Abstract
T-cell depletion associated with HIV infection or cytoreductive therapies triggers potential T-cell regenerative mechanisms such as peripheral T-lymphocyte expansion to weak antigenic stimuli and the increased availability of interleukin-7 (IL-7), a cytokine with potent antiapoptotic and proliferative activities. Deleterious mechanisms also associated with lymphopenia, such as increased Fas expression and apoptosis of T cell, however, may result in opposing effects. In this study, we show that Fas molecules, primarily associated with T-cell depletion in lymphopenic settings, may also contribute to compensatory T-cell expansion through transmitting costimulatory signals to suboptimally activated T cells. Proliferation of T lymphocytes in response to concomitant Fas and T-cell receptor (TCR) triggering was shown to be increased in HIV-infected individuals compared with noninfected controls. As IL-7 levels are often elevated in lymphopenic individuals in association with increased Fas expression, we analyzed whether IL-7 would influence Fas-mediated proliferative signals in T cells. We show that IL-7 is able to increase the efficacy of Fas to induce proliferation of suboptimally activated T cells. Thus, high IL-7 levels associated with lymphopenic conditions may simultaneously induce sensitivity to Fas-mediated apoptosis in nonactivated T cells and increase Fas-induced costimulatory signals in T cells recognizing low-affinity antigens.
Introduction
In T cell–depleted individuals, residual naive and memory T lymphocytes proliferate in response to self-peptide/MHC complexes1,2 and homeostatic cytokines.3,4 Expansion of peripheral T cells has been observed in animal models using T cells adoptively transferred into lymphopenic hosts, in immune deficiencies characterized by lymphopenia, and in patients receiving cytoreductive therapies against cancer, autoimmune disorders, or as pretreatment for bone marrow transplantation.3-5
Lymphopenia-induced homeostatic peripheral T-cell expansion (HPE) to low-affinity self-antigens improves peripheral T-cell counts and alleviates immunodeficiency that follows T-cell depletion. However, T-cell expansion triggered by lymphopenia may predispose to autoimmune disorders6 and may limit transplantation tolerance.7 Negative regulators of HPE, such as the increased sensitivity of T cells to activation-induced cell death (AICD)8,9 or the competition for homeostatic cytokines10 may constrain lymphopenia-associated autoimmunity and delay the process of immune reconstitution.
Fas (Apo-1/CD95) is a homotrimeric TNFR family member characterized by the presence of a death domain in its cytoplasmic tail. Fas molecules have been implicated in the maintenance of self-tolerance and T-cell homeostasis by transmitting apoptotic signals to repeatedly activated antigen-specific T cells, as well as to antigen-presenting dendritic cells (DCs) and activated B lymphocytes.11 Fas protein has also been identified as an important mediator of T-cell depletion in lymphopenic individuals. Fas expression and sensitivity of T cells to AICD is increased in HIV-infected individuals12-17 and in patients receiving cancer chemotherapy8 or treated by bone marrow transplantation.9 T cells undergoing HPE in lymphopenic mice were shown to be sensitive to Fas-mediated apoptosis, and Fas-deficient T cells expanded to higher numbers than wild-type cells.18
In addition to apoptosis induction, Fas has been recently implicated in the regulation of tissue repair,19,20 macrophage and DC activation,21,22 and neutrophil chemoattraction and activation.23 Moreover, Fas has been shown to act as a costimulatory molecule upon suboptimal T-cell activation in vitro by increasing IL-2 production and proliferation.24-28 The relevance of Fas-induced T-cell stimulatory signals during antigen-specific responses in vivo, or in clinical conditions associated with high Fas expression, and increased sensitivity to apoptosis are poorly characterized and require further investigations.
On the contrary to several known T-cell costimulatory receptors that promote naive T-cell activation, Fas may not be active on resting T cells. Sensitivity of T cells to Fas triggering increases gradually after T-cell activation due to the up-regulation of both Fas and Fas-L expression29,30 and the polarization of Fas receptors in the cell membrane.31,32 Down-regulation of the FLICE/caspase-8 inhibitory protein (FLIP), a prerequisite of Fas-induced T-cell apoptosis, is also dependent on previous T-cell activation.33
Based on these data, which identify Fas as an activation-dependent regulator of T cells, it is tempting to speculate that the costimulatory function of Fas receptors may be limited to conditions where resting T lymphocytes acquire increased sensitivity to Fas signals prior to T-cell receptor triggering. Two lines of experiments indicate that the cytokine IL-7 might play a key role in modulating the sensitivity of T cells to Fas ligation. First, we and others have shown that IL-7 increases Fas expression on naive and memory T cells and induces a cytoskeleton-dependent Fas polarization on the cell surface.14,34 This effect is accompanied by an increased sensitivity of T cells to Fas-mediated apoptosis upon experimental Fas receptor cross-linking14,34 or in HIV-infected cell cultures.35 Furthermore, it has also been demonstrated that peripheral T-cell depletion is associated with increased serum IL-7 levels in lymphopenic individuals including chemotherapy-treated and HIV-infected patients.4,36-40 In line with the in vitro data implicating IL-7 in Fas up-regulation, high IL-7 levels in the circulation of HIV-infected patients were associated with increased Fas expression on T cells and enhanced sensitivity to Fas-mediated apoptosis.14
Previous studies, demonstrating the role of Fas molecules exclusively in the process of T-cell depletion (TCD) in lymphopenic conditions,12-17 are contradictory with a potential role of IL-7 in priming T cells for costimulatory Fas-mediated signals. Nevertheless, in the present study, we show that Fas is able to act as an efficient costimulatory molecule on T cells of HIV-infected individuals stimulated through suboptimal T-cell receptor (TCR) triggering, and the costimulatory effect of Fas triggering on T cells is significantly higher in HIV-infected donors compared with noninfected controls. In this study, we raised the possibility that IL-7, elevated in response to TCD, might modify the sensitivity of T cells to Fas-mediated costimulatory signals. Indeed, we showed that IL-7 acts as a potent inducer of Fas costimulatory activity on suboptimally activated T cells. The combination of Fas signals with low level of TCR triggering on IL-7–treated T cells resulted in a significantly increased IL-2 production, sustained expression of IL-2Rα, and increased proliferation, compared with T cells with-out IL-7 treatment.
Our results suggest that the elevated Fas expression and the increased sensitivity of T cells to Fas signals reported to occur in T cell–depleted individuals may promote the proliferative effects of Fas triggering on T cells stimulated by low-affinity self- or foreign antigens. In addition, we identified a potential role for IL-7 in T-cell priming to Fas-mediated costimulation in lymphopenic conditions. Thereby, Fas, induced by high IL-7 levels, may contribute to T-cell regenerative pathways through increasing homeostatic proliferation of suboptimally activated T cells.
Methods
Patient samples and cell cultures
Blood samples were obtained from healthy donors and from 20 HIV-1–infected patients (11 men, 9 women; mean CD4+ T-cell count = 542 ±225 cells/μL). In the HIV-infected cohort, 16 patients were on combination therapy and 4 were treatment naive. Viral loads ranged between less than 50 to 214 000 copies/mL. The studies were performed with the approval of the ethical committee at Karolinska Institute. Approval was obtained from the Karolinska Institute institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll gradient centrifugation (Lymphoprep; Axis Shield PoC, Oslo, Norway). T lymphocytes were separated using the Pan T cell Isolation Kit (Miltenyi Biotech, Bergisch Gladbach, Germany). The selected cell populations contained 90% to 97% CD3+ lymphocytes as measured by fluorescence-activated cell sorting (FACS) analysis. Separation of naive and memory T cells was done using CD45RO-MicroBeads or CD45RA-MicroBeads, respectively (Miltenyi Biotech), in combination with the Pan T cell Isolation Kit. Human recombinant IL-7 (Peprotech, London, United Kingdom) was added to T-cell cultures at the concentration of 25 ng/mL. To generate dendritic cells, peripheral blood monocytes were separated using CD14-MicroBeads (Miltenyi Biotec) and cultured for 5 days in the presence of 75 ng/mL GM-CSF and 100 ng/mL IL-4 (Peprotech). All cultures were prepared using RPMI 1640 medium with l-glutamine containing 10% fetal calf serum and antibiotics.
Flow cytometric analysis of T cells
For flow cytometric measurements, phycoerytrin (PE)–conjugated anti-CD25; PE-cyanine-5 (Cy5)–conjugated anti-CD69, -CD4, and -CD8; allophycocyanin (APC)–conjugated anti-CD3; and the appropriate isotype control monoclonal antibodies (mAbs) were used (all from BD Pharmingen, San Diego, CA). Stained cells were fixed in 2% paraformaldehyde. Fluorescence intensities were measured by FACSsort (BD Biosciences, San Jose, CA) and data analyzed by WinMDI 2.8 software (Joseph Trotter, La Jolla, CA).
T-cell proliferation
Cell divisions were studied using carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA). T cells were cultured at 0.5 × 106 cells/mL density in 96-well plates and then activated using the combination of coated anti-CD3 (BD Pharmingen), anti-Fas (Clone CH-11; Nordic Biosite, Stockholm, Sweden), or control IgM (Sigma-Aldrich, St Louis, MO) antibodies. Carboxyfluorescein succinimidyl ester (CFSE)–labeled T cells were also cultured with autologous dendritic cells and IL-7 in the presence or absence of human recombinant FasL, cross-linked with an anti-His antibody (both from R&D Systems, Minneapolis, MN). After 4 days of culture, cells were recovered and analyzed by flow cytometry. Alternatively, T-cell proliferation was analyzed by detecting 3H-labeled thymidine incorporation (1 μCi [37 000 beq]/well; Nordic Biosite).
T-cell apoptosis
For apoptosis detection, T cells were treated with the combination of anti-CD3 and anti-Fas or control antibodies as already described. After 24 hours, cells were labeled with FITC- or Cy5-conjugated annexin-V (Pharmingen), fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
IL-2 production
IL-2 production of the activated T cells was analyzed using IL-2 enzyme-linked immunosorbent assay (ELISA; BD Pharmingen) according to the manufacturer's recommendation or, alternatively, using FITC-labeled anti–IL-2 antibodies (BD Pharmingen). Intracellular staining was performed on T cells reactivated with 50 ng/mL PMA and 500 ng/mL ionomycin (both from Sigma-Aldrich) for 6 hours.
Caspase activity
Caspase-8 and -3 activities were analyzed by flow cytometry on living activated T cells using Caspase-8 Detection Kit (Red-IETD-FMK) and Caspase-3 Detection Kit (FITC-DEVD-FMK), according to the instructions of the manufacturer (Calbiochem, Darmstadt, Germany). The role of caspase-3 and caspase-8 in T-cell apoptosis and proliferation was assessed using the Z-DEVD-FMK and the Z-IETD-FMK inhibitors, respectively (R&D Systems). The inhibitors were added to T cells 30 minutes prior activation at the concentrations of 5.6, 16.7, 50, or 150 μg/mL.
Results
Costimulatory function of Fas in T cells of HIV-infected patients
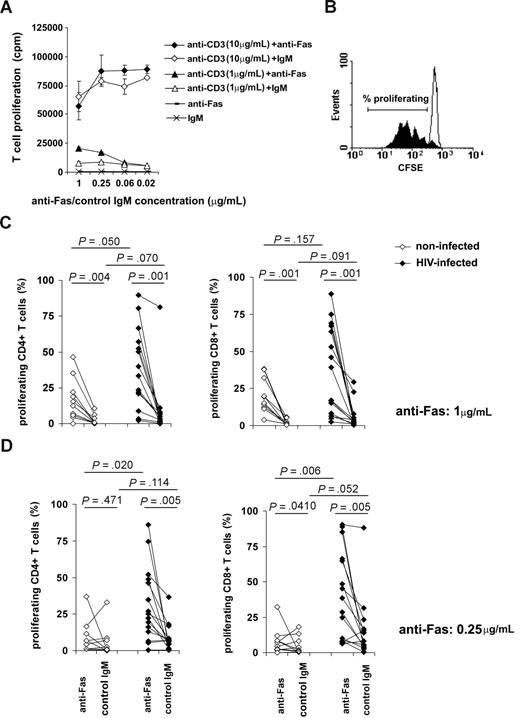
Fas has been extensively implicated in the process of progressive T-cell depletion during HIV infection12-17 as well as in other lymphopenic conditions.8,9 On the other hand, compensatory T-cell expansion has been suggested to occur in T cell–depleted individuals, indicating the coexistence of apoptotic and stimulatory factors acting on peripheral T cells.41 To the best of our knowledge, the potential role of Fas molecules in lymphopenia-induced T-cell proliferation has not been examined; therefore, we studied whether T cells from HIV-infected individuals could be stimulated through Fas cross-linking concomitant with suboptimal TCR stimulation. First, we sought to determine the optimal experimental conditions feasible for testing the potency of Fas to support T-cell proliferation. We stimulated peripheral blood T cells with different doses of anti-CD3 antibodies in the presence or absence of Fas cross-linking (Figure 1A). The costimulatory effect of anti-Fas antibodies on T-cell proliferation was apparent in the presence of weak CD3-mediated signals induced by 1 μg/mL anti-CD3 antibodies; whereas Fas-mediated costimulation was not observed when strong T-cell proliferation was triggered by 10 μg/mL anti-CD3 antibodies. Based on this experiment, we applied 1 μg/mL anti-CD3 Abs to induce suboptimal T-cell activation that can be modulated by costimulatory signals. T cells isolated from a group of HIV-infected individuals (n = 15) or from noninfected controls (n = 10) were labeled with CFSE and activated by 1 μg/mL anti-CD3 in the presence of anti-Fas or control antibodies administrated in 2 different concentrations. The percentage of proliferating T cells was calculated as shown on Figure 1B. Interestingly, we observed a generally decreased threshold for triggering proliferation of T cells isolated from HIV-infected donors; both CD4+ and CD8+ T cells showed increased proliferation in response to low dose of anti-CD3 compared with T cells of noninfected donors (Figure 1C,D). Anti-Fas antibodies administrated at the concentration of 1 μg/mL in parallel with suboptimal TCR triggering increased proliferation of T cells isolated from both HIV-infected or noninfected individuals (Figure 1C) without any major difference between the CD4+ and CD8+ subsets. Decreasing anti-Fas concentration to 0.25 μg/mL completely abolished the proliferative effect of Fas cross-linking on T cells in 9 of the10 noninfected controls, while the same anti-Fas concentration still induced a pronounced T-cell proliferation in cells derived from HIV-infected individuals (Figure 1D). These results demonstrate that Fas molecules expressed on T cells of HIV-infected individuals may act as potent costimulatory receptors able to induce T-cell proliferation upon suboptimal conditions of TCR triggering. Furthermore, the lower anti-Fas concentration needed for triggering T-cell proliferation in T cells of HIV-infected patients strongly suggested that HIV infection is associated with an increased sensitivity of T cells to proliferative signals transmitted by Fas receptors.
The effect of Fas cross-linking on proliferation of T cells isolated from HIV-infected or noninfected individuals. We analyzed the ability of anti-Fas antibodies to modulate T-cell proliferation in the presence of solid-phase bound anti-CD3 antibodies used at different doses. Proliferation of freshly isolated T cells was measured in response to 1 μg/mL or 10 μg/mL anti-CD3 in the presence or absence of Fas triggering. Thymidine incorporation was measured after 3 days of activation. Error bars represent standard deviation measured in triplicate samples, 1 representative experiment of 3 is shown. (A) To compare the proliferative effect of Fas cross-linking on T cells of HIV-infected and noninfected individuals, we activated CFSE-labeled T cells using 1 μg/mL coated anti-CD3 mAb in the presence or absence of Fas cross-linking for 4 days. The percentage of proliferating T cells was calculated as shown by a typical sample, where the filled histogram represents T cells activated in the presence of anti-Fas, and open histogram represents T cells activated in the presence of control IgM antibodies. (B) T cells isolated from a cohort of HIV-infected (closed symbols) or noninfected (open symbols) individuals were activated using 1 μg/mL anti-CD3 mAb, and 1 μg/mL (C) or 0.25 μg/m (D) anti-Fas or isotype control mAbs. Proliferation was analyzed in CD4+ and CD8+ T cells by flow cytometry; percentages of proliferating cells are shown.
The effect of Fas cross-linking on proliferation of T cells isolated from HIV-infected or noninfected individuals. We analyzed the ability of anti-Fas antibodies to modulate T-cell proliferation in the presence of solid-phase bound anti-CD3 antibodies used at different doses. Proliferation of freshly isolated T cells was measured in response to 1 μg/mL or 10 μg/mL anti-CD3 in the presence or absence of Fas triggering. Thymidine incorporation was measured after 3 days of activation. Error bars represent standard deviation measured in triplicate samples, 1 representative experiment of 3 is shown. (A) To compare the proliferative effect of Fas cross-linking on T cells of HIV-infected and noninfected individuals, we activated CFSE-labeled T cells using 1 μg/mL coated anti-CD3 mAb in the presence or absence of Fas cross-linking for 4 days. The percentage of proliferating T cells was calculated as shown by a typical sample, where the filled histogram represents T cells activated in the presence of anti-Fas, and open histogram represents T cells activated in the presence of control IgM antibodies. (B) T cells isolated from a cohort of HIV-infected (closed symbols) or noninfected (open symbols) individuals were activated using 1 μg/mL anti-CD3 mAb, and 1 μg/mL (C) or 0.25 μg/m (D) anti-Fas or isotype control mAbs. Proliferation was analyzed in CD4+ and CD8+ T cells by flow cytometry; percentages of proliferating cells are shown.
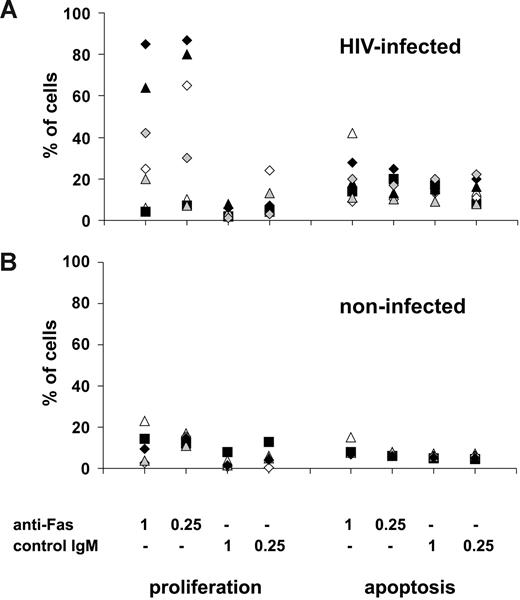
Although we observed a high rate of cell divisions when analyzing living T cells in the cultures, proliferation might still be accompanied by massive apoptosis, previously associated with HIV infection. To analyze whether the increased sensitivity of T cells to Fas-mediated apoptosis or the proliferative signals transmitted by Fas molecules would predominate in T-cell cultures, we compared Fas-induced apoptosis and proliferation of suboptimally activated T cells in a group of HIV-infected (n = 7) and noninfected (n = 5) individuals. Our results showed that in HIV-infected donors in whom Fas triggering resulted in enhanced proliferation (n = 5) of suboptimally activated T cells, the number of proliferating cells greatly exceeded the number of cells undergoing Fas-mediated apoptosis (Figure 2A). We observed in one HIV-infected patient only that the net effect of Fas triggering was cell loss due to Fas-induced apoptosis, and in another patient Fas-mediated triggering induced neither apoptosis nor proliferation (Figure 2A). As expected, Fas cross-linking on suboptimally activated T cells from noninfected donors resulted in low levels of proliferation and apoptosis, with a slightly higher ratio of proliferating compared with apoptotic cells in the presence of low anti-Fas antibody dose (Figure 2B). Overall, these results show that Fas acts as a potent costimulatory molecule in HIV-infected individuals, as the proliferative effects of Fas-mediated triggering of suboptimally activated T cells in most cases predominate over the induction of Fas-mediated apoptosis.
Fas-induced proliferation and apoptosis in HIV-infected patients. T cells isolated from a group of (A) HIV-infected patients (n = 7) and (B) noninfected controls (n = 5) were activated using 1 μg/mL anti-CD3 Abs combined with anti-Fas or control Abs used at 0.25- or 1-μg/mL concentrations. Apoptosis (measured by annexin-V staining after 24 hours of activation) and proliferation (measured after 4 days of activation using CFSE staining) were compared in the different cultures.
Fas-induced proliferation and apoptosis in HIV-infected patients. T cells isolated from a group of (A) HIV-infected patients (n = 7) and (B) noninfected controls (n = 5) were activated using 1 μg/mL anti-CD3 Abs combined with anti-Fas or control Abs used at 0.25- or 1-μg/mL concentrations. Apoptosis (measured by annexin-V staining after 24 hours of activation) and proliferation (measured after 4 days of activation using CFSE staining) were compared in the different cultures.
Interleukin-7 primes T cells for Fas-mediated costimulation
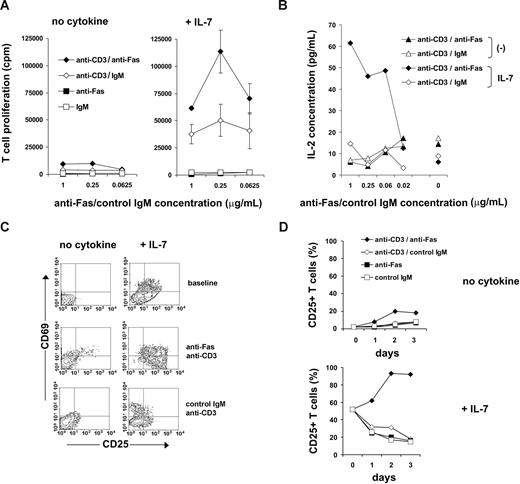
Our results demonstrate that, in addition to the well-characterized apoptosis-sensitive stage of peripheral T cells of HIV-infected individuals, these cells are also able to respond to proliferative Fas signals with high sensitivity. Several factors may contribute to increased T-cell apoptosis, including chronic activation, HIV-derived compounds triggering apoptotic pathways, as well as dysregulated cytokine levels. The concentration of IL-7 is often elevated in T cell–depleted individuals, and IL-7 has been shown to induce Fas up-regulation and cell surface reorganization on nonactivated T cells accompanied by increased sensitivity to Fas-mediated apoptosis.14 Accordingly, IL-7 may serve as a potential candidate for priming T cells to Fas-mediated proliferative signals in lymphopenic individuals. We analyzed whether IL-7 modulates the costimulatory action of Fas molecules on T-cell activation. Peripheral blood T cells cultured in the presence of 25 ng/mL IL-7 for 5 days showed higher levels of proliferation induced by suboptimal TCR signals compared with T cells freshly isolated or cultured for 5 days without IL-7 in line with the known T-cell costimulatory effects of IL-7 (Figure 3A and data not shown). Interestingly, triggering of surface Fas receptors dramatically enhanced the proliferation of IL-7–treated T cells activated by a suboptimal dose of anti-CD3 (Figure 3A), whereas only a minor effect was observed on Fas triggering of T cells cultured without IL-7 (Figure 3A), implicating Fas as a potent T-cell costimulatory molecule under conditions associated with increased IL-7 levels.
The effect of IL-7 on Fas-mediated T-cell costimulation. (A) T cells isolated from noninfected donors were cultured without any treatment (left panel) or in the presence of IL-7 (right panel) for 5 days. Thereafter, proliferation was triggered using 1 μg/mL anti-CD3 Abs combined with anti-Fas or control Abs. Thymidine incorporation was measured after 3 days of activation. Error bars represent standard deviation measured in triplicates; 1 representative experiment of 3 is shown. (B) T cells, freshly isolated or pretreated with 25 ng/mL IL-7 for 5 days, were activated with 1 μg/mL anti-CD3 Abs combined with anti-Fas or control Abs. IL-2 production was measured in culture supernatants using ELISA after 3 days of activation. One representative experiment of 3 is shown. (C) CD25 and CD69 expression was measured in freshly isolated or IL-7–pretreated T cells at baseline or after 3 days of activation using 1 μg/mL anti-CD3 in combination with anti-Fas or control mAbs. (D) Kinetics of CD25 expression is shown following activation of freshly isolated or IL-7–pretreated T cells using 1 μg/mL anti-CD3 combined with anti-Fas or control Abs. A representative result of 3 independent experiments is shown.
The effect of IL-7 on Fas-mediated T-cell costimulation. (A) T cells isolated from noninfected donors were cultured without any treatment (left panel) or in the presence of IL-7 (right panel) for 5 days. Thereafter, proliferation was triggered using 1 μg/mL anti-CD3 Abs combined with anti-Fas or control Abs. Thymidine incorporation was measured after 3 days of activation. Error bars represent standard deviation measured in triplicates; 1 representative experiment of 3 is shown. (B) T cells, freshly isolated or pretreated with 25 ng/mL IL-7 for 5 days, were activated with 1 μg/mL anti-CD3 Abs combined with anti-Fas or control Abs. IL-2 production was measured in culture supernatants using ELISA after 3 days of activation. One representative experiment of 3 is shown. (C) CD25 and CD69 expression was measured in freshly isolated or IL-7–pretreated T cells at baseline or after 3 days of activation using 1 μg/mL anti-CD3 in combination with anti-Fas or control mAbs. (D) Kinetics of CD25 expression is shown following activation of freshly isolated or IL-7–pretreated T cells using 1 μg/mL anti-CD3 combined with anti-Fas or control Abs. A representative result of 3 independent experiments is shown.
After T-cell activation, the secretion of IL-2 and the induction of CD25 (IL-2Rα chain) conferring T cells with high-affinity IL-2 binding are prerequisite for high rate of cell divisions, and both molecules are targeted by known costimulatory signals. On IL-7–treated T cells, Fas signals increased IL-2 production upon suboptimal activation (Figure 3B) without increasing the prevalence of IL-2–producing T cells as measured by intracellular IL-2 staining (data not shown). On the contrary, IL-2 production was not detected without IL-7 treatment. Signaling through Fas also induced minor up-regulation of membrane CD25 and that of the early T-cell activation marker CD69 on freshly isolated T cells triggered by suboptimal anti-CD3 Ab doses. IL-7 treatment alone resulted in partial CD25 up-regulation, in line with previous observations42 (Figure 3C), and the CD25 expression gradually decreased on these cells after suboptimal TCR stimulation in the absence of Fas signals. On the contrary, concomitant Fas and CD3 triggering resulted in the further increase of CD25+ T cells in culture, with stable CD25 expression on the majority (> 90%) of the cells detected 2 or 3 days after TCR triggering (Figure 3C,D). IL-7–induced Fas molecules may thus confer proliferative ability to suboptimally activated T cells due to increased IL-2 and IL-2Rα expression.
The effect of IL-7 on Fas costimulatory signals in T cells of HIV-infected individuals
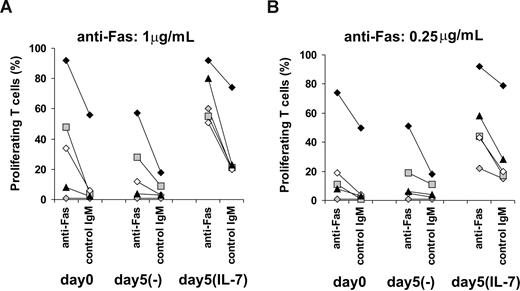
As we indicated, T cells of HIV-infected individuals are characterized by an increased sensitivity to costimulatory Fas signals compared with noninfected controls (Figure 1C,D). However, in vivo priming of T cells by IL-7 to receive proliferative Fas signals might be compromised due to the low levels of IL-7Rα available on several T cells in HIV-infected individuals.40,43-45 We analyzed whether T cells isolated from HIV-infected donors might be further sensitized to costimulatory Fas signals by IL-7. Peripheral T cells isolated from a group of HIV-infected individuals (n = 5) were activated using suboptimal TCR triggering combined with 1 μg/mL (Figure 4A) or 0.25 μg/mL (Figure 4B) anti-Fas antibodies at day 0 or, alternatively, after 5 days of culture in the presence or absence of IL-7. Importantly, Fas expression of T cells was increased 2- to 4-fold in the presence of IL-7 in the case of 3 HIV-infected individuals analyzed (data not shown), similarly to results obtained with noninfected individuals,14,34 indicating a preserved ability of IL-7 to modulate Fas expression of T cells in HIV-infected individuals. T cells of the different patients were characterized by different proliferative abilities and sensitivity to Fas signals at baseline (Figure 4A,B). Proliferative responses were slightly decreased after 5 days of culture without IL-7 and were maintained or elevated in cultures with IL-7. Priming to costimulatory Fas signals by IL-7 was apparent in the case of HIV-infected individuals characterized by low proliferative abilities at baseline or when Fas-induced signals were limited by lowering the dose of anti-Fas Abs (Figure 4A,B). These results showed that T cells of HIV-infected individuals, already primed to Fas costimulation in vivo, can be further sensitized by IL-7.
Sensitivity of T cells isolated from HIV-infected individuals to Fas-mediated costimulation in the presence or absence of IL-7. T cells isolated from a group of HIV-infected individuals (n = 5) were activated using 1 μg/mL anti-CD3 Abs combined with anti-Fas or control IgM used at (A) 1-μg/mL or (B) 0.25-μg/mL concentrations. Alternatively, T cells were cultured for 5 days in the presence or absence of 25 ng/mL IL-7 before activation. Proliferation was measured after 4 days of activation using CFSE staining. Different symbols represent individual donors.
Sensitivity of T cells isolated from HIV-infected individuals to Fas-mediated costimulation in the presence or absence of IL-7. T cells isolated from a group of HIV-infected individuals (n = 5) were activated using 1 μg/mL anti-CD3 Abs combined with anti-Fas or control IgM used at (A) 1-μg/mL or (B) 0.25-μg/mL concentrations. Alternatively, T cells were cultured for 5 days in the presence or absence of 25 ng/mL IL-7 before activation. Proliferation was measured after 4 days of activation using CFSE staining. Different symbols represent individual donors.
Fas triggering stimulates the proliferation of IL-7–treated T cells in the presence of autologous dendritic cells
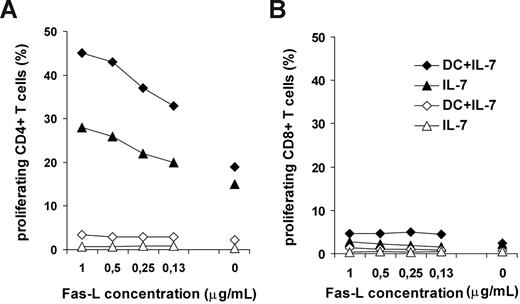
Recognition of self-peptide/MHC complexes by T cells contributes to homeostatic T-cell expansion in lymphopenic hosts.1,2 It is known that autologous DCs are able to trigger partial T-cell activation in vitro accompanied by proliferation.46,47 To address the question whether Fas receptors would play a stimulatory role in lymphopenia-induced T-cell expansion, we set up a model system for self-antigen–driven T-cell activation using autologous monocyte-derived DCs as antigen-presenting cells and high dose of IL-7. IL-7–pretreated or freshly isolated T cells were cultured together with autologous DCs for 4 days in the presence or absence of anti-Fas antibodies or recombinant FasL molecules, and thereafter T-cell proliferation was analyzed. Triggering through Fas receptors on IL-7–pretreated T cells using either antibodies or recombinant FasL resulted in a dose-dependent enhancement of CD4+ T-cell proliferation in the presence of autologous DCs, whereas CD8+ T cells did not proliferate under these conditions (Figure 5). Freshly isolated T cells did not proliferate in the presence of autologous DCs and IL-7 (Figure 5A). This experiment showed that Fas molecules expressed on IL-7–exposed T cells are able to transduce proliferative signals to T cells stimulated by low-affinity self-antigens. Furthermore, our results demonstrate the importance of chronic IL-7 stimulation in T-cell priming for costimulatory Fas signals, as IL-7 added to freshly isolated T cells was not able to sensitize T cells to Fas signals in the course of the experiment.
Effect of Fas triggering on the proliferative response of IL-7–treated T cells to self-antigens. IL-7–treated or freshly isolated T cells of noninfected donors were cultured in the presence of autologous dendritic cells. Fas receptors were triggered by recombinant FasL. Proliferation of freshly isolated (open symbols) or IL-7–pretreated (closed symbols) CD4+ (A) or CD8+ (B) T cells was analyzed in response to autologous DCs and IL-7 or to IL-7 only. Recombinant Fas-L was administrated at different concentrations to the cultures. Representative results of 3 independent experiments are shown.
Effect of Fas triggering on the proliferative response of IL-7–treated T cells to self-antigens. IL-7–treated or freshly isolated T cells of noninfected donors were cultured in the presence of autologous dendritic cells. Fas receptors were triggered by recombinant FasL. Proliferation of freshly isolated (open symbols) or IL-7–pretreated (closed symbols) CD4+ (A) or CD8+ (B) T cells was analyzed in response to autologous DCs and IL-7 or to IL-7 only. Recombinant Fas-L was administrated at different concentrations to the cultures. Representative results of 3 independent experiments are shown.
IL-7 induced sensitivity to Fas-mediated apoptosis or costimulation in different T-cell subsets
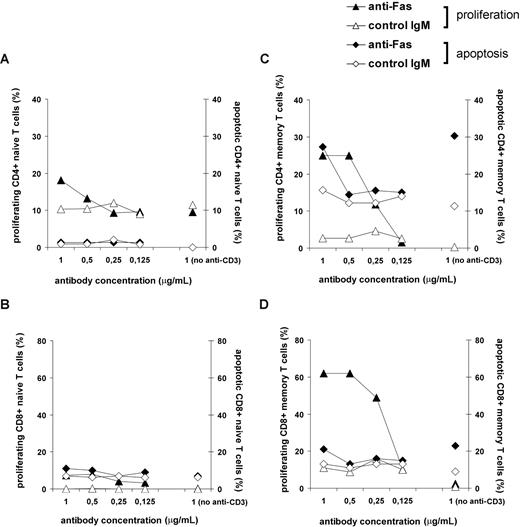
We and others have previously shown the priming of peripheral T cells to Fas-mediated apoptosis by high IL-7 doses.14,34 As we demonstrated in this study, IL-7–treated T cells are also characterized by an increased sensitivity to Fas-mediated costimulation (Figure 3), and it is also known that high IL-7 levels can lead to homeostatic T-cell proliferation under lymphopenic conditions. To determine whether Fas triggering on suboptimally activated T cells leads to apoptosis or proliferation in a T-cell subset–specific manner, we compared the sensitivity of different T-cell subpopulations to Fas-mediated apoptosis or costimulation after IL-7 treatment. Isolated naive and memory T cells, cultured in the presence of IL-7 for 5 days, were activated with a suboptimal dose of anti-CD3 mAb together with anti-Fas or control antibodies, and then the degree of apoptosis and proliferation of CD4+ and CD8+ T cells was monitored. We found that IL-7 treatment resulted in different sensitivity to apoptotic or proliferative Fas-mediated signals in the various T-cell subpopulations. Naive CD4+ (Figure 6A) and CD8+ (Figure 6B) exhibited minimal level of apoptosis or proliferation to suboptimal TCR triggering irrespective of the presence or absence of Fas signals. Within the CD4+ memory pool, comparable numbers of apoptotic and dividing cells were observed as a result of Fas signaling (Figure 6C). Remarkably, the proliferative effect of Fas cross-linking on suboptimally activated CD8+ memory T cells greatly exceeded the induction of apoptosis (Figure 6D).
Sensitivity of different T-cell subsets to Fas-mediated apoptosis or costimulation as a result of IL-7 treatment. IL-7–treated naive and memory T cells of noninfected subjects were activated in the presence of 1 μg/mL anti-CD3 mAb and with the indicated concentrations of anti-Fas or control IgM Abs. We followed T-cell apoptosis measured after 24 hours of activation and T-cell proliferation measured at day 4 with T cells isolated from the same donor and activated at the same conditions. Apoptosis was detected using annexin-V staining and proliferation was analyzed using CFSE staining on CD4+ (A) and CD8+ (B) naive subsets or CD4+ (C) and CD8+ (D) memory subsets. A representative experiment of 4 is shown.
Sensitivity of different T-cell subsets to Fas-mediated apoptosis or costimulation as a result of IL-7 treatment. IL-7–treated naive and memory T cells of noninfected subjects were activated in the presence of 1 μg/mL anti-CD3 mAb and with the indicated concentrations of anti-Fas or control IgM Abs. We followed T-cell apoptosis measured after 24 hours of activation and T-cell proliferation measured at day 4 with T cells isolated from the same donor and activated at the same conditions. Apoptosis was detected using annexin-V staining and proliferation was analyzed using CFSE staining on CD4+ (A) and CD8+ (B) naive subsets or CD4+ (C) and CD8+ (D) memory subsets. A representative experiment of 4 is shown.
Segregation of apoptotic and proliferative Fas signals at the level of caspase-8 and caspase-3 activation
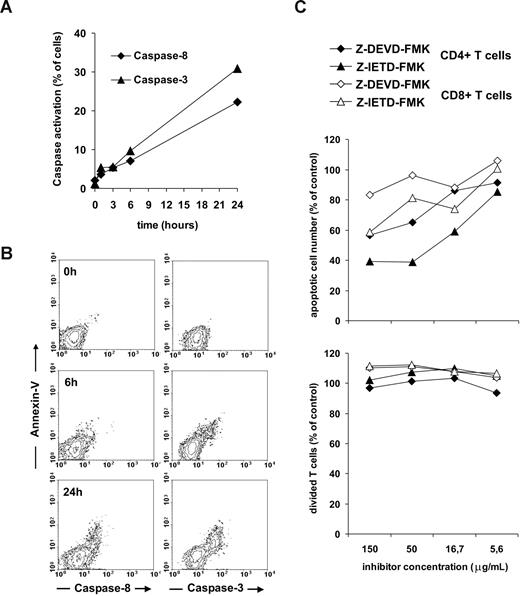
It is intriguing to speculate how Fas receptors are able to transduce either apoptosis-inducing or proliferative signals to IL-7–treated T cells. The role of caspases is well characterized in activation-induced T-cell apoptosis but controversial in proliferative Fas signals. Caspase-3 has been indicated as an important component of T-cell activation signaling,25 however in another study caspases were shown to play a minor role in Fas-mediated T-cell costimulation.27 We sought to determine possible differences in key signaling pathways originated from Fas molecules in T cells undergoing apoptosis or increased cell division upon concomitant TCR and Fas cross-linking. The detection of caspase activation using fluorescent substrate analogues enabled us to follow caspase-8 and caspase-3 activation and apoptosis in the same cultures. Using this approach, we detected a slow activation of both caspases following Fas triggering on IL-7–pretreated T cells (Figure 7A). Caspase-8 and caspase-3 activation was closely associated with the acquisition of annexin-V positivity, indicating a role of both caspases in T-cell apoptosis but not in proliferation or survival (Figure 7B). In line with these data, application of caspase-8 and caspase-3 inhibitors to IL-7–treated T cells exposed to suboptimal doses of anti-CD3 combined with anti-Fas antibodies resulted in reduced apoptosis but did not affect the proliferative response to Fas-mediated activation (Figure 7C).
Role of caspase-8 and caspase-3 in Fas-mediated apoptotic and proliferative signaling of IL-7–treated T cells. T cells of noninfected individuals, pretreated by 25 ng/mL IL-7, were activated using 1 μg/mL coated anti-Fas and anti-CD3 mAbs. Caspase-8 and caspase-3 activation was measured by flow cytometry in the course of the 24-hour activation period (A). The association of caspase-8 and caspase-3 activation with annexin-V binding was monitored (B). Inhibitors of caspase-3 (Z-DEVD-FMK) and caspase-8 (Z-IETD-FMK) were added to T cells at different doses, and 30 minutes later the cells were activated with 1 μg/mL coated anti-Fas and anti-CD3 mAbs. The ratio of apoptotic cells was determined in the cultures after 24 hours using annexin-V staining; proliferation was measured after 4 days of activation using CFSE staining (C). A representative experiment of 3 is shown.
Role of caspase-8 and caspase-3 in Fas-mediated apoptotic and proliferative signaling of IL-7–treated T cells. T cells of noninfected individuals, pretreated by 25 ng/mL IL-7, were activated using 1 μg/mL coated anti-Fas and anti-CD3 mAbs. Caspase-8 and caspase-3 activation was measured by flow cytometry in the course of the 24-hour activation period (A). The association of caspase-8 and caspase-3 activation with annexin-V binding was monitored (B). Inhibitors of caspase-3 (Z-DEVD-FMK) and caspase-8 (Z-IETD-FMK) were added to T cells at different doses, and 30 minutes later the cells were activated with 1 μg/mL coated anti-Fas and anti-CD3 mAbs. The ratio of apoptotic cells was determined in the cultures after 24 hours using annexin-V staining; proliferation was measured after 4 days of activation using CFSE staining (C). A representative experiment of 3 is shown.
Discussion
Several findings implicate the progressive immune activation and the subsequent increase of activation-induced T-cell apoptosis as major components in HIV-1 pathology.48-50 The apoptosis of noninfected bystander T cells is increased in patients, the increase of activated/memory T cells in periphery is associated with high sensitivity to AICD ex vivo, and the activation status of peripheral T cells has been identified as a good indicator of disease progression.12,51-53 Primate models also suggested that immune activation might contribute to HIV immunopathology. In contrast to rhesus macaques, where SIV infection leads to high viral loads associated with immune activation and progression to AIDS, in sooty mangabeys persistently high viral loads and massive initial loss of CD4 memory T cells is followed by limited immune activation and immunopathology.54,55
T cell–depleted conditions and the associated regenerative mechanisms, such as the increase of IL-7 concentration, might contribute to further immune activation due to the decreased activation threshold of peripheral T cells that leads to compensatory expansions to low-affinity antigens. Lymphopenia-induced expansion of mature T cells in the periphery contributes to T-cell regeneration in patients undergoing cancer chemotherapy or bone marrow transplantation, and compensatory mechanisms leading to CD8+ T-cell expansion in parallel with CD4+ T-cell depletion have been also shown in HIV-infected individuals.3,6,7,41 The immune reconstitution inflammatory syndrome (IRIS), observed in several HIV-infected patients after the initiation of antiretroviral therapy, demonstrates an increased responsiveness of T cells to peripheral antigens.6 T cells that spontaneously proliferate in a lymphopenic environment recognize low-affinity self- or foreign antigens and receive stimulatory signals from homeostatic cytokines, such as IL-7. Self-recognition may induce autoimmune T-cell responses, and, indeed, a link has been established between lymphopenia and autoimmunity or high IL-7 levels and autoimmunity.6,56,57 On the other hand, T-cell expansions in lymphopenic individuals are controlled by regulatory pathways inducing sensitivity to apoptosis through reduced Bcl-2 levels and elevated Fas ex-pression.8,12,49 The significance of these negative regulatory pathways in T-cell regeneration and the exact regulation of the concerted T-cell apoptotic and proliferative signals in lymphopenic conditions are yet to be clarified.
Fas is known as a dual function receptor able to transfer apoptosis-inducing signals or act as a costimulatory molecule for suboptimally activated T cells.24-28 So far, the increase of Fas expression has been exclusively interpreted as a mechanism that contributes to T-cell depletion in lymphopenic individuals. Nevertheless, in the present paper, we showed vigorous proliferation of T cells isolated from HIV-infected individuals in response to concomitant Fas and TCR triggering, significantly higher than the proliferation observed in the case of noninfected controls. Lymphopenia-induced Fas expression and sensitivity may thus lead to a stage characterized not only by increased apoptosis but also by sharpened sensitivity of T cells to proliferative signals.
Previously we have established a potential link between lymphopenia and elevated Fas expression in T cells showing that IL-7, a cytokine available at increased concentrations in T cell–depleted individuals, increases Fas expression on both naive and memory T cells.14 However, administration of high doses of IL-7 to lymphopenic hosts was shown to induce T-cell restoration rather than apoptosis,58-60 suggesting that the IL-7–induced sensitivity to Fas-mediated apoptosis may not ultimately lead to T-cell depletion.
In this study, we addressed the question of whether Fas molecules, expressed on resting T cells as a result of bystander activation by high IL-7 doses associated with HIV infection, can enhance T-cell proliferation acting as costimulatory receptors. Our results showed that IL-2 secretion, high-affinity IL-2 receptor expression, and proliferation of suboptimally activated T cells were significantly enhanced by Fas cross-linking if T-cell activation was preceded by IL-7 treatment. IL-7 primed T cells for stimulatory Fas signals when T-cell activation was achieved by self-antigen-presenting DCs or, alternatively, by suboptimal doses of anti-CD3 antibodies. The CD8+ memory pool showed the highest potential to multiply in response to costimulatory Fas signals reflecting the strongest regenerative ability of this subset observed in chemotherapy-treated patients61 or in chronic HIV infection.41 IL-7–induced Fas molecules may thus transmit proliferative signals to T cells receiving suboptimal TCR signals.
Dissection of Fas-mediated signals leading to apoptosis or to increased proliferation and decreased apoptosis was indicated by an early study using different Fas-targeting monoclonal antibodies,62 raising a potential therapeutic relevance of such agents in conditions characterized by increased apoptosis. Apoptotic pathways mediated by Fas receptors involve caspase-8 and caspase-3 activation. However, as we showed on T cells primed by IL-7 to proliferative Fas signals, these signaling components were not activated in T cells surviving initial Fas cross-linking, and caspase activation was dispensable for proliferation induced by Fas cross-linking on suboptimally activated T cells. These findings demonstrated major differences in Fas signal components activated in T cells undergoing apoptosis or increased proliferation under the same conditions.
If so, how are Fas molecules, induced under lymphopenic conditions, able to transduce distinct signals leading either to apoptosis or proliferation of T cells apparently sensitive for both? Based on our previous14 and present results, we hypothesize that IL-7, without playing an instructive role, primes T cells to both apoptotic and proliferative Fas signals. On the other hand, FasL expression and TCR stimulation might have a significant impact on whether IL-7–induced Fas molecules induce apoptosis or increase proliferation. High IL-7 concentration may potentiate Fas-mediated T-cell apoptosis in conditions characterized by elevated FasL expression and lack of TCR signals. In this respect, HIV infection may represent a potentially apoptosis-prone condition, characterized by increased and polarized Fas expression induced by IL-7 and other bystander stimuli and increased FasL levels. On the other hand, in the case of T cells receiving TCR signals through recognition of low-affinity antigens, IL-7–induced Fas molecules may transmit costimulatory signals leading to increased proliferation, compensatory for concurrent apoptosis. IL-7, by inducing sensitivity to Fas signals, may thus create a selective environment for T cells where the ones able to recognize low-affinity antigens will proliferate better when the Fas molecules are triggered, while at the same time Fas signals may increase apoptosis of nonactivated T cells.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swedish Medical Research Council, the Swedish International Development Cooperation Agency, Department of Research Cooperation, and the EU Fp6 Network of Excellence Europrise. B.R. received the Hungarian State Eotvos Fellowship and the Magyary Zoltan Postdoctoral Fellowship. S.S. is supported through a fellowship from the EU Marie Curie Programme (contract MEST-CT-2005-020872)
Authorship
Contribution: B.R. designed and performed research, and wrote the paper; N.V. designed and performed research; S.S., C.F., and N.R. performed research; A.A. provided patient samples; E.R. and F.C. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesca Chiodi, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Nobels väg 16, Stockholm 17177, Sweden; e-mail: francesca.chiodi@ki.se.
References
Author notes
*B.R. and N.V. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal