Abstract
Therapy-related acute myelogenous leukemia (t-AML) is an important late adverse effect of alkylator chemotherapy. Susceptibility to t-AML has a genetic component, yet specific genetic variants that influence susceptibility are poorly understood. We analyzed an F2 intercross (n = 282 mice) between mouse strains resistant or susceptible to t-AML induced by the alkylator ethyl-N-nitrosourea (ENU) to identify genes that regulate t-AML susceptibility. Each mouse carried the hCG-PML/RARA transgene, a well-characterized initiator of myeloid leukemia. In the absence of ENU treatment, transgenic F2 mice developed leukemia with higher incidence (79.4% vs 12.5%) and at earlier time points (108 days vs 234 days) than mice in the resistant background. ENU treatment of F2 mice further increased incidence (90.4%) and shortened median survival (171 vs 254 days). We genotyped F2 mice at 384 informative single nucleotide polymorphisms across the genome and performed quantitative trait locus (QTL) analysis. Thirteen QTLs significantly associated with leukemia-free survival, spleen weight, or white blood cell count were identified on 8 chromosomes. These results suggest that susceptibility to ENU-induced leukemia in mice is a complex trait governed by genes at multiple loci. Improved understanding of genetic risk factors should lead to tailored treatment regimens that reduce risk for patients predisposed to t-AML.
Introduction
Approximately 5% to 10% of all acute myeloid leukemia (AML) cases are the result of prior genotoxic therapy.1,2 Like other secondary malignancies, therapy-related AML (t-AML) responds poorly to treatment, and the median survival is only 6 to 12 months.2,3 The poor prognosis and the iatrogenic nature of t-AML provide impetus for determining risk factors that contribute to t-AML susceptibility. On the basis of the type of preceding treatment, t-AML can be broadly classified as alkylator-associated or topoisomerase II inhibitor–associated. Alkylator-associated t-AML generally arises after a latency of 3 to 5 years and frequently evolves from a myelodysplastic syndrome (MDS).4 Alkylating agents such as nitrosoureas and cyclophosphamide are widely used in the treatment of solid tumors (eg, breast, ovarian, and lung carcinomas) as well as leukemias and lymphomas. Because therapeutic options exist for most patients, personalized treatment plans based on t-AML risk factors could significantly reduce the incidence of this serious adverse outcome.
There are several known nongenetic risk factors for t-AML. Chemotherapy dose is correlated with risk of t-AML.5 Long-term, low-dose alkylator treatment and short-term, high-dose treatment (in the context of stem cell transplantation) confer the greatest risk of t-AML.5-7 There is also an association with primary cancer type. In a large single institution series, hematologic malignancies (eg, non-Hodgkin lymphoma, Hodgkin disease, myeloma, acute lymphoblastic leukemia) composed 171 (56%) of 306 primary malignancies preceding t-AML.8 However, there are documented cases of t-AML arising from alkylator treatment of a wide variety of tumors. Age is another important risk factor. The most recent evidence indicates that in the context of stem cell transplantation, patients older than 40 years are at increased risk of developing t-AML.9
In addition to these risk factors, several lines of evidence suggest there is a genetic component to t-AML susceptibility. First, a higher incidence of cancer is found in first-degree relatives of patients with secondary AML compared with relatives of patients with de novo AML.2 In addition, some familial forms of cancer predisposition confer an increased risk of t-AML (eg, neurofibromatosis type 1 and Li Fraumeni syndrome).10,11 These rare familial syndromes cause highly penetrant disease but account for a small number of t-AML cases. In addition, association studies have suggested that common polymorphisms in drug metabolism genes (eg, cytochrome p450 enzymes and phase II conjugation enzymes) and DNA repair pathways can contribute to t-AML susceptibility (reviewed by Seedhouse and Russell12 ). In 2 studies, the CYP3A4*1B allele was underrepresented in patients with t-AML compared with patients with de novo AML13 or with a control cohort.14 However, other studies of CYP3A4*1B comparing children treated for ALL who did or did not develop t-AML15 and comparing t-AML cases with normal controls16 showed no association. The results from studies of several other candidate genes are similarly mixed, and a comprehensive understanding of the important genetic factors influencing t-AML susceptibility remains elusive.
Because susceptibility factors for t-AML are largely unknown, an unbiased, genomewide screen could identify important targets for further study. Inbred mice provide a powerful platform for identifying the genetic basis of complex traits. We previously screened 20 inbred strains and reported that susceptibility to t-AML in mice is heritable.17 In that study, SWR/J mice were found to be susceptible to t-AML, whereas C57BL/6J and C3H/HeJ mice were resistant.17 On the basis of these results, we designed an F2 intercross to define regions of the genome important for t-AML susceptibility in mice.
Methods
Mice
Transgenic hCG-PML/RARA (PR) mice were maintained in a C57BL/6J×C3H/HeJ F1 (B6C3F1) background.18 Genotyping was performed as previously described.19 SWR/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in a specific pathogen-free facility. All studies were conducted with the approval of the Animal Studies Committee at Washington University School of Medicine.
Mutagenesis protocol
Ethyl-N-nitrosourea (ENU; Sigma-Aldrich, St Louis, MO) was administered at 9 and 10 weeks of age (200 mg/kg intraperitoneally, total dose), as previously described.17 Mice were pretreated with hydrocortisone (2.5 mg intraperitoneally every other day × 3) at 8 weeks of age (ie, 1 week before ENU treatment) to decrease the incidence of thymic lymphomas.
Hematologic analysis
Mice were killed when moribund or when they reached 14 months of age. Postmortem analysis included spleen weight, complete blood counts, flow cytometric analysis, and histologic evaluation of blood and bone marrow smears. Complete blood counts were obtained with a Forcyte veterinary cell counter (Oxford Sciences, Oxford, CT). For flow cytometry, bone marrow and spleen cells were stained with directly conjugated antibodies to B220, CD3, CD45, c-kit, GR1, CD34, CD4, or CD8 (BD Biosciences, San Jose, CA). Scatter gated events (10 000) were acquired on a flow cytometer (FACScan; BD Biosciences) and analyzed with FlowJo software (version 4.5.1; TreeStar, Ashland, OR). Flow cytometry data are reported as means plus or minus SD. For histologic evaluation, tissues were fixed in 10% formalin and embedded in paraffin. Sections (5 μm) were stained with H&E and reviewed by a veterinary pathologist. Peripheral blood, bone marrow smears, and spleen touch preps were stained with May-Grünwald/Giemsa and manually scored.
Survival analysis
The cumulative probability of leukemia-free survival was estimated by the Kaplan-Meier method. Mice were censored at the time of death if postmortem analysis did not show leukemia (eg, lymphoma, myeloproliferative disorder without features of acute leukemia, or no significant abnormalities at the time of killing). Differences in survival between cohorts was assessed by the log-rank test.
SNP selection
Custom oligonucleotide pools (Illumina, San Diego, CA) were designed for 384 informative single nucleotide polymorphisms (SNPs) chosen from the Wellcome Trust-CTC mouse genotype set20 to provide a 4-cM resolution map across the mouse genome (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). All selected SNPs are polymorphic between SWR/J and B6C3F1 and monomorphic between C57BL/6J and C3H/HeJ so that the SWR/J allele can be unambiguously identified at each locus. Tail DNA prepared from the parental (SWR/J and B6C3F1), F1 (n = 10), and F2 mice (n = 282) were arrayed with replicates in 96-well plates. Using the GoldenGate protocol, these samples were amplified in an allele-specific manner and hybridized to Sentrix arrays (Illumina). Genotype calls were made using BeadStudio software (Illumina). Twenty-seven of the 384 SNPs were excluded for poor call rates, leaving 357 markers for analysis. All markers were tested for deviation from Hardy-Weinberg (HW) proportions using a chi-square test with 1 degree of freedom. Markers diverging from HW were checked for excessive recombination, and none was found.
Genetic map
R/QTL21 was used to detect possible genotyping errors and to estimate the map distances in centimorgans (cM). Because all animals were male, the map distances for the X chromosome were calculated by counting the recombinants for each animal and converting them to map distances. Genotypes with error logarithmic odds (LOD) scores greater than 4 were treated as missing and imputed using methods from Haley and Knott.22 The average intermarker map distance is 3.89 cM. The largest intermarker distance is 46 cM on chromosome 10, corresponding to the largest physical distance between markers (51.8 Mb). The map distance was similar to that found in the Oxford Wellcome Trust database.20 The genotypes at each marker were coded as 1 (SWR/J allele homozygote), 0 (heterozygote), and −1 (B6C3F1 allele homozygote). Only transgene-positive mice were used in the study, resulting in a selection of only a portion of the variation in the chromosome immediately around the transgene in chromosome 2. Consequently, quantitative trait loci (QTLs) near this transgene integration site will not be detected in this analysis.
QTL analysis
We searched for QTLs in the F2 mice associated with 3 quantitative traits: leukemia-free survival, white blood cell (WBC) count, and spleen weight. The QTLs were mapped using interval mapping methods as first proposed by Lander and Botstein.23 Intervals were imputed for the autosomal data22 and the X chromosome24 every 1 cM between the 357 observed markers, resulting in 1658 observed and imputed locations across the genome (physical and genetic map distances provided in Table S1). All analyses were performed in R (http://www.r-project.org). WBC count and spleen weight were log transformed and analyzed with standard linear regression, whereas the survival data were analyzed using the Cox Proportional Hazards Model25 by implementing the coxph function from the Survival package in R (http://www.rqtl.org).
Models
Four models were used to test our hypothesis that genotype influences t-AML susceptibility. For each model, the additive genotypic value (a; half the mean difference between the homozygotes) and the dominance genotypic value (d; deviation of the heterozygote mean from the midpoint of the homozygotes, commonly described as interaction within a locus) were calculated. A large “a” signifies a large difference between homozygotes. The dominance ratio (calculated as d/a) signifies no dominance if d/a is less than 1, complete dominance if d/a is approximately 1, and overdominance if d/a is more than 1. The covariate model pools a and d across treatment groups. A significant result indicates genotypic effects independent of treatment status. The interaction model tests for interaction between treatment status and genotypic effects. A significant result implies a genotypic effect that is different in treated compared with untreated mice. The final 2 models, +ENU and −ENU, examine each treatment group separately and were used only for interpretation of the full interaction model. Significance thresholds at genomewide and chromosome-wide levels were calculated by performing 1000 permutations of the phenotypic data while leaving the genotypic data intact. Thresholds for all 4 models were essentially the same for each phenotype; therefore, the average threshold of all 4 models was used for each phenotype. Averaging the thresholds did not add or eliminate any QTLs. The significance thresholds for the survival data were calculated from the covariate model combined for both groups and also within the untreated group.
Gene list
The 1 LPR (likelihood probability ratio) confidence interval was determined for all QTL peaks. The interval was then converted from the genetic map to physical map location using the known physical location of observed SNPs. When 1 LPR confidence intervals ended at an imputed location, the physical location of the imputed point was approximated by using the observed SNPs flanking the imputed location to create a linear transform from the genetic to the physical map. The list of genes was generated by searching physical map intervals using the Ensembl Biomart search function.26 For prioritization purposes, genes within QTLs were queried for Medline citations using the MeSH terms “myelodysplastic syndrome,” “leukemia, myeloid,” “DNA repair,” “metabolic detoxication, drugs,” and “alkylating agents.” The number of citations was summed to generate a “MeSH score” for each gene.
Results
SWR/J alleles accelerate development of leukemia initiated by PML/RARA
In a screen for susceptibility to alkylator-induced myeloid leukemia in mice, we previously identified several sensitive (eg, SWR/J) and resistant (eg, C57BL/6J, C3H/HeJ) strains.17 To identify regions of the SWR/J genome that influence t-AML susceptibility, we designed an F2 intercross experiment. We “sensitized” the screen toward development of myeloid leukemia by breeding in the PR transgene. The PR transgene places the bcr-1 isoform of the t(15;17) PML/RARA fusion cDNA under the regulatory control of the human cathepsin G promoter.18 All PR+ mice develop a myeloproliferative disorder (MPD) characterized by modest splenomegaly and an increase in the proportion of mature and immature myeloid cells in the peripheral blood and bone marrow.18 In addition, MPD evolves into acute leukemia in 6% to 30%18,27 of PR+ mice after a latency of 6 to 13 months.18 The long latency and low penetrance of AML suggests that, although PR is sufficient for MPD, transformation to AML requires additional cooperating mutations. We used this potent initiator of myeloid leukemia to probe for germ line alleles that influence susceptibility to t-AML. Parental SWR/J and PR+ B6C3F1 mice were crossed to create the F1 generation. PR+ and PR− F1 littermates were bred, and single transgene-positive male F2 mice were treated (n = 141) or untreated (n = 141) with ENU and killed and analyzed when moribund or when they reached 14 months of age. Only male mice were included, because we previously observed a high incidence of ENU-induced uterine tumors in several strains leading to loss of power to detect myeloid leukemias with longer latency.17 A separate cohort of B6C3F1 transgene-positive male mice (n = 24 untreated, n = 21 ENU treated) was generated for comparison.
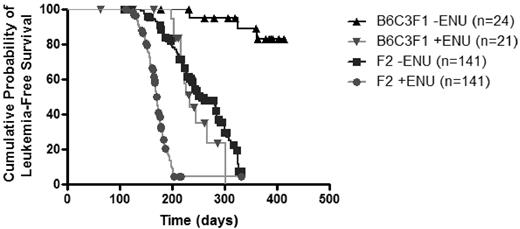
Consistent with previous reports,18 all PR+ B6C3F1 mice developed MPD and 12.5% progressed to AML (Figure 1; Table 1). In contrast, untreated PR+ F2 mice developed AML at a higher frequency (79.4%, P < .001) and shorter latency (108 days vs 234 days) and had significantly reduced survival (P < .001 by log-rank test) with a median survival of 254 days. These results suggest that SWR/J alleles increase susceptibility to PR-initiated leukemia and confirm our prior observation that the SWR/J genome contains factors that predispose mice to myeloid leukemia.
Kaplan-Meier survival curves of treated and untreated B6C3F1 PR+ and F2 mice. Untreated mice in the resistant parental background (B6C3F1 PR+) develop leukemia after a long latency and with low penetrance (▴). ENU treatment of PR+ mice in the B6C3F1 background increases leukemia penetrance and decreases latency (▾). Intercrossing SWR/J alleles has an effect equipotent to ENU treatment in B6C3F1 mice (▾ vs ■, P = .34; ▴ vs ▾ or ▴ vs ■, P < .001). Treatment with ENU further increases the rate and the incidence of leukemogenesis in F2 mice (■ vs ●, P < .001).
Kaplan-Meier survival curves of treated and untreated B6C3F1 PR+ and F2 mice. Untreated mice in the resistant parental background (B6C3F1 PR+) develop leukemia after a long latency and with low penetrance (▴). ENU treatment of PR+ mice in the B6C3F1 background increases leukemia penetrance and decreases latency (▾). Intercrossing SWR/J alleles has an effect equipotent to ENU treatment in B6C3F1 mice (▾ vs ■, P = .34; ▴ vs ▾ or ▴ vs ■, P < .001). Treatment with ENU further increases the rate and the incidence of leukemogenesis in F2 mice (■ vs ●, P < .001).
Summary of hematologic and survival data from nonleukemic and leukemic mice
| . | n . | Spleen wt, g . | WBC count, 109/L . | Hb level, g/L . | Platelet count, 109/L . | Latency, d . | Median survival, d . | Incidence, % . |
|---|---|---|---|---|---|---|---|---|
| B6C3F1 −ENU | 23 | |||||||
| Nonleukemic | 20 | 0.29 ± 0.35 | 7.73 ± 3.63 | 125.0 ± 25.8 | 911.6 ± 215.9 | |||
| Leukemic | 3 | 1.63 ± 0.48* | 213.0 ± 147.4* | 89.7 ± 21.6† | 458.7 ± 78.5‡ | 234 | NA§ | 12.5 |
| B6C3F1 +ENU | 21 | |||||||
| Nonleukemic | 6 | 0.24 ± 0.14 | 9.07 ± 6.70 | 120.3 ± 17.8 | 1032 ± 414.1 | |||
| Leukemic | 12 | 0.90 ± 0.33‡ | 56.85 ± 69.59 | 76.2 ± 29.2† | 425.7 ± 245.4‡ | 199 | 233 | 57.1 |
| Lymphoma | 3 | 1.09 ± 0.71 | 61.11 ± 25.43 | 86.7 ± 39.9 | 527 ± 80.0 | |||
| F2 −ENU | 141 | |||||||
| Nonleukemic | 29 | 0.25 ± 0.04 | 7.95 ± 5.56 | 119.7 ± 41.0 | 930 ± 482 | |||
| Leukemic | 112 | 1.01 ± 0.61* | 35.47 ± 71.43 | 102.7 ± 28.4† | 584 ± 298* | 108 | 254 | 79.4 |
| F2 +ENU | 137 | |||||||
| Nonleukemic | 4 | 0.17 ± 0.07 | 6.50 ± 5.73 | 139.8 ± 50.2 | 632.5 ± 209.2 | |||
| Leukemic | 127 | 1.45 ± 0.63* | 115.2 ± 154.9 | 84.4 ± 29.6* | 440.2 ± 214.6 | 111 | 171 | 90.4 |
| Lymphoma | 6 | 1.51 ± 0.76 | 288.9 ± 392.0 | 94.8 ± 32.5 | 506.5 ± 328.8 |
| . | n . | Spleen wt, g . | WBC count, 109/L . | Hb level, g/L . | Platelet count, 109/L . | Latency, d . | Median survival, d . | Incidence, % . |
|---|---|---|---|---|---|---|---|---|
| B6C3F1 −ENU | 23 | |||||||
| Nonleukemic | 20 | 0.29 ± 0.35 | 7.73 ± 3.63 | 125.0 ± 25.8 | 911.6 ± 215.9 | |||
| Leukemic | 3 | 1.63 ± 0.48* | 213.0 ± 147.4* | 89.7 ± 21.6† | 458.7 ± 78.5‡ | 234 | NA§ | 12.5 |
| B6C3F1 +ENU | 21 | |||||||
| Nonleukemic | 6 | 0.24 ± 0.14 | 9.07 ± 6.70 | 120.3 ± 17.8 | 1032 ± 414.1 | |||
| Leukemic | 12 | 0.90 ± 0.33‡ | 56.85 ± 69.59 | 76.2 ± 29.2† | 425.7 ± 245.4‡ | 199 | 233 | 57.1 |
| Lymphoma | 3 | 1.09 ± 0.71 | 61.11 ± 25.43 | 86.7 ± 39.9 | 527 ± 80.0 | |||
| F2 −ENU | 141 | |||||||
| Nonleukemic | 29 | 0.25 ± 0.04 | 7.95 ± 5.56 | 119.7 ± 41.0 | 930 ± 482 | |||
| Leukemic | 112 | 1.01 ± 0.61* | 35.47 ± 71.43 | 102.7 ± 28.4† | 584 ± 298* | 108 | 254 | 79.4 |
| F2 +ENU | 137 | |||||||
| Nonleukemic | 4 | 0.17 ± 0.07 | 6.50 ± 5.73 | 139.8 ± 50.2 | 632.5 ± 209.2 | |||
| Leukemic | 127 | 1.45 ± 0.63* | 115.2 ± 154.9 | 84.4 ± 29.6* | 440.2 ± 214.6 | 111 | 171 | 90.4 |
| Lymphoma | 6 | 1.51 ± 0.76 | 288.9 ± 392.0 | 94.8 ± 32.5 | 506.5 ± 328.8 |
Data are means plus or minus SD (all such values).
WBC indicates white blood cell; Hb, hemoglobin; ENU, ethyl-N-nitrosourea; NA, not applicable.
P < .001 for 2-tailed t tests comparing nonleukemic with leukemic mice.
P < .05 for 2-tailed t tests comparing nonleukemic with leukemic mice.
P < .01 for 2-tailed t tests comparing nonleukemic with leukemic mice.
Median survival not reached.
The leukemias detected in untreated F2 mice were characterized by splenomegaly, leukocytosis, anemia, and thrombocytopenia (Table 1). Flow cytometric analysis showed that, compared with untreated nonleukemic F2 mice, untreated leukemic F2 mice had a higher proportion of c-kit+CD45+ cells (16.25% ± 11.62% vs 11.22% ± 10.55%; P = .045) in the bone marrow, decreased B220+ cells (25.16% ± 13.35% vs 41.29% ± 8.28%;P < .001), increased Gr1+CD34+ cells (17.48% ± 16.39% vs 10.54% ± 13.44%; P = .046), and increased Gr1+ cells (63.46% ± 26.15% vs 47.95% ± 19.76%; P = .004) in the spleen. Histologically, nonleukemic F2 mice had normal splenic architecture and marked myeloid expansion in the bone marrow, an expected consequence of the PR transgene. Myeloid maturation was preserved, with the predominant forms being mature neutrophils. In contrast, leukemic F2 mice had an accumulation of immature, neoplastic myeloid cells in both the peripheral blood and bone marrow (Table 2). Splenic architecture was effaced by infiltrating neoplastic cells. In summary, the phenotype of leukemias arising in untreated F2 PR+ mice strongly resembles the acute leukemia with promyelocytic features previously described in PR+ B6C3F1 mice.18
Differential counts comparing nonleukemic and leukemic PR+ F2 mice
| . | −ENU . | +ENU . | ||
|---|---|---|---|---|
| Nonleukemic, n = 6 . | Leukemic, n = 6 . | Nonleukemic, n = 4 . | Leukemic, n = 6 . | |
| Peripheral blood | ||||
| Myeloid precursors | ||||
| Early | 0 (0) | 6 (1-15)* | 0 (0) | 3 (0-7) |
| Mid | 1.67 (0-3) | 25 (6-44)† | 4.25 (1-7) | 24.83 (14-36)† |
| Late | 36.5 (26-67) | 38.33 (16-85) | 44 (31-63) | 55 (22-72) |
| Lymphocytes | 56.67 (28-67) | 25.67 (3-46)† | 47 (25-61) | 13.33 (5-31)† |
| Erythroid precursors | 0.17 (0-1) | 2.5 (0-8) | 1.25 (0-2) | 2 (0-6) |
| Eosinophils | 1.33 (0-3) | 0.67 (0-3) | 0.25 (0-1) | 0.17 (0-1) |
| Monocytes | 3.67 (2-6) | 1.83 (0-3) | 3.25 (1-5) | 1.67 (0-4) |
| Bone marrow | ||||
| Myeloid precursors | ||||
| Early | 5 (3-7) | 33.29 (16-50)‡ | 5 (4-7) | 26.17 (7-64) |
| Mid | 11.5 (5-20) | 12.57 (5-22) | 15.25 (8-20) | 17.17 (10-33) |
| Late | 55.5 (45-64) | 34.14 (11-58)* | 54.25 (33-62) | 32.83 (10-65) |
| Lymphocytes | 8.5 (5-12) | 2.86 (1-5)‡ | 7.25 (4-15) | 2.5 (1-4) |
| Erythroid precursors | 17 (14-22) | 12.43 (5-21) | 15 (6-21) | 20.17 (9-33) |
| Eosinophils | 2.17 (0-7) | 4.43 (0-25) | 2.5 (0-4) | 1 (0-4) |
| Monocytes | 0.33 (0-2) | 0.29 (0-2) | 0.75 (0-2) | 0.17 (0-1) |
| . | −ENU . | +ENU . | ||
|---|---|---|---|---|
| Nonleukemic, n = 6 . | Leukemic, n = 6 . | Nonleukemic, n = 4 . | Leukemic, n = 6 . | |
| Peripheral blood | ||||
| Myeloid precursors | ||||
| Early | 0 (0) | 6 (1-15)* | 0 (0) | 3 (0-7) |
| Mid | 1.67 (0-3) | 25 (6-44)† | 4.25 (1-7) | 24.83 (14-36)† |
| Late | 36.5 (26-67) | 38.33 (16-85) | 44 (31-63) | 55 (22-72) |
| Lymphocytes | 56.67 (28-67) | 25.67 (3-46)† | 47 (25-61) | 13.33 (5-31)† |
| Erythroid precursors | 0.17 (0-1) | 2.5 (0-8) | 1.25 (0-2) | 2 (0-6) |
| Eosinophils | 1.33 (0-3) | 0.67 (0-3) | 0.25 (0-1) | 0.17 (0-1) |
| Monocytes | 3.67 (2-6) | 1.83 (0-3) | 3.25 (1-5) | 1.67 (0-4) |
| Bone marrow | ||||
| Myeloid precursors | ||||
| Early | 5 (3-7) | 33.29 (16-50)‡ | 5 (4-7) | 26.17 (7-64) |
| Mid | 11.5 (5-20) | 12.57 (5-22) | 15.25 (8-20) | 17.17 (10-33) |
| Late | 55.5 (45-64) | 34.14 (11-58)* | 54.25 (33-62) | 32.83 (10-65) |
| Lymphocytes | 8.5 (5-12) | 2.86 (1-5)‡ | 7.25 (4-15) | 2.5 (1-4) |
| Erythroid precursors | 17 (14-22) | 12.43 (5-21) | 15 (6-21) | 20.17 (9-33) |
| Eosinophils | 2.17 (0-7) | 4.43 (0-25) | 2.5 (0-4) | 1 (0-4) |
| Monocytes | 0.33 (0-2) | 0.29 (0-2) | 0.75 (0-2) | 0.17 (0-1) |
Data are mean (range) counts per 100 nucleated cells.
Early indicates blasts and promyelocytes; Mid, myelocytes and metamyelocytes; Late, bands and neutrophils.
P < .05 for 2-tailed unpaired t tests of nonleukemic versus leukemic counts.
P < .01 for 2-tailed unpaired t tests of nonleukemic versus leukemic counts.
P < .001 for 2-tailed unpaired t tests of nonleukemic versus leukemic counts.
ENU treatment interacts with genotype to promote leukemogenesis
ENU-treated PR+ B6C3F1 mice developed leukemia at a higher rate than untreated littermate PR+ mice (57.1% vs 12.5%; P = .004) and had significantly shorter survival (P < .001), although their survival was not significantly different from untreated F2 mice (P = .34; Figure 1; Table 1). This suggests that ENU treatment of PR+ mice in the resistant background and exposure to SWR/J alleles are roughly equipotent, although not necessarily mechanistically equivalent, in promoting leukemia.
ENU also interacts with SWR/J alleles in F2 mice to increase leukemia incidence. Compared with treated B6C3F1 mice, treated F2 mice develop leukemia at a much higher incidence (90.4% vs 57.1%, P < .001), with shorter latency (111 days vs 199 days), and shorter median survival (171 days vs 233 days). Similarly, treated F2 animals developed leukemia with a higher incidence than untreated F2 animals (90.4% and 79.4%; P < .01) and had decreased median survival (171 days vs 254 days).
The phenotype of leukemia in treated and untreated F2 mice was similar, although ENU-treated leukemic F2 mice had higher spleen weight, higher WBC counts, lower hemoglobin levels, and lower platelet counts (Table 1). Compared with F2 treated nonleukemic mice, F2 treated leukemic mice had an increased proportion of c-Kit+CD45+ cells (22.74% ± 15.75% vs 6.80% ± 6.13%; P = .047) in the bone marrow, decreased B220+ cells (20.98% ± 11.30% vs 47.31% ± 15.12%; P < .001), and decreased CD3+ cells (3.50% ± 2.92% vs 10.39% ± 5.47%; P < .001) in the spleen. The leukemic cells in treated and untreated F2 mice had similar morphology in peripheral blood, bone marrow, and spleen (not shown).
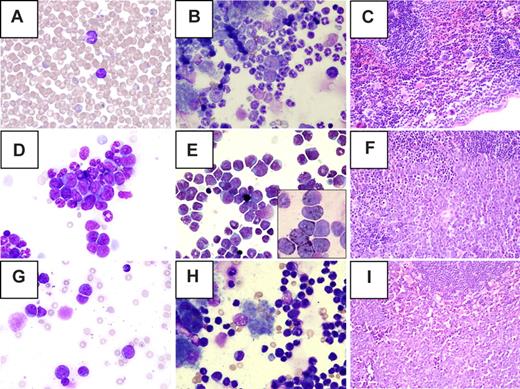
T-cell lymphomas were detected in a small number of ENU-treated mice (n = 3 B6C3F1, n = 6 F2) These mice had a high percentage of immature lymphoid cells in the peripheral blood and a monomorphic population of small lymphocytes in the bone marrow (Figure 2). Splenic architecture was effaced by an infiltrate of immature lymphoid cells. Flow cytometric analysis showed a large population of CD4+CD8+ cells in both the spleen (38.97% ± 28.18%) and bone marrow (data not shown).
Morphology of tumors detected in ENU-treated PR+ F2 mice. (A-C) Myeloproliferative disease, (D-F) myeloid leukemia, (G-I) lymphoma, (A,D,G) peripheral blood, (B,E,H) bone marrow, and (C,F,I) spleen. (A) Normal lymphocyte and neutrophil. (B) Mild myeloid hyperplasia (secondary to the PR transgene) and normal maturation of all lineages. (C) Normal splenic architecture. (D) Clusters of immature myeloid cells in the peripheral blood. (E) Left shift and expansion of the myeloid lineage in the bone marrow. (Inset) Myeloperoxidase positive blasts. (F) Effacement of splenic architecture by leukemic blasts. (G) Immature lymphoid cells in peripheral blood. (H) Infiltration of bone marrow by malignant lymphocytes. (I) Effacement of splenic architecture by lymphoma cells. Original magnification: ×50 (A), ×40 (C,F,I); ×100 (all others). Images were obtained using a BX40 microscope (Olympus, Center Valley, PA) with UPlanFl 40×/0.75, Plan 50×/0.90 oil iris, and UplsnFl 100×/1.30 oil ∞/0.17 objective lenses. DP70 camera and DP controller software version 2.2.1.227 (Olympus) were used for image acquisition. Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image preparation.
Morphology of tumors detected in ENU-treated PR+ F2 mice. (A-C) Myeloproliferative disease, (D-F) myeloid leukemia, (G-I) lymphoma, (A,D,G) peripheral blood, (B,E,H) bone marrow, and (C,F,I) spleen. (A) Normal lymphocyte and neutrophil. (B) Mild myeloid hyperplasia (secondary to the PR transgene) and normal maturation of all lineages. (C) Normal splenic architecture. (D) Clusters of immature myeloid cells in the peripheral blood. (E) Left shift and expansion of the myeloid lineage in the bone marrow. (Inset) Myeloperoxidase positive blasts. (F) Effacement of splenic architecture by leukemic blasts. (G) Immature lymphoid cells in peripheral blood. (H) Infiltration of bone marrow by malignant lymphocytes. (I) Effacement of splenic architecture by lymphoma cells. Original magnification: ×50 (A), ×40 (C,F,I); ×100 (all others). Images were obtained using a BX40 microscope (Olympus, Center Valley, PA) with UPlanFl 40×/0.75, Plan 50×/0.90 oil iris, and UplsnFl 100×/1.30 oil ∞/0.17 objective lenses. DP70 camera and DP controller software version 2.2.1.227 (Olympus) were used for image acquisition. Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image preparation.
QTL analysis shows significant peaks for survival, spleen weight, and WBC count
Because survival in this leukemia model was significantly influenced by genetic background, we performed genotype:phenotype association mapping to identify regions of the mouse genome containing genes that regulate this trait. In addition to survival, we sought to determine whether spleen weight and WBC count were influenced by genotype. These variables were included because splenomegaly28 and high WBC counts29 are markers of poor prognosis in human AML. Thirteen QTLs were found that affected the traits of interest in the F2 population, including 5 loci affecting leukemia-free survival (MleuX.Y, where X specifies chromosome and Y specifies the QTL on the chromosome), 5 affecting spleen weight (SplX.Y), and 3 affecting white blood cell count (WbcX.Y) (Table 3). Two QTLs (Mleu1.1 and Spl11.1) were significant at the genomewide threshold; the remainder were significant at the chromosome threshold. Seven QTLs provided evidence for significant genotype by treatment interaction, indicating that their effects were either restricted to the ENU-treated population (Mleu1.1, Wbc1.1, Wbc15.1), restricted to the untreated population (MleuX.1, Spl11.1), or had opposite effects in the 2 populations with either a negative (Spl16.1) or a positive (Spl6.1) effect in the treated population. Four QTLs had significant effects only in either the treated (Mleu11.1, Mleu19.1, Spl11.2) or the untreated (Mleu2.1) population, but the difference in effects between populations failed to reach statistical significance. Finally, 2 QTLs (Spl7.1, Wbc19.1) had significant, coequal genetic effects in the treated and untreated populations. Thus, the majority of QTLs mapped here have different effects in ENU-treated compared with untreated mice.
Quantitative trait loci associated with survival, spleen weight, and WBC count
| QTL . | Peak map location (1 LPR CI) . | Closest SNP . | Model . | LPR . | R2 . | a (SE) . | d (SE) . |
|---|---|---|---|---|---|---|---|
| Mleu1.1 | 42.837 (39-46) | rs13476036 | +ENU | 3.94* | 0.125 | −0.51 (0.17)† | 0.62 (0.20)† |
| Mleu2.1 | 47.000 (37-55.3) | rs3669077 | −ENU | 2.53‡ | 0.082 | 0.56 (0.15)† | −0.28 (0.20) |
| Mleu11.1 | 54.044 (50-57) | rs13481161 | +ENU | 2.48‡ | 0.081 | −0.10 (0.13) | −0.64 (0.19)† |
| Mleu19.1 | 24.989 (14-46) | rs3655407 | +ENU | 2.99‡ | 0.096 | 0.45 (0.12)† | 0.13 (0.19) |
| MleuX.1 | 59.634 (49-ter) | rs13484113 | −ENU | 2.26‡ | 0.055 | 0.27 (0.10)† | — |
| Spl6.1 | 65.000 (53-ter) | rs13479087 | Interact | 2.62‡ | 0.036 | −0.17 (0.09) | 0.18 (0.11) |
| Spl6.1 | 65.000 (53-ter) | rs13479087 | +ENU | 1.82 | 0.060 | 0.18 (0.07)† | −0.15 (0.07) |
| Spl6.1 | 65.000 (53-ter) | rs13479087 | −ENU | 1.05 | 0.035 | −0.17 (0.10) | 0.18 (0.10) |
| Spl7.1 | 11.000 (0-20) | rs6239372 | Covariate | 3.13‡ | 0.042 | −0.20 (0.07)† | 0.13 (0.08) |
| Spl11.1 | 1.000 (0–6) | rs13480834 | −ENU | 4.24* | 0.135 | 0.38 (0.09)† | −0.20 (0.13) |
| Spl11.2 | 42.310 (37-54) | rs6384104 | +ENU | 2.47‡ | 0.081 | −0.15 (0.07)† | 0.14 (0.07)† |
| Spl16.1 | 16.505 (7-31) | rs4167843 | Interact | 2.48‡ | 0.034 | 0.13 (0.08) | 0.29 (0.11)† |
| Spl16.1 | 16.505 (7-31) | rs4167843 | +ENU | 1.20 | 0.041 | −0.14 (0.07)† | −0.12 (0.10) |
| Spl16.1 | 16.505 (7-31) | rs4167843 | −ENU | 1.47 | 0.049 | 0.13 (0.10) | 0.29 (0.13)† |
| Wbc1.1 | 24.000 (17-31) | rs6206420 | +ENU | 3.30‡ | 0.129 | −0.69 (0.20)† | −0.27 (0.27) |
| Wbc15.1 | 23.879 (16-34) | rs13482609 | +ENU | 2.75‡ | 0.109 | 0.68 (0.19)† | 0.00 (0.25) |
| Wbc19.1 | 47.000 (36-ter) | rs3718687 | Covariate | 2.85‡ | 0.042 | 0.43 (0.13)† | 0.21 (0.18) |
| QTL . | Peak map location (1 LPR CI) . | Closest SNP . | Model . | LPR . | R2 . | a (SE) . | d (SE) . |
|---|---|---|---|---|---|---|---|
| Mleu1.1 | 42.837 (39-46) | rs13476036 | +ENU | 3.94* | 0.125 | −0.51 (0.17)† | 0.62 (0.20)† |
| Mleu2.1 | 47.000 (37-55.3) | rs3669077 | −ENU | 2.53‡ | 0.082 | 0.56 (0.15)† | −0.28 (0.20) |
| Mleu11.1 | 54.044 (50-57) | rs13481161 | +ENU | 2.48‡ | 0.081 | −0.10 (0.13) | −0.64 (0.19)† |
| Mleu19.1 | 24.989 (14-46) | rs3655407 | +ENU | 2.99‡ | 0.096 | 0.45 (0.12)† | 0.13 (0.19) |
| MleuX.1 | 59.634 (49-ter) | rs13484113 | −ENU | 2.26‡ | 0.055 | 0.27 (0.10)† | — |
| Spl6.1 | 65.000 (53-ter) | rs13479087 | Interact | 2.62‡ | 0.036 | −0.17 (0.09) | 0.18 (0.11) |
| Spl6.1 | 65.000 (53-ter) | rs13479087 | +ENU | 1.82 | 0.060 | 0.18 (0.07)† | −0.15 (0.07) |
| Spl6.1 | 65.000 (53-ter) | rs13479087 | −ENU | 1.05 | 0.035 | −0.17 (0.10) | 0.18 (0.10) |
| Spl7.1 | 11.000 (0-20) | rs6239372 | Covariate | 3.13‡ | 0.042 | −0.20 (0.07)† | 0.13 (0.08) |
| Spl11.1 | 1.000 (0–6) | rs13480834 | −ENU | 4.24* | 0.135 | 0.38 (0.09)† | −0.20 (0.13) |
| Spl11.2 | 42.310 (37-54) | rs6384104 | +ENU | 2.47‡ | 0.081 | −0.15 (0.07)† | 0.14 (0.07)† |
| Spl16.1 | 16.505 (7-31) | rs4167843 | Interact | 2.48‡ | 0.034 | 0.13 (0.08) | 0.29 (0.11)† |
| Spl16.1 | 16.505 (7-31) | rs4167843 | +ENU | 1.20 | 0.041 | −0.14 (0.07)† | −0.12 (0.10) |
| Spl16.1 | 16.505 (7-31) | rs4167843 | −ENU | 1.47 | 0.049 | 0.13 (0.10) | 0.29 (0.13)† |
| Wbc1.1 | 24.000 (17-31) | rs6206420 | +ENU | 3.30‡ | 0.129 | −0.69 (0.20)† | −0.27 (0.27) |
| Wbc15.1 | 23.879 (16-34) | rs13482609 | +ENU | 2.75‡ | 0.109 | 0.68 (0.19)† | 0.00 (0.25) |
| Wbc19.1 | 47.000 (36-ter) | rs3718687 | Covariate | 2.85‡ | 0.042 | 0.43 (0.13)† | 0.21 (0.18) |
QTL locations and gene effects are shown for survival (MleuX.Y, where X is the chromosome and Y is the number along the chromosome), spleen weight (SplX.Y), and white blood cell count (WbcX.Y).
LPR CI indicates likelihood probability ratio confidence interval; R2, proportion of variance for the model; a, gene effects for the additive genotypic value; d, gene effects for the dominance genotypic value; SE, standard error of the estimate.
Significant at the genomewide threshold.
Significant at P = .05.
Significant at the chromosome-wide threshold.
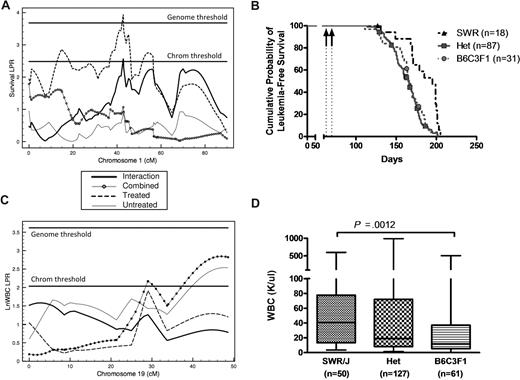
For the survival QTLs, all significant additive effects, except for Mleu1.1 (Figure 3), were positive, indicating that the SWR allele typically predisposes to myeloid leukemia. At Mleu11.1, heterozygotes had superior survivorship relative to both SWR and B6C3F1 homozygotes. Treatment by genotype interaction was significant at Mleu1.1 and MleuX.1, where the effect was limited to the ENU-treated population or the untreated population, respectively. Even though the differences between treatments failed to reach significance in the genome scan, the effects of Mleu2.1 was stronger in the untreated mice, whereas Mleu11.1 and Mleu19.1 had stronger effects in the treated mice.
Quantitative trait locus (QTL) mapping for survival and WBC count in leukemic PR+ F2 mice. QTL and survival curves were generated from analysis of F2 mice. (A) Survival QTL (Mleu1.1) in the treated model on chromosome 1 at 42.8 cM that is significant at the genomewide level. (B) Survival of ENU-treated F2 mice is significantly (P < .001) affected by genotype at Mleu1.1. Arrows indicate ENU treatment at 9 and 10 weeks of age. (C) WBC count QTL (Wbc19.1) on chromosome 19 at 47.0 cM that is significant in the covariate model at the chromosome-wide level. (D) At Wbc19.1, SWR/J homozygotes have a higher mean WBC count than B6C3F1 homozygotes (79 ± 128 vs 48 ± 96, respectively, P < .01) independent of treatment status. Data are plotted as median (horizontal bar), 25th to 75th percentile (box) and range (whiskers).
Quantitative trait locus (QTL) mapping for survival and WBC count in leukemic PR+ F2 mice. QTL and survival curves were generated from analysis of F2 mice. (A) Survival QTL (Mleu1.1) in the treated model on chromosome 1 at 42.8 cM that is significant at the genomewide level. (B) Survival of ENU-treated F2 mice is significantly (P < .001) affected by genotype at Mleu1.1. Arrows indicate ENU treatment at 9 and 10 weeks of age. (C) WBC count QTL (Wbc19.1) on chromosome 19 at 47.0 cM that is significant in the covariate model at the chromosome-wide level. (D) At Wbc19.1, SWR/J homozygotes have a higher mean WBC count than B6C3F1 homozygotes (79 ± 128 vs 48 ± 96, respectively, P < .01) independent of treatment status. Data are plotted as median (horizontal bar), 25th to 75th percentile (box) and range (whiskers).
The 5 spleen weight QTLs mapped to chromosomes 6, 7, 11 (2 QTLs), and 16. The SWR/J allele typically results in lower spleen weights, especially in the treated population, although it leads to higher spleen weight at Spl6.1 in the treated population and at Spl11.1 and Spl16.1 in the untreated population. QTLs Spl6.1, Spl11.1, and Spl16.1 show significantly different effects in the 2 treatment groups, the SWR/J allele having a positive, recessive effect on spleen weight in the treated group but a negative, recessive effect in the untreated group at Spl6.1, a positive effect in the untreated population and no effect in the treated population at Spl11.1, and a negative, dominant effect in the treated population and a positive, overdominant effect in the untreated population at Spl16.1. At Spl7.1, the SWR/J allele has a negative effect in both treatment groups, whereas the SWR/J allele at Spl11.2 has a negative, recessive effect restricted to the treated group.
The 3 white blood cell count QTLs map to chromosomes 1 (Wbc1.1), 15 (Wbc15.1), and 19 (Wbc19.1). At Wbc1.1, the SWR/J allele leads to a reduction in WBC count in ENU-treated animals but has no effect in untreated animals. This difference in gene effects between treated and untreated groups is also statistically significant. At Wbc15.1, the SWR/J allele leads to higher WBC count in the treated group, but has no effect in the untreated group. At Wbc19.1, at the distal end of chromosome 19, the SWR/J allele leads to higher WBC count in both the treated and untreated populations (Figure 3).
Because the total number of genes contained within these QTLs is large (3474 genes in 13 QTLs), we used a bioinformatic strategy (described in “Methods”) to systematically identify the subset of genes with known connections to myeloid leukemogenesis. The most frequently cited genes (Table 4) represent high priority candidate t-AML susceptibility factors.
Candidate quantitative trait genes prioritized by MeSH analysis
| Gene symbol . | Gene name . | QTL . | MeSH Score . |
|---|---|---|---|
| Trp53 | Transformation related protein 53 | Spl11.2 | 110 |
| Prkdc | Protein kinase, DNA-activated, catalytic polypeptide | Spl16.1 | 39 |
| Xrcc5 | X-ray repair complementing defective repair in Chinese hamster cells 5 | Wbc1.1 | 29 |
| Sfpi1 | SFFV proviral integration 1 | Mleu2.1 | 25 |
| Ercc1 | Excision repair cross-complementing rodent repair deficiency, complementation group 1 | Spl7.1 | 21 |
| Nf1 | Neurofibromatosis 1 | Spl11.2 | 17 |
| Ercc2 | Excision repair cross-complementing rodent repair deficiency, complementation group 2 | Spl7.1 | 14 |
| Piga | Phosphatidylinositol glycan, class A | MleuX.1 | 13 |
| Myc | Myelocytomatosis oncogene | Wbc15.1 | 11 |
| Bcl2 | B-cell leukemia/lymphoma 2 | Mleu1.1 | 9 |
| Trp53bp1 | Transformation-related protein 53 binding protein 1 | Mleu2.1 | 8 |
| Ddb2 | Damage-specific DNA binding protein 2 | Mleu2.1 | 6 |
| Fas | Fas (TNF receptor superfamily member) | Mleu19.1 | 6 |
| Gene symbol . | Gene name . | QTL . | MeSH Score . |
|---|---|---|---|
| Trp53 | Transformation related protein 53 | Spl11.2 | 110 |
| Prkdc | Protein kinase, DNA-activated, catalytic polypeptide | Spl16.1 | 39 |
| Xrcc5 | X-ray repair complementing defective repair in Chinese hamster cells 5 | Wbc1.1 | 29 |
| Sfpi1 | SFFV proviral integration 1 | Mleu2.1 | 25 |
| Ercc1 | Excision repair cross-complementing rodent repair deficiency, complementation group 1 | Spl7.1 | 21 |
| Nf1 | Neurofibromatosis 1 | Spl11.2 | 17 |
| Ercc2 | Excision repair cross-complementing rodent repair deficiency, complementation group 2 | Spl7.1 | 14 |
| Piga | Phosphatidylinositol glycan, class A | MleuX.1 | 13 |
| Myc | Myelocytomatosis oncogene | Wbc15.1 | 11 |
| Bcl2 | B-cell leukemia/lymphoma 2 | Mleu1.1 | 9 |
| Trp53bp1 | Transformation-related protein 53 binding protein 1 | Mleu2.1 | 8 |
| Ddb2 | Damage-specific DNA binding protein 2 | Mleu2.1 | 6 |
| Fas | Fas (TNF receptor superfamily member) | Mleu19.1 | 6 |
All genes within QTLs were queried for Medline citations using the MeSH terms “myelodysplastic syndrome,” “leukemia, myeloid,” “DNA repair,” “metabolic detoxication, drugs,” and “alkylating agents.” Citations for each gene were summed to generate a “MeSH score.” Genes with more than 5 citations are shown here.
Discussion
Studies in mice17,30 and humans31,32 support the concept that susceptibility to myeloid leukemia has a genetic component. In addition, environmental factors (eg, genotoxic therapy) and gene-by-environment interactions contribute to t-AML susceptibility. Intermediate phenotypes relevant for t-AML include the response to DNA damage, the efficiency of drug metabolism, and/or the frequency of cells vulnerable to transformation. Correspondingly, QTLs influencing stem cell number30 and genes involved in DNA repair and drug metabolism12-14 have been associated with t-AML susceptibility. Human studies have identified a limited number of genetic variants that are more prevalent in affected persons, but because of the complex nature of t-AML susceptibility, it is likely that important genetic determinants have not yet been identified. Inbred mouse strains provide an experimental system for whole genome scans using a tractable number of animals. This approach has the potential to identify contributors to t-AML susceptibility (or other complex traits) that would not have been selected a priori for a candidate gene approach. We previously reported that susceptibility to alkylator-associated AML in inbred mice is a heritable trait.17 Here, we report the results of an F2 intercross (sensitized by including the hCG-PML/RARA transgene) between strains of mice previously identified as susceptible (SWR/J) or resistant (C57BL/6J and C3H/HeJ) to developing alkylator-induced AML. Transgenic F2 mice were highly susceptible to leukemia with (90.4% incidence) or without (79.4%) alkylator therapy, confirming the original observation that the SWR/J genome contains alleles that predispose mice to myeloid malignancy. A genomewide analysis in this cohort identified 13 quantitative trait loci (QTLs) significantly associated with survival, spleen weight, or WBC count.
The genotypic effects mapped here are substantial but still of individually small effect. The average significant additive and dominance genotypic values are approximately 0.4 to 0.5 standard deviation units across traits. Most of the single locus gene effects differed depending on whether the animal was treated with ENU. For the single locus QTLs, only 2 loci, Spl7.1 and Wbc19.1, had significant congruent effects in both treated and untreated populations. Two other QTLs, Spl6.1 and Spl16.1, had significant opposite effects in the treated and untreated populations. Of the remaining QTLs, 6 were only apparent in the treated population and 3 in the untreated population. Clearly, the effects of genes on AML in our mouse model depend highly on whether the animals are treated with ENU.
The presence of multiple, significant QTLs governing survival, spleen weight, and WBC count suggests that these are complex traits, supporting the hypothesis that genetic polymorphism at multiple loci are important for t-AML susceptibility. The 1 LPR confidence intervals for 2 pairs of QTLs (Mleu19.1 and Wbc19.1, and Mleu11.1 and Spl11.2) overlap, suggesting a possible shared genetic basis for these traits. The SWR/J allele at Mleu19.1 and Wbc19.1 leads to decreased survival in ENU-treated mice and to higher WBC counts independent of treatment status. Because the SWR/J allele for these QTLs leads to higher WBC counts and poorer survival, a single locus may affect both of these traits. At Mleu11.1, heterozygotes have a significantly longer survival compared with either homozygote, whereas at Spl11.2, SWR/J mice have significantly smaller spleen weight when killed. The proportion of variance (R2) accounted for by each QTL ranges from 3.4% to 13.5%, suggesting that most of the variance seen in these models cannot be explained by a single QTL. The 3 survival QTLs detected in the ENU-treated model account for 30% of the variance seen in this model. The remaining variance is attributable to other environmental causes or genes with effect sizes too small for our study to detect.
As predicted, additive genotypic values for most of the QTLs we detected suggest that SWR/J alleles promote phenotypes associated with a worse outcome in this leukemia model (ie, decreased survival and increased WBC count). This is consistent with our original observation that SWR/J mice are more susceptible to ENU-induced myeloid malignancies (AML, MDS, granulocytic sarcoma).17 However, the additive genotypic values for 1 survival QTL (Mleu1.1), 3 spleen weight QTLs (Spl7.1, Spl11.2, and Spl16.1), and 1 WBC count QTL (WBC1.1) indicate that SWR/J alleles at these QTLs lead to increased survival and decreased spleen weight or WBC count. Several plausible explanations for these counterintuitive results are possible. With respect to survival, t-AML susceptibility is a complex trait, so, although the overall effect of the SWR/J background increases susceptibility, it is possible that some individual genes within SWR/J lead to enhanced survival. Three of 5 spleen weight QTLs suggested that lower spleen weight was associated with a homozygous SWR/J genotype at those QTLs. For these QTLs, it is possible that once the leukemic transformation has occurred, SWR/J homozygous mice succumb more quickly than their B6C3F1 counterparts, leaving less time for infiltration of the spleen before being killed. One could envision a similar scenario for WBC1.1. A final explanation may be that epistatic interactions between SWR/J and B6C3F1 alter the expected phenotype of F2 mice, effectively giving the appearance that SWR/J alleles enhance survival (or spleen weight), although the effect is primarily due to interactions between unlinked SWR/J and B6C3F1 genes.
Approximately 5% of human AML cases with t(15;17) arise after chemotherapy,33 including alkylator treatment in the majority of patients,34 establishing the clinical relevance of this model. However, a potential caveat of the design is that it may be biased to detect QTLs that interact primarily with PML/RARA. Because all mice in the present study were transgene positive, we cannot directly determine the effect of PML/RARA on these results. However, the large effect of ENU in the F2 population and the fact that most of the QTLs detected are treatment dependent suggest that these findings are largely alkylator associated and not solely PML/RARA dependent. In addition, we have confirmed in a larger cohort our previous observation that nontransgenic SWR/J mice are susceptible to ENU-induced myeloid malignancies (M. J. Walter and T.G., manuscript in preparation). Therefore, the susceptibility loci mapped in this F2 cross are likely to be relevant for AML pathogenesis in the presence or absence of PML/RARA.
As expected in this F2 intercross, the QTL regions we describe are large and contain many genes with established roles in leukemogenesis. Fine-mapping these QTLs will likely uncover other genes with no known connections to AML biology. Candidate genes will require further study to determine their contribution to the QTL phenotypes and their role in t-AML susceptibility. Bcl2 (located 144 Kbp from the peak of Mleu1.1) is a particularly interesting candidate because high BCL2 expression is associated with poor survival in patients with AML.35 Of note, a genetic interaction between Bcl2 and PML/RARA has been shown previously.36 Coexpression of Bcl2 and PML/RARA from the MRP8 promoter in transgenic mice produced a greater impairment in myeloid maturation at the promyelocyte stage and decreased median time to leukemia (127 days vs 257 days) compared with mice transgenic for MRP8-PML/RARA alone.36 This provides strong circumstantial evidence suggesting that Bcl2 may be the quantitative trait gene driving Mleu1.1, the most significant survival QTL detected in our F2 intercross. Because ENU triggers an apoptotic response in a number of tissues,37-39 differences in the Bcl2 coding sequence or expression levels between SWR/J and B6C3F1 might affect the response to apoptotic signals in early myeloid cells. Altered Bcl2 expression could affect the balance between proapoptotic and antiapoptotic signals, thereby promoting survival of cells that have accumulated significant DNA damage. Such cells may be more prone to subsequent mutations that contribute to leukemogenesis.
Discovering the specific genetic variations that underlie these QTLs may provide information that can be used to predict t-AML susceptibility prospectively in patients. Such knowledge would be a valuable tool in designing treatments to minimize the risk of adverse outcomes such as t-AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St Louis, MO, for services provided by the High Speed Cell Sorter Core and the Bioinformatics Core. The Siteman Cancer Center is supported in part by a National Cancer Institute (NCI) Cancer Center Support Grant P30 CA91842.
This work was supported by the National Institutes of Health (grants P01 CA101937 and T35 DK074375) and a Howard Hughes Medical Institute Medical Research Training Fellowship (R.K.F.).
National Institutes of Health
Authorship
Contribution: T.A.G., T.J.L., and J.M.C. designed the study; M.I., R.K.F., and D.E. performed experiments; M.I., F.K., and R.K.F. collected data; R.K.F., F.K., and T.M. analyzed data; R.K.F. and T.A.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy Graubert, Washington University School of Medicine, Division of Oncology, Stem Cell Biology Section, Campus Box 8007, 660 S Euclid Ave, St Louis, MO 63110; e-mail: graubert@medicine.wustl.edu.