To the editor:
A 3-month-old girl, the first child of consanguineous parents with a family history of unexplained febrile episodes including a death from infection of the eldest brother of the father at 8 months of age, was admitted to our hospital for gastroenteritis. She had a mild leukocytosis (27.4 × 109/L) that was due mainly to an increased number of lymphocytes. After oral rehydration, the child recovered rapidly, and she was discharged after the fever had disappeared. We subsequently learned from the laboratory data that the clinical symptoms had been caused by cytomegalovirus (CMV). In addition, we found that the lymphocyte subsets causing the leukocytosis were extremely unbalanced; 90% were CD56dim natural killer (NK) cells, 10% were B cells, while T cells were virtually absent (0.07 × 109/L). Genetic analysis showed early stop codons in the patient's genes encoding the α-chain of the interleukin-7 (IL-7) receptor, a defect known to be associated with this particular T−B+NK+ SCID-phenotype.1,2
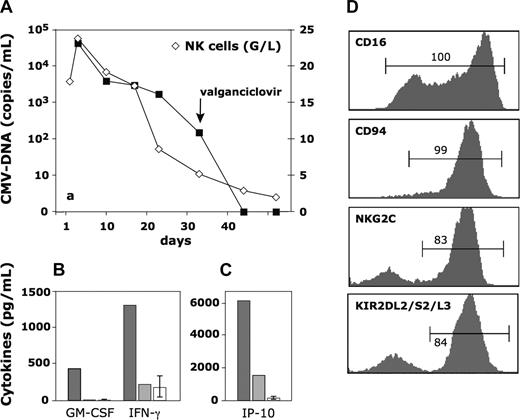
The correlation between viral load, number of NK cells, and cytokine serum-levels during the follow up was very suggestive of a causal relation (Figure 1A-C). The extremely high number of NK cells (23.8 × 109/L) present at the peak of viremia (4.1 × 104 copies CMV-DNA/mL of serum) remained elevated for approximately 2 weeks. At that moment, NK cell–derived3 cytokines granulocyte macrophage–colony stimulating factor (GM-CSF) and interferon-γ (IFN-γ) and the ensuing IFN-γ inducible protein-10 were at very high levels (Figure 1B,C). The viral load, already reduced by one log, decreased further to 150 copies/mL at 4 to 5 weeks and could not be detected anymore after the 6th week. By then, the number of NK cells had dropped to 2 × 109/L and the cytokines had returned to normal levels (Figure 1B,C, gray bars).
Kinetics of NK-cell expansion and cytokine serum-levels in relation to viral load, and phenotype of the expanded NK cells. (A) Number of CMV-DNA copies (■-■) and number of NK cells (◇-◇). The number of NK in the last sample (day 50) is at the upper limit of that in normal age-matched controls. → indicates the first day of oral valganciclovir given to maintain CMV suppression. (B,C) Serum cytokine-levels during viremia ( , day 9 in panel A) and in a late CMV-DNA free sample (
, day 9 in panel A) and in a late CMV-DNA free sample ( , day 43 in panel A). □ depicts the mean value plus or minus SD in 10 healthy controls. (D) FACS analysis of the respective marker with gates on CD3-CD56+ lymphocytes.
, day 43 in panel A). □ depicts the mean value plus or minus SD in 10 healthy controls. (D) FACS analysis of the respective marker with gates on CD3-CD56+ lymphocytes.
Kinetics of NK-cell expansion and cytokine serum-levels in relation to viral load, and phenotype of the expanded NK cells. (A) Number of CMV-DNA copies (■-■) and number of NK cells (◇-◇). The number of NK in the last sample (day 50) is at the upper limit of that in normal age-matched controls. → indicates the first day of oral valganciclovir given to maintain CMV suppression. (B,C) Serum cytokine-levels during viremia ( , day 9 in panel A) and in a late CMV-DNA free sample (
, day 9 in panel A) and in a late CMV-DNA free sample ( , day 43 in panel A). □ depicts the mean value plus or minus SD in 10 healthy controls. (D) FACS analysis of the respective marker with gates on CD3-CD56+ lymphocytes.
, day 43 in panel A). □ depicts the mean value plus or minus SD in 10 healthy controls. (D) FACS analysis of the respective marker with gates on CD3-CD56+ lymphocytes.
The phenotype of the NK cells expanded at the peak of infection had several interesting features (Figure 1D). Half the population expressed the low-affinity Fcγ-receptor type 3 (CD16) only at low density, a phenomenon characteristic of activated CD56dim NK cells.4 All cells expressed CD94 of which most was dimerized with NKG2C. NK cells expressing activating CD94-NKG2C receptors, which are rare (< 1%) in CMV− individuals,5 may be triggered directly by CMV-infected fibroblasts.6 Furthermore, the killer cell immunoglobulin-like receptors (KIR) repertoire appeared to be biased as more than 80% of NK cells were stained by a monoclonal antibody specific for KIR2DL2/2DS2/2DL3, while the other KIR, all present in the patient's genome, were expressed by less than 10% of NK cells (not shown).
To what extent NK cells contribute to human antiviral responses is unknown. Patients with diminished NK-cell functions may suffer from severe viral infections,7-10 but this may also be due to lack of guidance of the adaptive immune system by NK cell–derived cytokines. Here, we report a T−B+NK+ SCID patient who recovered from CMV disease without antiviral therapy after a significant expansion of IFN-γ–producing CD16+CD94-NKG2C+ NK cells had occurred. To our knowledge, this case provides the first direct evidence that human NK cells can effectively control CMV infection in the absence of T cells.
Authorship
We thank Dr J.-M. Tiercy for KIR genotyping and Mrs Solange Vischer for expert technical assistance.
E.R. is supported by a grant of the Swiss National Science Foundation (#3100-112612) and by the “Dr Henri Dubois-Ferrière-Dinu Lipatti” Foundation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taco W. Kuijpers, MD, PhD, Emma Children's Hospital, Academic Medical Center (Room G8-205), Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: t.w.kuijpers@amc.uva.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal