Abstract
Polycythemia vera (PV) is associated with high morbidity and mortality for thrombosis. We hypothesized that in PV altered sensitivity to aspirin might be related to dysfunction of the endothelial repair and/or of the nitric oxide (NO) system. Urinary thromboxane (TX) A2 metabolite (TXM), endothelial colony-forming cells (ECFCs), plasma asymmetric dimethylarginine (ADMA) and von Willebrand factor (VWF) were measured in 37 PV patients on low-dose aspirin and 12 healthy controls. Patients showed an approximately 2-fold increase in median TXM and plasma ADMA levels (P < .001), while ECFC numbers were reduced by approximately 7-fold (P < .001) as compared with nonaspirinated control. These differences were more pronounced in patients with previous thrombosis. An 8-week course of aspirin did not affect ECFCs in 6 controls. VWF and TXM correlated directly with ADMA, and inversely with ECFCs. By multiple regression analysis, lower ECFC quartiles (beta = −0.39; SE = 0.17; P = .028) and higher VWF levels (beta = 0.338, SE = 0.002, P = .034) were independent predictors of higher TXM quartiles (R2 = 0.39). Serum TXB2, measured in 22 patients, was approximat-ly 10-fold higher than aspirin-treated controls. PV patients appear to have an unbalanced ECFC/NO axis, and an apparent altered sensitivity of platelet TXA2 production, all potentially contributing to aspirin-insensitive TXM formation. Thus, additional antithrombotic strategies may be beneficial in PV.
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative disorder associated with thrombosis-related high morbidity and mortality.1 Clinical, biochemical, and pharmacologic evidence indicates that PV patients have enhanced in vivo platelet activation. Indeed, platelet-related microvascular disturbances, such as erythromelalgia and visual impairment, as well as enhanced urinary excretion of thromboxane (TX) A2 metabolites (TXM), an in vivo index of platelet activation2 have been observed in PV patients. Consistently, low-dose aspirin, which selectively inhibits cyclooxygenase (COX)–1–dependent TXA2 biosynthesis in platelets,3 improved microvascular symptoms,4 lowered TXM, and was able to prevent by approximately 60% a composite of venous and arterial thrombotic complications, but was unable to significantly reduce cardiovascular mortality, overall mortality, and nonfatal arterial complications.5 Whether persistent platelet activation in PV is primary or secondary to disease-dependent factors (ie, erythrocytosis or chronic endothelial damage) remains to be elucidated.
Endothelial dysfunction, assessed as impaired endothelium-dependent vasodilation, has been observed in PV, even in the absence of overt arterial disease.6 Endothelial progenitor cells (EPCs) derive from the hemangioblast,7 and physiologically contribute to endothelial repair.8 Impaired EPC mobilization or depletion may promote endothelial dysfunction and cardiovascular disease.9 Indeed, reduced circulating EPCs predict cardiovascular events in patients with coronary artery disease.10 Moreover, in these patients, the number of EPCs inversely correlates with circulating levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide (NO) synthase inhibitor,11 underlining the functional relationship between EPCs and the NO system. Notably, ADMA levels are increased in patients at high cardiovascular risk.12 Along these lines, circulating von Willebrand factor (VWF) levels have been often used as an index of endothelial damage in cardiovascular disorders.13
We investigated whether alterations of circulating EPCs, ADMA, or VWF levels might exist in PV. In parallel, we studied TXA2 biosynthesis in vivo by measuring TXM and examined whether EPCs or ADMA might be associated with a low-dose-aspirin-insensitive residual TXA2 production.
Methods
Study subjects
Thirty-seven patients [25 males, mean age 75.6 ± 11 years, range 41-82, mean disease duration 7 ± 6.8 years, range 1–28, median [intequartile range] hematocrit 49 [46.5-51.0]%, hemoglobin 15.8 [14.7-16.8] mg/dL, white-cell count 7400 (6200-11 500) × 106/L, platelet count 278 [195-395] × 109/L], with a diagnosis of PV according to the Polycythemia Vera Study Group,14,15 were recruited on an outpatient basis. All patients had been on low-dose (100 mg/od) aspirin for at least 1 year, 7 were being phlebotomized, and 22 were on hydroxyurea (HU) to keep their hematocrit under 48%.14 The remaining 8 patients had not undergone cytoreduction (HU or phlebotomy) in the previous 2 months, based on the individual clinical judgement of the referring physician. Twenty patients had arterial hypertension, 3 had type 2 diabetes mellitus, and 5 had hypercholesterolemia. Thirteen patients had previous thrombosis (6 months to 5 years before the study: 3 myocardial infarctions, 3 transient ischemic attacks, 5 deep vein thromboses, and 2 superficial thrombophlebites). Nine patients were being treated with cytoreduction (HU, phlebotomy) at the time of the cross-sectional evaluation: 8 with HU and 1 (previous superficial thrombophlebitis) with phlebotomy. The remaining 4 patients were receiving only aspirin, in addition to warfarin (3 subjects with previous deep vein thromboses) or ticlopidine (1 patient with previous transient ischemic attack). None of them had taken any antiinflammatory drug in the 10 days preceding the study. Twelve healthy volunteers (7 males, aged 65.8 ± 5.2 years, range 59-73) free of drugs known to affect platelet function were enrolled as controls. Six of them were evaluated for EPCs before and after 8-week low-dose aspirin (100 mg/od) treatment. The protocol was approved by the Pescara Hospital Ethics Committee, and the study was carried out and informed consent was obtained in accordance with the Declaration of Helsinki, as revised in 2004.
Isolation of endothelial progenitor cell and colony-forming assays
EPCs were studied as previously described,9,16 by counting colonies derived after 7 days of culturing from adherent mononuclear circulating cells, and termed endothelial colony-forming cells (ECFCs). Peripheral blood mononuclear cells (PBMCs) were separated from whole blood (50 mL, 0.38% sodium citrate) by Ficoll-Paque gradient (GE Healthcare, Milan, Italy) and 5 × 106 cells were seeded onto fibronectin (Fn)–coated (Sigma-Aldrich, Milan, Italy) 6-well plates in M199 medium (Mascia Brunelli, Milan, Italy) supplemented with autologous serum (2%) obtained by incubating platelet rich plasma with CaCl2 (23 mM, 1h, 37°). After 48 hours, the nonadherent cells were removed and fresh medium containing bovine brain extract endothelial cell growth factor (ECGF, 50 μg/mL) and heparin (10 μg/mL) was added. Endothelial cells forming colonies (ECFCs) were enumerated at day 7 in a minimum of 3 wells under a light microscope.
Plasma measurements of ADMA and VWF
Plasma ADMA levels were measured using a commercial kit (DLD Diagnostika, Hamburg, Germany), following manufacturer's instructions.17 VWF was measured in citrated plasma samples drawn from a subgroup of 29 patients by an immunoturbidometric assay (VWF antigen; Instrumentation Laboratory, Milan, Italy) and an automatic photometer (Top; Instrumentation Laboratory). Results were expressed in percent of normality. The calibration plasma for the reference curve was from Instrumentation Laboratory. In addition, Normal Control, Special Test Controls Level 1 (that contains a level of VWF:Ag within the normal range) and Special Test Controls Level 2 (that contains a level of VWF:Ag within the abnormal range), both from Instrumentation Laboratory, were also used as quality controls. The range of normality was assessed using 100 samples from healthy blood donors (50%-140%).
Thromboxane-related measurements
One-mL blood samples were transferred into glass tubes without anticoagulant, incubated for 1 hour at 37°C, and centrifuged at 1200g for 10 minutes at room temperature. The supernatant serum was stored at −20°C, until assayed for thromboxane (TX) B2.18 Urinary 11-dehydro-TXB2 was measured by a previously validated radioimmunoassay.19
Statistical analysis
Student t, Mann-Whitney U, or Kruskal-Wallis tests were performed to assess differences among the groups, as appropriate. The Spearman Rank correlation test was used for calculating relationships among variables. The differences between baseline and posttreatment values were analyzed with the Wilcoxon signed-rank test. Because the distribution of ECFCs and TXM was skewed (Shapiro-Wilk test W = 0.61, P < .001, and W = 0.938, P = .04), data were also analyzed by quartiles. A stepwise multiple linear regression analysis was performed with quartiles of TXM as the dependent variable. Only 2-tailed P values less than .05 were significant. Data are expressed as median and interquartile range (IQR; 25th to 75th percentile). Analyses were performed using SPSS (Chicago, IL) version 13.0.
Results
Despite ongoing aspirin treatment, PV patients showed a 2-fold increase in median TXM excretion as compared with nonaspirinated controls, with 18 (49%) patients exceeding the 95th percentile of control excretion. TXM excretion was independent of the type of treatment and unrelated to hematocrit, platelet or leukocyte counts, and disease duration (Figure 1A and data not shown).
Urinary 11-dehydro-TXB2 excretion and number of ECFCs in polycythemia vera patients. (A) Urinary 11-dehydro-TXB2 excretion in the entire polycythemia vera patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12). ● represents patients with a history of previous thrombotic events.  represents the 95th percentile of 11-dehydro-TXB2 values in the control group. (B) Number of ECFCs in the entire patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12) and in controls after a 8-week treatment with low-dose aspirin (100 mg/od) (n = 6). ● represents patients with previous thrombotic events.
represents the 95th percentile of 11-dehydro-TXB2 values in the control group. (B) Number of ECFCs in the entire patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12) and in controls after a 8-week treatment with low-dose aspirin (100 mg/od) (n = 6). ● represents patients with previous thrombotic events.
Urinary 11-dehydro-TXB2 excretion and number of ECFCs in polycythemia vera patients. (A) Urinary 11-dehydro-TXB2 excretion in the entire polycythemia vera patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12). ● represents patients with a history of previous thrombotic events.  represents the 95th percentile of 11-dehydro-TXB2 values in the control group. (B) Number of ECFCs in the entire patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12) and in controls after a 8-week treatment with low-dose aspirin (100 mg/od) (n = 6). ● represents patients with previous thrombotic events.
represents the 95th percentile of 11-dehydro-TXB2 values in the control group. (B) Number of ECFCs in the entire patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12) and in controls after a 8-week treatment with low-dose aspirin (100 mg/od) (n = 6). ● represents patients with previous thrombotic events.
PV patients displayed a reduced number of ECFCs (P < .001 vs controls), independently of the type of treatment (Figure 1B), hematocrit, platelet or leukocyte count, or disease duration. Low-dose aspirin treatment per se was unable to modify ECFCs in 6 healthy subjects treated for 8 weeks with 100 mg/od aspirin (from 7.5 [4-10.1] to 9.5 [7.7-10] ECFCs, median [IQR], P = .34; Figure 1B).
Patients also showed a significant, 2-fold increase in ADMA levels (P < .001 vs controls, Figure 2), which were inversely correlated with ECFCs (Rho = −0.67, P < .001), but did not correlate with platelet or leukocyte count, hematocrit, or disease duration. In addition, plasma VWF levels (74 ± 40%, mean ± SD, n = 29) were not significantly correlated with hematocrit, platelet, or leukocyte count.
Plasma ADMA levels in polycythemia vera patients. Plasma ADMA levels in the entire patient population (n = 37), divided according to the ongoing treat-ment (aspirin [ASA] alone, aspirin plus phlebothomy [Phl], or plus hydroxyurea [HU], as well as in healthy controls (n = 12). ● represents patients with previous thrombotic events.
Plasma ADMA levels in polycythemia vera patients. Plasma ADMA levels in the entire patient population (n = 37), divided according to the ongoing treat-ment (aspirin [ASA] alone, aspirin plus phlebothomy [Phl], or plus hydroxyurea [HU], as well as in healthy controls (n = 12). ● represents patients with previous thrombotic events.
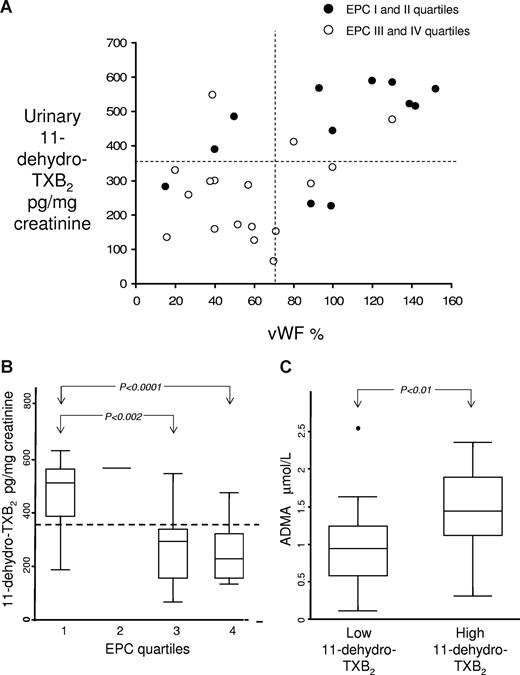
In the whole patient population, TXM and VWF levels were inversely correlated with ECFCs (Rho = −0.53, P = .001, Rho = −0.45, P = 0,014, for TXM and VWF, respectively) and directly with ADMA levels (Rho = 0.39, P = .01). In addition, VWF levels were significantly directly correlated with TXM (Rho = 0.49, P = .007, Figure 3A), while the correlation with ADMA was at the limit of significance (Rho = 0.36, P = .057). Multiple regression analysis indicated that lower ECFC quartiles (beta = −0.61; SE = 0.13; P = .001) were the only predictors of higher TXM quartiles, independently of age, hematocrit, platelet and white blood cell count, disease duration, cardiovascular risk factors, previous thrombotic events, or ADMA levels (Figure 3B). Likewise, based on the coefficient of determination (R2) of the multiple regression model, it appears that the number of ECFCs can account for more than one-third (∼37%) of the variability in TXM excretion (R2 = 0.37). Patients with the highest TXM showed also the highest ADMA levels and the lowest ECFCs (Figure 3B,C). Multiple regression analysis including plasma VWF as a continuous covariate, demonstrated that lower ECFC quartiles (beta = −0.39; SE = 0.17; P = .028) and higher VWF levels (beta = 0.338; SE = 0.002; P = .034) were the only predictors of higher TXM quartiles (R2 = 0.39). Among the 13 patients with VWF and11-dehydro-TXB2 levels in the first and second quartiles, 12 (92.3%) had ECFC number above the median. In contrast, only 2 (22.2%) of the 9 patients with VWF and 11-dehydro-TXB2 levels in the third and fourth quartiles exhibited ECFC numbers above the median (Figure 3A).
Relationship between urinary II-dehydro- TXB2 levels and their potential determinants in polycythemia vera patients. (A) Correlation between VWF levels and urinary 11-dehydro-TXB2 excretion in 29 polycythemia vera patients according to quartiles of ECFCs. ● represents subjects in the first and second quartiles for ECFC number. ○ represent subjects in the third and fourth quartiles for ECFC number. ¦ and  mark the boundaries of median values of both VWF and 11-dehydro-TXB2. (B) Urinary 11-dehydro-TXB2 levels according to quartiles of ECFC number in polycythemia vera patients. Box and whisker plots of urinary 11-dehydro-TXB2 levels divided according to quartiles of ECFC number.
mark the boundaries of median values of both VWF and 11-dehydro-TXB2. (B) Urinary 11-dehydro-TXB2 levels according to quartiles of ECFC number in polycythemia vera patients. Box and whisker plots of urinary 11-dehydro-TXB2 levels divided according to quartiles of ECFC number.  , representing the 95th percentile of 11-dehydro-TXB2 values in the control group, discriminates subjects with low versus high 11-dehydro-TXB2 values. Overall significance (by Kruskal-Wallis test): P = .043. (C) ADMA levels in polycythemia vera patients, according to 11-dehydro-TXB2 values below or above the 95th percentile of control values. Box and whisker plots of plasma ADMA levels in PV patients, stratified according to 11-dehydro-TXB2 values below or above the 95th percentile of 11-dehydro-TXB2 values in the control group.
, representing the 95th percentile of 11-dehydro-TXB2 values in the control group, discriminates subjects with low versus high 11-dehydro-TXB2 values. Overall significance (by Kruskal-Wallis test): P = .043. (C) ADMA levels in polycythemia vera patients, according to 11-dehydro-TXB2 values below or above the 95th percentile of control values. Box and whisker plots of plasma ADMA levels in PV patients, stratified according to 11-dehydro-TXB2 values below or above the 95th percentile of 11-dehydro-TXB2 values in the control group.
Relationship between urinary II-dehydro- TXB2 levels and their potential determinants in polycythemia vera patients. (A) Correlation between VWF levels and urinary 11-dehydro-TXB2 excretion in 29 polycythemia vera patients according to quartiles of ECFCs. ● represents subjects in the first and second quartiles for ECFC number. ○ represent subjects in the third and fourth quartiles for ECFC number. ¦ and  mark the boundaries of median values of both VWF and 11-dehydro-TXB2. (B) Urinary 11-dehydro-TXB2 levels according to quartiles of ECFC number in polycythemia vera patients. Box and whisker plots of urinary 11-dehydro-TXB2 levels divided according to quartiles of ECFC number.
mark the boundaries of median values of both VWF and 11-dehydro-TXB2. (B) Urinary 11-dehydro-TXB2 levels according to quartiles of ECFC number in polycythemia vera patients. Box and whisker plots of urinary 11-dehydro-TXB2 levels divided according to quartiles of ECFC number.  , representing the 95th percentile of 11-dehydro-TXB2 values in the control group, discriminates subjects with low versus high 11-dehydro-TXB2 values. Overall significance (by Kruskal-Wallis test): P = .043. (C) ADMA levels in polycythemia vera patients, according to 11-dehydro-TXB2 values below or above the 95th percentile of control values. Box and whisker plots of plasma ADMA levels in PV patients, stratified according to 11-dehydro-TXB2 values below or above the 95th percentile of 11-dehydro-TXB2 values in the control group.
, representing the 95th percentile of 11-dehydro-TXB2 values in the control group, discriminates subjects with low versus high 11-dehydro-TXB2 values. Overall significance (by Kruskal-Wallis test): P = .043. (C) ADMA levels in polycythemia vera patients, according to 11-dehydro-TXB2 values below or above the 95th percentile of control values. Box and whisker plots of plasma ADMA levels in PV patients, stratified according to 11-dehydro-TXB2 values below or above the 95th percentile of 11-dehydro-TXB2 values in the control group.
Taking into account only patients with a previous thrombotic event, the correlation between ECFCs and ADMA was even more pronounced (Rho = −0.81, P = .001), and the majority of these patients showed the lowest ECFCs and the highest ADMA and TXM levels (Figures 1,2). Indeed, 9 of 13 thrombotic patients showed levels of TXM exceeding the 95th percentile of control median (Figure 1A). Moreover, VWF levels were significantly higher in this subgroup of patients, as compared with subjects without any previous thrombotic event (100 [75.5-130] vs 52 [39-75]%, P = .012).
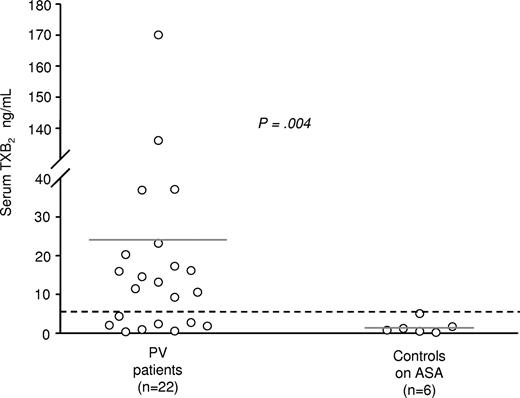
In a subgroup of 22 patients, 2 indexes of thromboxane biosynthesis were measured: serum TXB2 as an index of maximal biosynthetic capacity of platelet COX-1,18,20 vis-à-vis with TXM, which is an index of the actual production of TXA2 in the whole body. In these patients mean serum TXB2 values were 24.8 (± 43) ng/mL, whereas in 6 healthy volunteers mean serum TXB2 levels were 1.5 (± 1.7) ng/mL (Figure 4). Fourteen of 22 patients had values higher than the upper limit of healthy volunteers (4.9 ng/mL, mean + 2SD). However, TXM values were not significantly different among patients above or below this threshold of serum TXB2 (P = .19).
Serum TXB2 levels in polycythemia vera patients. Serum TXB2 levels were measured in polycythemia vera patients (n = 22) as well as in healthy controls (n = 6) after an 8-week treatment with low-dose aspirin (100 mg/od).  represents the upper limit of values in healthy volunteers (4.9 ng/mL, mean + 2SD).
represents the upper limit of values in healthy volunteers (4.9 ng/mL, mean + 2SD).
Serum TXB2 levels in polycythemia vera patients. Serum TXB2 levels were measured in polycythemia vera patients (n = 22) as well as in healthy controls (n = 6) after an 8-week treatment with low-dose aspirin (100 mg/od).  represents the upper limit of values in healthy volunteers (4.9 ng/mL, mean + 2SD).
represents the upper limit of values in healthy volunteers (4.9 ng/mL, mean + 2SD).
Discussion
The present study shows for the first time that PV is characterized by a reduced potential for endothelial regeneration and/or repair regardless of low-dose aspirin therapy. We have consistently observed in PV patients a reduced number of circulating ECFCs, which correlated with disturbances of NO metabolism and VWF release. We also denoted in aspirin-treated PV patients a substantial residual TXA2 formation in vivo, significantly higher than in either nonaspirinated or aspirinated healthy controls. These findings were more evident in patients with a previous thrombotic event.
Although we could not estimate the degree of inhibition exerted by low-dose aspirin on TXA2 biosynthesis in patients, because pre-aspirin TXM levels were not available, it is conceivable that diverse, disease-related mechanisms may overcome low-dose aspirin capability to fully inhibit TXA2 formation, at least in a fraction of patients. Given the selective suppression of platelet COX-1–dependent TXA2 exerted by low-dose aspirin,3 a residual TXA2 generation in vivo might derive either from extra-platelet, cellular COX-1 and/or COX-2, or from incomplete, disease-based platelet suppression.21,22 In fact, COX-2 is scarcely sensitive to low-dose aspirin23 and cells can newly synthesize COX-1 to replace the enzyme permanently inactivated by aspirin. To address this issue, we measured in a subgroup of patients serum TXB2, according to the whole blood assay described by Patrono et al18 This is an ex vivo biochemical index of maximal enzymatic activity of platelet COX-1 stimulated by thrombin generated during blood clotting. In absolute values, serum TXB2 in patients was higher than expected (24.8 ± 43 ng/mL), as compared with values determined in healthy controls in earlier studies,18 as well as in controls recruited for the present investigation (1.5 ± 1.7 ng/mL). This observation is in full agreement with a previous report by the GISP showing absolute TXB2 values of approximately 25 ng/mL in aspirin-treated PV patients,24 corresponding to approximately 95% inhibition exerted by low-dose aspirin. We know from previous studies that to achieve a complete inhibition of TXM in vivo, an almost-complete (≥ 99%) inhibition of serum TXB2 is a necessary condition, because the relationship between inhibition of TXA2 generation in vivo and serum TXB2 is rather nonlinear.25 Thus, at least a fraction of the higher-than-expected levels of TXM in vivo might derive from an incomplete suppression of platelet TXB2. This might originate either from enhanced availability of COX-1 or even COX-2 in a condition where platelet turnover might be also affected.21 In this regard, the observation that there was no significant correlation between absolute values of serum TXB2 and TXM may indicate that extra-platelet sources of TXA2 are likely to be involved.
PV patients displayed a dramatic reduction in ECFCs (Figure 1B). Because we evaluated colonies derived from adherent mononuclear cells, which do not have hematopoietic origin and are negative for the JAK2 V617F mutation,16,26 it is unlikely that the ECFC defect in PV arises from the clonal disorder. The observation that ECFC number in patients was independent of cytoreduction or indexes of disease activity (blood cell counts) is also consistent with this hypothesis. Based on the multivariate analysis, ECFCs and VWF were independent predictors of TXM. These results may indicate that in PV residual in vivo platelet activation may be in relation with perturbation of key endothelium repair mechanisms. In this respect, a direct correlation between VWF levels and TXM (Figure 3A) may arise from either unspecific release of VWF from Weibel-Palade bodies of senescent/apoptotic endothelial cells (in this case the increase in TXM may be a consequence of endothelial damage) or by the capability of TXA2 to directly stimulate VWF release from Weibel-Palade bodies.27
ADMA levels were increased in our PV patients (Figure 2). Circulating ADMA levels at least double in patients at high cardiovascular risk.11,12,28 ADMA is an endogenous inhibitor of endothelial NO synthase.12 Recent evidence suggests that even small modifications of ADMA levels affect vascular production of NO,28 which is known to regulate EPC mobilization.29 Thus, not only EPC production but also mobilization might be hampered in PV. Notably, ADMA can concentration-dependently inhibit EPC proliferation and differentiation in vitro.30 This is in agreement with our observation of a strong negative correlation between ECFCs and ADMA in PV patients. On the other hand, patients with the highest ADMA levels also displayed the highest TXM levels (Figure 3C). Thus, an impairment of the NO system may also contribute to residual aspirin-insensitive TXB2 generation in PV patients. Although the mechanisms involved in ADMA up-regulation in PV remain to be fully elucidated, oxidative stress may play a role within this context. It has been reported that oxidative stress may impair ADMA degradation.31 It is conceivable that an oxidative unbalance may occur in PV. Indeed, a spontaneous increase in oxygen consumption by leukocytes has been observed in PV.32 Moreover, pathologic erythropoiesis can be linked to an altered oxygen balance.33 On the other hand, enhanced oxidative status in vivo, through the nonenzymatic production of isoprostanes, might indeed activate platelets via an aspirin-insensitive pathway.34 We are currently evaluating whether there might be a link between platelet activation and in vivo oxidation in PV.
Thus, an emerging scenario is that impaired endothelial repair, endogenous NO suppression, higher-than-expected levels of serum TXB2 from platelets and residual, low-dose-aspirin–insensitive TXM excretion appear to be closely associated in PV, contributing to high cardiovascular risk in PV. Consistently, incomplete TXM suppression in aspirin-treated high-risk patients, has been shown to predict myocardial infarction or cardiovascular death.35
In conclusion, our study unravels a previously unappreciated relationship between impaired endothelial repair, endogenous NO suppression, and residual, low-dose-aspirin–insensitive TXM excretion in PV. This might affect the risk of cardiovascular events in this setting, suggesting that antithrombotic strategies in addition to or alternative to low-dose aspirin might be worth investigating in PV.36
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Carlo Patrono for his invaluable suggestions and for critical reading of the manuscript. We also thank Dr Natale Vazzana for expert editorial assistance.
This work was supported by European Commission Sixth Framework Programme funding (LSHM-CT-2004-0050333). This publication reflects only the authors' views. The Commission is not liable for any use that may be made of information herein.
Authorship
Contribution: F.S. performed research, analyzed data, and wrote the paper; M.R. designed research and wrote the paper; A.R. performed research in the laboratory; A.D. enrolled patients and controls; A.F. performed research; G.L. and F.F. enrolled patients and controls; S.L. and D.M. performed research in the laboratory; R.DeC. measured von Willebrand factor levels in plasmas; B.R. performed research and wrote the paper; and G.D. designed research, contributed reagents and analytical tools, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni Davì, MD, Center of Excellence on Aging, G. d'Annunzio University Foundation, Via Colle dell'Ara 66013 Chieti, Italy; e-mail: gdavi@unich.it.

![Figure 1. Urinary 11-dehydro-TXB2 excretion and number of ECFCs in polycythemia vera patients. (A) Urinary 11-dehydro-TXB2 excretion in the entire polycythemia vera patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12). ● represents patients with a history of previous thrombotic events. represents the 95th percentile of 11-dehydro-TXB2 values in the control group. (B) Number of ECFCs in the entire patient population (n = 37), and divided according to the ongoing treatment [aspirin (ASA) alone, aspirin plus phlebothomy (Phl), or plus hydroxyurea (HU)] as well as in healthy controls (n = 12) and in controls after a 8-week treatment with low-dose aspirin (100 mg/od) (n = 6). ● represents patients with previous thrombotic events.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-11-123091/6/m_zh80170822910001.jpeg?Expires=1767764628&Signature=JfgFfmfjm6Wd705R8eDRj4nME7bvt-SIKuBpAXAddWo11AG4Bz14q-r5ncPHHm61kB-1ONW1sJjgdrkhd-Yp8yKbIM6y0zg4karrXlO26y8BeZ8z-iXvHnOtTTd6F5zuKYoMekqvhtX~TvqLzy0xCwodqNm-mXM4v3sUkpZpreQg~FffWz1Bm1b9PRBYm9f3lnN6RjCfunDmimeFdFHTqIe2X37CPD7nbU1ZD4iQOxqJpNG~ohdojqqLUAYPbWb5t7up3iq5kIFds4VhPQUxRD9eyoMNq-snYIjFjRMBbQj2XSMBCqwGGak067ElpgKAQV7NtGq1heQEb2Kc2GJ3Mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Plasma ADMA levels in polycythemia vera patients. Plasma ADMA levels in the entire patient population (n = 37), divided according to the ongoing treat-ment (aspirin [ASA] alone, aspirin plus phlebothomy [Phl], or plus hydroxyurea [HU], as well as in healthy controls (n = 12). ● represents patients with previous thrombotic events.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-11-123091/6/m_zh80170822910002.jpeg?Expires=1767764628&Signature=RZ18RWP2levxPt24QgIcx4WKKoJCDbPDzyo6Vus1p3fSF4~i8A2q-lciKl6eQusYAlSBRQp~7pSZeCypcWqHxKB2WIvKaJ9qkF9qqbhXPbQAllNclB322~AyTjNeNxr5VoqlypkCn4Ub91oxiuFKA8KMTp-6QcSs94-E3WM03PD36h6HyOjqEY6jWQ8gxticLF-l4tmH-W3n4TsdqU0otHOm0YP50~zBTDhgzP6spYrW6aZFzHEmMpVimaIe-LwTsW9HxyT9nXz6NtoGu0Z7DayXk-Y3pgbqWoYk0yESemPdvYfaJpeo~arsPO4ERzc9yG7GH0MmTNKz1HveO6RUJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal