These data strongly support the view that impaired lymphocyte homing to LNs of Slc35c1−/− mice causes reduced or delayed immune responses in these organs, whereas unperturbed lymphocyte homing to the spleen allows normal spleen responses in the LAD II model mice.
Humoral responses to a T-cell–dependent antigen
| Route of immunization . | IgM, μg/mL* . | IgG, μg/mL* . | ||
|---|---|---|---|---|
| +/+ . | −/− . | +/+ . | −/− . | |
| Subcutaneous | ||||
| Day 0 | < 0.1 | < 0.1 | < 0.01 | < 0.01 |
| Day 3 | 2.22 ± 0.25 | 0.93 ± 0.21 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| Day 5 | 3.01 ± 0.35 | 1.47 ± 0.32 | 0.11 ± 0.05 | 0.03 ± 0.01 |
| Day 7 | 3.23 ± 0.25 | 1.78 ± 0.18 | 1.76 ± 0.21 | 0.21 ± 0.04 |
| Intravenous | ||||
| Day 0 | < 0.1 | < 0.1 | < 0.01 | < 0.01 |
| Day 5 | 5.0 ± 0.25 | 5.5 ± 0.21 | 0.1 ± 0.05 | 0.1 ± 0.07 |
| Day 9 | 5.0 ± 0.18 | 6.0 ± 0.22 | 0.1 ± 0.07 | 0.1 ± 0.07 |
| Day 14 | 8.0 ± 1.27 | 8.5 ± 0.35 | 4.5 ± 0.10 | 5.0 ± 0.21 |
| Route of immunization . | IgM, μg/mL* . | IgG, μg/mL* . | ||
|---|---|---|---|---|
| +/+ . | −/− . | +/+ . | −/− . | |
| Subcutaneous | ||||
| Day 0 | < 0.1 | < 0.1 | < 0.01 | < 0.01 |
| Day 3 | 2.22 ± 0.25 | 0.93 ± 0.21 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| Day 5 | 3.01 ± 0.35 | 1.47 ± 0.32 | 0.11 ± 0.05 | 0.03 ± 0.01 |
| Day 7 | 3.23 ± 0.25 | 1.78 ± 0.18 | 1.76 ± 0.21 | 0.21 ± 0.04 |
| Intravenous | ||||
| Day 0 | < 0.1 | < 0.1 | < 0.01 | < 0.01 |
| Day 5 | 5.0 ± 0.25 | 5.5 ± 0.21 | 0.1 ± 0.05 | 0.1 ± 0.07 |
| Day 9 | 5.0 ± 0.18 | 6.0 ± 0.22 | 0.1 ± 0.07 | 0.1 ± 0.07 |
| Day 14 | 8.0 ± 1.27 | 8.5 ± 0.35 | 4.5 ± 0.10 | 5.0 ± 0.21 |
Slc35c1−/− and wild-type littermate mice were immunized subcutaneously or intravenously with 100 μg KLH at day 0. At the indicated times the concentrations of KLH-specific antibodies in the sera were determined by ELISA. Values (± SD) are from 6 mice in each group.
Discussion
In this report we have analyzed in vivo leukocyte trafficking in mice deficient for the GDP-fucose transporter Slc35c1 and show strongly defective leukocyte rolling in inflamed cremaster muscle venules, impaired neutrophil trafficking to the inflamed peritoneum, virtually absent lymphocyte homing to LNs, but normal homing of lymphocytes to the spleen.
Similar to results reported in an earlier in vivo study in wich leukocytes from patients with LAD II were injected into inflamed vessels of the rat mesentery,13 we found an almost complete absence of leukocyte rolling in TNF-α (2-4 hours)–stimulated cremaster muscle venules of Slc35c1-deficient mice. Leukocyte rolling in this setting is mostly dependent on interactions of P- and E-selectin with fucosylated core-2–decorated O-glycans on selectin ligands.41 A total of 2 fucosyltransferases, FucT-VII and FucT-IV, have been implicated in the posttranslational fucosylation of inflammatory selectin ligands in vivo. In mice double-deficient for these enzymes, leukocyte rolling in inflamed venules of the ear and the cremaster muscle was almost entirely lost.23,44 Together with our results, this implies that the GDP-fucose transporter is essential for maintaining sufficient transport of GDP-fucose into the Golgi apparatus, where this nucleotide sugar is used for posttranslational fucosylation of selectin ligands by FucT-VII and -IV.
In addition to the profound defect in leukocyte rolling, we also found a dramatic reduction in firm leukocyte arrest, although the observed reduction was less pronounced than anticipated. Furthermore, neutrophil recruitment to the inflamed peritoneum was also reduced by 89% in Slc35c1−/− mice. This shows that both leukocyte adhesion and transmigration into tissue are largely dependent on Slc35c1 expression. The residual adhesion and recruitment seen in Slc35c1−/− mice may in part be due to the 5-fold increase of neutrophils in the circulation of these mice. Taking this neutrophilia into account, a very low adhesion and recruitment efficiency can be calculated for Slc35c1−/− mice. Whether residual leukocyte arrest was preceded by occasional rolling events which escaped our detection or whether arrest was completely independent of rolling is unknown. Residual adhesion has been observed in P-, E-, and L-selectin triple-deficient mice, suggesting that selectin-independent mechanisms exist for the successful transition of free-flowing leukocytes via leukocyte rolling to firm leukocyte arrest.45,46 We and others have reported that α4-integrin can mediate selectin-independent leukocyte rolling and adhesion during inflammation.47,48 This mechanism may be responsible for the occurrence of residual adhesion in the absence of Slc35c1.
The reduction of neutrophil recruitment to the inflamed peritoneum in Slc35c1−/− mice is very similar to that induced by blocking P- and L-selectins (89%-90%)34,49 and to that seen in FucT-IV/-VII double-deficient mice (95%).23 Thus, the peritonitis data confirm that these 4 proteins appear to be largely dependent on Slc35c1 with little or no contribution of additional GDP-fucose transport systems. Price et al14 measured neutrophil emigration into the inflamed skin of a patient with LAD II. A 94% to 98% reduction of neutrophil recruitment was found. These results are consistent with our data from the murine peritonitis model.
Besides the perturbated neutrophil function in Slc35c1−/− mice, we show for the first time that the absence of a functional GDP-fucose transporter also affects a critical function of lymphocytes, the homing to LNs. We found that absolute numbers of lymphocytes that homed to PLNs and MLNs in Slc35c1−/− mice were reduced by 99% and 98%, respectively. This homing defect is at least as strong as that in FucT-IV/-VII double-deficient mice (reduction of 99% and 96%, respectively).23 This implies that the enzymatic activity of the 2 fucosyltransferases in high endothelial cells fully depends on Slc35c1, and that no other GDP-fucose transport activity is involved in these cells. Interestingly, lymphocytes show 18% residual in vitro binding to HEVs of frozen MLN sections obtained from Slc35c1−/− mice. We show that this residual binding is largely dependent on L-selectin. The basis for the residual binding of L-selectin is unclear. L-selectin has been found to bind to nonfucosylated structures like heparan sulfate and sulfatide.50-53 However, such structures have not been reported to mediate leukocyte binding to HEVs. Alternatively, a very low residual fucosylation of HEVs that may have escaped histochemical detection19 may support the low in vitro L-selectin binding. Whatever the mechanism for the residual in vitro binding via L-selectin may be, it does not translate into equivalent lymphocyte homing in vivo. One explanation for this difference may be that the in vitro binding assay was performed under static conditions, whereas lymphocyte homing to LNs takes place under dynamic flow conditions. Furthermore, it has to be emphasized that LNs in Slc35c1−/− mice are small and strongly hypocellular, which may additionally affect lymphocyte homing.
It is interesting to compare lymphocyte homing in Slc35c1−/− mice with homing in the presence of L-selectin blocking mAb Mel-14 and in L-selectin–deficient mice. Mel-14 was shown to almost completely inhibit homing to PLNs,54,55 and L-selectin deficiency reduced homing to PLNs by 99% under similar conditions.56 The profound reduction in lymphocyte homing to PLNs in L-selectin–deficient mice is identical to the reduction in Slc35c1−/− mice. Thus, the latter can be fully explained by the lack of L-selectin ligand function in vivo.
The situation is somewhat different for homing to MLNs, which is higher in the presence of mAb Mel-14 and in L-selectin−/− mice (17% and 12% of normal, respectively)54-56 than in Slc35c1−/− mice (2% of normal). This is accompanied by an almost normal cellularity of MLNs in L-selectin−/− mice,56 whereas Slc35c1−/− MLNs are strongly hypocellular. It can be concluded that fucosylation must have a role for MLNs that goes beyond generating functional L-selectin ligands. Possible mechanisms may include L-selectin–independent leukocyte recruitment from the blood, leukocyte entry via the lymph, an influence on cell proliferation within MLNs, or an effect on structural elements like fucosylated collagens in the LNs.
Two reports have shown that patients with LAD II can mount normal antibody responses.14,15 Our data on lymphocyte homing and T cell–dependent antibody responses in Slc35c1−/− mice provide an explanation for this finding, suggesting that at least during the early phase of an adaptive immune response the spleen substitutes for the barely accessible LNs in LAD II.
It should be noted that the strong LN homing defect in L-selectin−/− mice does not preclude the LNs from partcipating in later phases of an immune response,39 probably because a low influx of cells is sufficient once activation signals have impaired the egress of lymphocytes from the LNs.57 Such an effect may also apply to Slc35c1−/− mice and patients with LAD II.
Homing of lymphocytes to the spleen appears to be fundamentally different from homing to LNs. No HEV-like structures have yet been identified in the spleen and migration of lymphocytes to the spleen is independent of L-selectin.25,58,59 Moreover, lymphocyte homing to the spleen appears to be independent from E- and P-selectin, P-selectin glycoprotein ligand-1, MAdCAM-1, and the modifying enzymes FucT-IV and FucT-VII.23,25,58,59
It is not well understood how lymphocytes enter the white pulp, where they can mount adaptive immune responses. It is believed that sinosoidal structures in or near the marginal zone which surrounds the white pulp are the transit area for lymphocytes that leave the circulation.60-63 Importantly, these honeycomb-like structures are not lined by endothelial cells.63
Although all these data suggest that spleen homing is independent from the conventional role of fucosylation (ie, allowing interactions between selectins and their fucosylated ligands), several studies have shown that the sulphated L-fucose polymer fucoidan efficiently inhibits lymphocyte homing to the spleen.24-26 Moreover, fucoidan alters the distribution of the homed cells within the spleen, allowing fewer cells to enter the white pulp.25,26 These data suggested that fucoidan inhibits cellular recognition of fucosylated and/or sulphated structures that would normally promote entry to the spleen.
We now show that lymphocyte homing to the splenic white pulp is not perturbed in the strongly hypofucosylated Slc35c1−/− mice. This finding argues against a role of fucosylation in spleen homing. Thus, the data obtained with fucoidan may rather be interpreted as an effect of the multiple sulfate groups that are present in this compound.
Based on our data, we predict that lymphocyte homing to the spleen is grossly normal in patients with LAD II. This opens the possibility that the spleen may provide some, although perhaps limited, adaptive immunity in patients with LAD II. Spleen-mediated immunity may explain why the immunodeficiency in LAD II is milder than one would expect from the complete lack of selectin interactions that is seen in this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Barry Wolitzky (MitoKor, San Diego, CA) for providing the E-selectin blocking antibody 9A9, Viktor Wixler (University of Münster) and Birgit Kempe for help with immunizations, and Dietmar Vestweber (Max Planck Institute for Molecular Biomedicine, Münster, Germany) and Kerstin Lühn (University of Oxford, United Kingdom) for very helpful discussions.
This work was supported by the SFB 293 of the Deutsche Forschungsgemeinschaft (DFG) (M.K.W.), by the Max Planck Society (M.K.W.), by DFG grant SP621/3-1 (M.S.), by a fellowship of the International Graduate Research School “Molecular Basis of Dynamic Cell Processes,” GRK 1050 (S.Y.), by Ludwig-Maximilians-University Innovativ BioImaging, and by a PhD fellowship of the Ev. Studienwerk e.V. Villigst (C.C.H.).
Authorship
Contribution: S.Y., D.F., B.P., D.S., C.J., U.I., and R.K. performed research. C.H. and C.K. performed research and generated and contributed Slc35c1-deficient mice. M.G.B. provided vital new reagents. M.K.W. and M.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin K. Wild, Max Planck Institute for Molecular Biomedicine, Röntgenstr. 20, 48149 Münster, Germany; e-mail: mwild@mpi-muenster.mpg.de; or Markus Sperandio, Walter Brendel Center of Experimental Medicine, Ludwig-Maximilians-Universität, Marchioninistr. 15, 81377 München, Germany; e-mail: markus.sperandio@med.uni-muenchen.de.
References
Author notes
S.Y. and D.F. contributed equally to this work.
M.S. and M.K.W. contributed equally as senior authors of this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal