Abstract
Allogeneic hematopoietic cell transplantation (HCT) is the only known curative modality for patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL). Sixty-seven patients with HLA-matched sibling donors received fractionated total body irradiation (FTBI) and high-dose VP16, whereas 11 patients received FTBI/VP16/cyclophosphamide, and 1 patient received FTBI/VP16/busulfan. The median age was 36 years. At the time of HCT, 49 patients (62%) were in first complete remission (CR1) and 30 patients (38%) were beyond CR1 (> CR1). The median follow-up was 75 months (range, 14-245 months). The 10-year overall survival for the CR1 and beyond CR1 patients was 54% and 29% (P = .01), respectively, and event-free survival was 48% and 26% (P = .02), respectively. There was no significant difference in relapse incidence (28% vs 41%, P = .28), but nonrelapse mortality was significantly higher in the beyond CR1 patients, (31% vs 54%, P = .03, respectively). By univariate analysis, factors affecting event-free and overall survival were white blood cell count at diagnosis (< 30 × 109/L vs > 30 × 109/L) and disease status (CR1 vs > CR1). The median time to relapse for CR1 and for beyond CR1 patients was 12 months and 9 months, respectively. Our results indicate that FTBI/VP16 with or without cyclophosphamide confers long-term survival in Ph+ ALL patients and that disease status at the time of HCT is an important predictor of outcome.
Introduction
In patients with acute lymphoblastic leukemia (ALL), the frequency of the Philadelphia chromosome (Ph+) or translocation (9,22) increases with age and is detected in approximately 5% of children and in approximately 30% of adults.1 Despite high response rates to induction chemotherapy, relapse occurs frequently resulting in a 5-year overall survival (OS) of only 0% to 20%. The only known curative therapy for patients with Ph+ ALL is allogeneic hematopoietic cell transplantation (HCT), with this treatment conferring the most benefit when patients undergo transplantation early in the course of their disease.2-5
In 1987 we reported the first series of 10 patients with Ph+ ALL who were treated with high-dose conditioning regimens followed by hematopoietic cell transplantation (HCT) from histocompatible siblings.6 Six of the 10 patients became long-term disease-free survivors.7 That same year we described a new preparatory regimen consisting of fractionated total body irradiation (FTBI) and VP16.8 This regimen was found to be a potent combination for patients with acute leukemia and other hematologic malignancies.9-12 Later, the regimen was enhanced by adding cyclophosphamide to FTBI and VP16, especially for patients with advanced-stage hematologic malignancies.13 The current report details the experience in 79 patients with Ph+ ALL who were treated during the past 2 decades at the City of Hope National Medical Center and at Stanford University Medical Center.
Methods
Patient characteristics
The databases from the Stanford Blood and Marrow Transplantation Program and City of Hope National Medical Center were screened for patients with the diagnosis of Ph+ acute lymphoblastic leukemia. Pediatric (n = 10) and adult (n = 69) patients who underwent allogeneic HCT from 1985 to 2005 for the diagnosis of Ph+ ALL were identified for the purpose of this analysis. All patients had B-lineage ALL and HLA-matched siblings were the hematopoietic cell donors for all patients. The patient characteristics are listed in Table 1. Seventy-nine patients comprised this analysis and ranged in age from 2 to 57 years (median, 36 years). Thirty-two patients (40%) also had other chromosomal abnormalities in the addition to translocation (9,22). The median white blood cell count (WBC) at diagnosis was 26 × 109/L (range, 1.2-420 × 109/L). The majority of patients underwent induction chemotherapy with the DVAP (daunorubicin, vincristine, asparaginase, prednisone) regimen, whereas other patients received variations of this regimen that also included high-dose cytarabine, 6-mercaptopurine, idarubicin, and/or cyclophosphamide. All patients received intrathecal chemotherapy and the majority of patients (77%) received external beam radiotherapy before HCT to the cranial area as part of central nervous system disease prophylaxis. Seventeen patients received imatinib during induction chemotherapy before HCT, with one of these 17 patients receiving imatinib after HCT for maintenance therapy. Patients were either treated on research protocols or included in this analysis of the regimens as approved by the Institutional Review Boards of the Stanford University School of Medicine and the City of Hope National Medical Center and the Scientific Review Committees of the Cancer Centers of the 2 respective institutions. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patient characteristics
| Characteristic . | . |
|---|---|
| No. of patients | 79 |
| Median age at HCT, y (range) | 36 (2-57) |
| Patient sex, no. | |
| Female | 23 |
| Male | 56 |
| Donor sex, no. (%) | |
| Female | 28 (35) |
| Male | 51 (65) |
| Median WBC at diagnosis, × 109/L (range) | 26 (12-420) |
| History of CNS disease, no. (%) | |
| Yes | 13 (16) |
| No | 63 (80) |
| Unknown | 3 (4) |
| Radiation therapy before HCT, no. (%) | |
| Yes | 61 (77) |
| No | 13 (16) |
| Unknown | 5 (6) |
| Imatinib before HCT, no. (%) | |
| Yes | 17 (22) |
| No | 62 (78) |
| Disease status at HCT, no. (%) | |
| First complete remission | 49 (62) |
| Beyond CR1 | 30 (38) |
| Hematopoietic cell source, no. (%) | |
| Bone marrow | 43 (54) |
| Peripheral blood | 36 (46) |
| Conditioning regimen, no. (%) | |
| FTBI/VP16 | 67 (85) |
| FTBI/VP16/CY | 11 (14) |
| FTBI/VP16/BU | 1 (1) |
| GVHD prophylaxis, no. (%) | |
| CSA/MTX | 37 (47) |
| CSA/PSE | 23 (29) |
| CSA/PSE/MTX | 9 (11) |
| CSA/MMF | 4 (5) |
| TAC/SIROLIMUS | 3 (4) |
| CSA/MMF/MTX | 1 (1) |
| Unknown | 2 (3) |
| Characteristic . | . |
|---|---|
| No. of patients | 79 |
| Median age at HCT, y (range) | 36 (2-57) |
| Patient sex, no. | |
| Female | 23 |
| Male | 56 |
| Donor sex, no. (%) | |
| Female | 28 (35) |
| Male | 51 (65) |
| Median WBC at diagnosis, × 109/L (range) | 26 (12-420) |
| History of CNS disease, no. (%) | |
| Yes | 13 (16) |
| No | 63 (80) |
| Unknown | 3 (4) |
| Radiation therapy before HCT, no. (%) | |
| Yes | 61 (77) |
| No | 13 (16) |
| Unknown | 5 (6) |
| Imatinib before HCT, no. (%) | |
| Yes | 17 (22) |
| No | 62 (78) |
| Disease status at HCT, no. (%) | |
| First complete remission | 49 (62) |
| Beyond CR1 | 30 (38) |
| Hematopoietic cell source, no. (%) | |
| Bone marrow | 43 (54) |
| Peripheral blood | 36 (46) |
| Conditioning regimen, no. (%) | |
| FTBI/VP16 | 67 (85) |
| FTBI/VP16/CY | 11 (14) |
| FTBI/VP16/BU | 1 (1) |
| GVHD prophylaxis, no. (%) | |
| CSA/MTX | 37 (47) |
| CSA/PSE | 23 (29) |
| CSA/PSE/MTX | 9 (11) |
| CSA/MMF | 4 (5) |
| TAC/SIROLIMUS | 3 (4) |
| CSA/MMF/MTX | 1 (1) |
| Unknown | 2 (3) |
CNS indicates central nervous system; CY, cyclophosphamide; BU, busulfan; CSA, cyclosporin A; MTX, methotrexate; PSE, prednisone; MMF, mycophenolate mofetil; and TAC, tacrolimus.
Cytogenetic analysis and fluorescence in situ hybridization analyses
Karyotype analysis on pretreatment and follow-up bone marrow and peripheral blood samples was performed using standard methods. Dual-color fluorescence in situ hybridization (FISH) analyses were performed on bone marrow samples as per the manufacturer's instructions (Abbott/Vysis, Downers Grove, IL).
Polymerase chain reaction
The presence of bcr-abl mRNA by polymerase chain reaction (PCR) was measured before and after HCT in most patients, although the time points of study were not consistent among patients. PCR analyses were performed as previously described with some patients expressing the p190 variant and others expressing the p210 form. In addition, the presence of bcr-abl RNA copies was measured either qualitatively and/or quantitatively among some patients.
Conditioning regimen and graft-versus-host disease prophylaxis
All patients underwent high-dose conditioning that included fractionated total body irradiation before HCT.14,15 As listed in Table 1, the majority of patients (85%) received the preparatory regimen of fractionated total body irradiation (FTBI) 1320 cGy delivered in 11 fractions over 4 days followed by etoposide (VP16) at 60 mg/kg administered as a single infusion over 4 hours. Eleven patients (14%) received the combination of FTBI/VP16/cyclophosphamide with the cyclophosphamide (CY) dose of 60 mg/kg, and one patient received FTBI/VP16/busulfan for the purpose of omitting CY due to the patient's history of severe hemorrhagic cystitis from previous CY exposure. Forty-three patients (54%) received bone marrow as the hematopoietic cell source, with 36 patients (46%) receiving filgrastim-mobilized peripheral blood hematopoietic cells. The grafts were not T-cell depleted.
Various regimens were administered for graft-versus-host disease (GVHD) prophylaxis as per institutional trials conducted during the time period of analysis. The most commonly used regimens consisted of cyclosporine and methotrexate on days +1, +3, +6, and +11 (47%) followed by cyclosporine and prednisone (29%). Table 1 lists all regimens administered.
Statistical analysis
Survival estimates were calculated based on the product-limit method, and 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate.16 Differences between survival curves were assessed by the log rank test. The significance of demographic/treatment features collected from HCT recipients was assessed using survival analysis and univariate Cox regression analysis.17 The list of features included was determined from a literature review that identified factors found to be associated with survival and disease relapse in patients treated with allogeneic HCT. The values/levels of each variable, whether continuous or categoric, were based on findings published by large cooperative group and multicentered studies. Statistical significance was defined at the P less than or equal to .05 level.
Results
Overall survival and event-free survival
The median follow-up for all patients was 75 months (range, 14-245 months). When analyzed by disease status, the median follow ups for the first complete remission (CR1) patients and beyond CR1 patients were 75 months (range, 14-180 months) and 127 months (range 16-245 months), respectively. Disease status at the time of HCT had a significant impact on both OS and event-free survival (EFS). The estimated 10-year OSs for the CR1 patients and beyond CR1 patients were 54% and 29%, respectively (P = .01; Figure 1A) and for EFS were 48% and 26%, respectively (P = .02). As the time of accrual spanned more than 20 years, OS was examined by decade of HCT (1985-1995 [n = 35] vs 1996-2005 [n = 44]) with no significant difference seen among these 2 time periods (5-year OS: 43% vs 46%, P = .74, respectively; Figure 1B). In addition, the outcomes of the 10 pediatric/adolescent patients who were younger than18 years at the time of HCT were compared with the 69 adult patients. OS and relapse incidence were not found to be significantly different between these 2 groups (10-year OS: 44% for both groups, P = .91; and 10-year relapse incidence was 42% vs 34%, P = .61).
Overall survival. (A) Overall survival by remission status at transplantation. (B) Overall survival by decade of transplantation (1985-1995 [n = 35]; 1996-2005 [n = 44]).
Overall survival. (A) Overall survival by remission status at transplantation. (B) Overall survival by decade of transplantation (1985-1995 [n = 35]; 1996-2005 [n = 44]).
The impact of imatinib on transplantation outcomes was also assessed. Of the 17 patients who received imatinib before HCT, 15 patients entered HCT in CR1. It was not possible to accurately assess the impact of imatinib on transplantation outcomes compared with the rest of the cohort as the median follow-up of the 17 imatinib-treated patients was only 5 months (range, 1-53 months) and the comparative sample sizes are unbalanced. However, of these 17 patients, 6 patients are alive and in continuous CR. Of the 11 patients who have expired, 1 patient died from relapse and 10 patients succumbed from nonrelapse mortality (NRM)–related causes.
By univariate analysis, pre-HCT factors found to affect both OS and EFS were WBC at diagnosis (< 30 × 109/L vs > 30 × 109/L, P = .03 and P = .04, respectively), disease status at HCT (CR1 vs > CR1, P = .01 and P = .02, respectively) and development of grades 2 to 4 acute GVHD (P = .04 and P = .01, respectively) (Table 2). When tested by multivariate analysis, WBC at diagnosis and disease status remained significant for both OS and EFS, whereas grades 2 to 4 acute GVHD was significant for DFS with a trend seen with OS (Table 3). The presence of chromosomal abnormalities in addition to t(9;22) did not significantly impact survival outcomes.
Univariate analysis of risk factors
| Variable . | Overall survival . | Event-free survival . | Relapse rate . | Nonrelapse mortality . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| WBC at diagnosis, ×109/L (less than 30, n=42; more than 30, n=36; na=1) | 1.97 (1.08-3.58) | .03 | 1.83 (1.03-3.24) | .04 | 1.87 (0.76-4.63) | .17 | 1.87 (0.88-3.98) | .10 |
| Additional cytogenetic abnormalities (yes, n=32; no, n=33; na=14) | 0.70 (0.35-1.44) | .33 | 0.86 (0.44-1.68) | .66 | 1.33 (0.46-3.84) | .60 | 0.62 (0.26-1.49) | .28 |
| CNS disease, (yes, n= 3; no, n=63; na=13) | 1.27 (0.60-2.70) | .53 | 1.65 (0.80-3.37) | .17 | 2.59 (0.92-7.28) | .07 | 0.99 (0.36-2.68) | .98 |
| Prior radiation therapy (yes, n=13; no n=61; na=5) | 2.02 (0.99-4.15) | .05 | 1.81 (0.89-3.68) | .10 | 1.25 (0.36-4.32) | .72 | 2.25 (0.94-5.36) | .07 |
| Age at HCT (younger than 36 y, n=39; 36 y or older, n=40) | 1.19 (0.65-2.16) | .57 | 1.29 (0.72-2.29) | .39 | 0.97 (0.39-2.39) | .95 | 1.66 (0.78-3.51) | .19 |
| Disease status at HCT (CR1, n=49; beyond CR1, n=30) | 2.14 (1.17-3.91) | .01 | 1.98 (1.11-3.52) | .02 | 1.90 (0.76-4.77) | .17 | 2.12 (0.99-4.53) | .05 |
| Source of HCs (bone marrow, n=43; peripheral blood, n=36) | 1.04 (0.57-1.90) | .89 | .91 (.51-1.62) | .75 | 0.83 (0.33-2.06) | .69 | 0.93 (0.44-1.98) | .85 |
| Acute GVHD* (yes, n=26; no, n=51) | 1.88 (1.03-3.41) | .04 | 2.05 (1.16-3.64) | .01 | 1.32 (0.50-3.51) | .57 | 2.72 (1.29-5.77) | <.01 |
| Chronic GVHD† (yes, n=25; no, n=42) | 0.81 (0.39-1.67) | .57 | 0.69 (0.35-1.37) | .29 | 0.71 (0.28-1.79) | .46 | 0.83 (0.30-2.29) | .72 |
| Variable . | Overall survival . | Event-free survival . | Relapse rate . | Nonrelapse mortality . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| WBC at diagnosis, ×109/L (less than 30, n=42; more than 30, n=36; na=1) | 1.97 (1.08-3.58) | .03 | 1.83 (1.03-3.24) | .04 | 1.87 (0.76-4.63) | .17 | 1.87 (0.88-3.98) | .10 |
| Additional cytogenetic abnormalities (yes, n=32; no, n=33; na=14) | 0.70 (0.35-1.44) | .33 | 0.86 (0.44-1.68) | .66 | 1.33 (0.46-3.84) | .60 | 0.62 (0.26-1.49) | .28 |
| CNS disease, (yes, n= 3; no, n=63; na=13) | 1.27 (0.60-2.70) | .53 | 1.65 (0.80-3.37) | .17 | 2.59 (0.92-7.28) | .07 | 0.99 (0.36-2.68) | .98 |
| Prior radiation therapy (yes, n=13; no n=61; na=5) | 2.02 (0.99-4.15) | .05 | 1.81 (0.89-3.68) | .10 | 1.25 (0.36-4.32) | .72 | 2.25 (0.94-5.36) | .07 |
| Age at HCT (younger than 36 y, n=39; 36 y or older, n=40) | 1.19 (0.65-2.16) | .57 | 1.29 (0.72-2.29) | .39 | 0.97 (0.39-2.39) | .95 | 1.66 (0.78-3.51) | .19 |
| Disease status at HCT (CR1, n=49; beyond CR1, n=30) | 2.14 (1.17-3.91) | .01 | 1.98 (1.11-3.52) | .02 | 1.90 (0.76-4.77) | .17 | 2.12 (0.99-4.53) | .05 |
| Source of HCs (bone marrow, n=43; peripheral blood, n=36) | 1.04 (0.57-1.90) | .89 | .91 (.51-1.62) | .75 | 0.83 (0.33-2.06) | .69 | 0.93 (0.44-1.98) | .85 |
| Acute GVHD* (yes, n=26; no, n=51) | 1.88 (1.03-3.41) | .04 | 2.05 (1.16-3.64) | .01 | 1.32 (0.50-3.51) | .57 | 2.72 (1.29-5.77) | <.01 |
| Chronic GVHD† (yes, n=25; no, n=42) | 0.81 (0.39-1.67) | .57 | 0.69 (0.35-1.37) | .29 | 0.71 (0.28-1.79) | .46 | 0.83 (0.30-2.29) | .72 |
HR indicates hazard ratio; WBC, white blood cell count; na, data not available; CNS, central nervous system; HC, hematopoietic cell; HCT, hematopoietic cell transplantation; and GVHD, graft-versus-host disease.
Two patients died before engraftment.
Missing cGVHD data for 1 patient, and 11 patients died within 100 days.
Multivariate analysis of risk factors
| Variable . | Overall survival . | Event-free survival . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| WBC at diagnosis, ×109/L (less than 30, n=42; more than 30, n = 36; na = 1) | 1.92 (1.02-3.61 | .05 | 1.79 (0.98-3.29) | .06 |
| Prior radiation therapy (yes, n = 13; no, n = 61; na = 5) | 1.44 (0.67-3.12) | .35 | 1.35 (0.64-2.86) | .43 |
| Disease status at HCT (CR1, n = 49; beyond CR1, n = 30) | 2.14 (1.09-4.15) | .03 | 1.99 (1.06-3.74) | .03 |
| Acute GVHD (yes, n = 26; no, n = 51) | 1.77 (0.95-3.32) | .07 | (1.01-3.36) | .05 |
| Variable . | Overall survival . | Event-free survival . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| WBC at diagnosis, ×109/L (less than 30, n=42; more than 30, n = 36; na = 1) | 1.92 (1.02-3.61 | .05 | 1.79 (0.98-3.29) | .06 |
| Prior radiation therapy (yes, n = 13; no, n = 61; na = 5) | 1.44 (0.67-3.12) | .35 | 1.35 (0.64-2.86) | .43 |
| Disease status at HCT (CR1, n = 49; beyond CR1, n = 30) | 2.14 (1.09-4.15) | .03 | 1.99 (1.06-3.74) | .03 |
| Acute GVHD (yes, n = 26; no, n = 51) | 1.77 (0.95-3.32) | .07 | (1.01-3.36) | .05 |
HR indicates hazard ratio; WBC, white blood cell count; HCT, hematopoietic cell transplantation; and GVHD, graft-versus-host disease.
Graft-versus-host disease and hematopoietic cell source
Acute GVHD was observed in 39 (49%) patients with grades 2 to 4 acute GVHD occurring in 28 (35%) patients. Twenty-six of the 68 patients evaluable developed chronic GVHD with extensive manifestations seen in 9 of these patients. As mentioned previously, the presence of acute GVHD was an adverse factor for both OS and EFS. The development of chronic GVHD did not significantly affect survival or relapse.
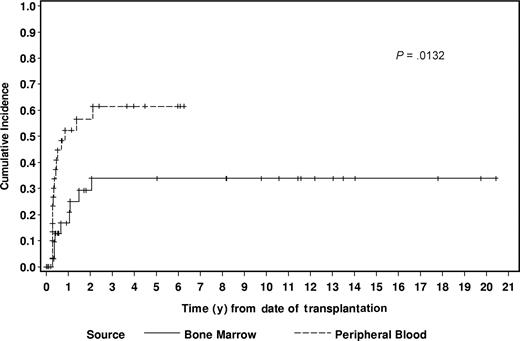
Bone marrow and peripheral blood were the sources of hematopoietic cells (HCs) in 54% (n = 43) and 46% (n = 36) of patients, respectively. The cumulative incidence of aGVHD was 27% and 50% among the BM and PB recipients (P = .07), respectively. The HC source significantly increased the risk of chronic GVHD as the 2-year cumulative incidences were 29% in the BM recipients and 57% in the PB recipients (P = .01; Figure 2). By univariate analysis, source of HCs did not significantly affect OS, EFS, relapse rate, or NRM (Table 2).
Relapse and causes of death
A total of 44 patients have died. The cumulative incidence of NRM for all patients was 35%, with infection (n = 10) and cardiopulmonary complications (n = 8) representing the most frequent causes of NRM. The other causes of nonrelapse death included acute GVHD (n = 5), chronic GVHD (n = 3), and diabetes mellitus (n = 1), and one patient died in remission from an unknown cause. All deaths due to NRM occurred within 3 years after HCT with the exception of one patient who died approximately18 years after HCT from diabetes-related complications. When analyzed by disease status at HCT, NRM was significantly higher among the beyond CR1 versus the CR1 patients, 54% versus 31% (P = .05), respectively (Figure 3A). The inferior OS and EFS for the beyond CR1 patients was attributed to the higher NRM. The development of grades 2 to 4 acute GVHD also significantly increased NRM (P = .01). NRM did not significantly differ when analyzed by decade of HCT. The 5-year NRM was 36% for the patients who underwent transplantation between 1985 and 1995 and also 36% between 1996 and 2005 (P = .93).
Outcomes by remission status. (A) Nonrelapse mortality by remission status at transplantation. (B) Relapse incidence by remission status at transplantation.
Outcomes by remission status. (A) Nonrelapse mortality by remission status at transplantation. (B) Relapse incidence by remission status at transplantation.
Sixteen patients have died from relapse, yielding a relapse mortality of 20%. The median time to relapse for the CR1 patients was 12 months (range, 1-27 months) and for the beyond CR1 patients was 9 months (range, 3-19 months). The latest observed relapse in our entire cohort was seen at 27 months after HCT. For patients in CR1 and beyond CR1, the relapse rates were 28% and 41% (P = .17), respectively (Figure 3B). There was no difference in 10-year relapse incidence between the pediatric and adult subgroups (42% vs 34%, P = .61, respectively). By univariate analysis, hematopoietic cell source, the presence of acute or chronic GVHD, WBC at diagnosis, or additional cytogenetic abnormalities did not affect relapse incidences (Table 2).
Cytogenetic and molecular analyses
By cytogenetic analyses, all patients were Ph+ at diagnosis. Additional clonal abnormalities were seen in 32 patients at diagnosis, but this finding did not significantly affect outcomes. All long-term survivors remain in a complete cytogenetic remission by sequential analyses.
Molecular diagnoses of either minor or major bcr-abl transcripts were performed in most but not all patients. Over the 20-year course of our study, various molecular techniques were introduced, ranging from qualitative PCR to highly specific quantitative PCR. PCR data were available in 57 patients. Thirty-one patients were PCR negative and 26 patients were PCR positive at the time of HCT. When analyzed by the presence or absence of PCR positivity for the bcr-abl fusion transcript, PCR status at the time of HCT was not predictive of outcome. Comparing the outcomes of the PCR-negative and PCR-positive patients, the 10-year OS, EFS, and relapse incidences were 43% versus 46% (P = .99), 40% versus 39% (P = .69), and 30% versus 43% (P = .37), respectively. All long-term survivors remain PCR negative except for one patient who experienced a molecular relapse. This particular patient has responded to imatinib and currently is in continued molecular remission.
Discussion
Although patients with Ph+ ALL typically respond to upfront combination chemotherapy, their remissions are rarely durable. The median EFS after combination chemotherapy is only approximately 8 months, with a 5-year OS of 0% to 20%.5,18 Due to these dismal outcomes, allogeneic HCT has been considered the optimal treatment after induction chemotherapy for patients with a suitable donor. Our report summarizes the outcomes of 79 patients with Ph+ ALL who underwent allogeneic HCT at Stanford University and the City of Hope National Medical Center. This retrospective review is the largest reported uniform series to date of Ph+ ALL patients who received an FTBI/VP16-containing regimen followed by allografting from matched sibling donors and also contains the longest median follow-up time compared with all other published series.
Despite the inherently poor prognosis of patients with Ph+ ALL, our results demonstrate that many patients can be cured if allogeneic HCT is performed early during the course of the disease. As reported by other groups, our results underscore the powerful prognostic impact of remission status, as patients who underwent transplantation in CR1 fared significantly better in terms of survival and NRM compared with patients who underwent transplantation beyond CR1. The 10-year OS of 54% and EFS of 48% for the CR1 patients after lengthy follow-up compares very favorably to other reports pertaining to Ph+ ALL patients who underwent HCT in CR1 (Table 4). The Center for International Blood and Marrow Transplant Research published a registry analysis of 67 Ph+ ALL patients who underwent allogeneic HCT with matched related donors.19 The 2-year leukemia-free survival and relapse incidences were not significantly different between patients who were in CR1 versus beyond CR1 at HCT (38% vs 41%, and 34% vs 32%, respectively). As seen in our study, most relapses occurred within 10 months. A retrospective series from Japan reported the outcomes of 93 Ph+ ALL patients in CR1 who received allogeneic HCT from either related or unrelated donors.3 With a median follow-up of 5 years, OS was 34% for CR1 patients compared with 21% for patients who underwent transplantation beyond CR1. Results were not separately analyzed by donor type—thus, it is not known whether the patients who had matched related donors fared as well as seen in our group.
Allogeneic HCT for Philadelphia chromosome–positive acute lymphoblastic leukemia
| Group (year) . | n . | Median age, y . | Remission status . | Donor . | Conditioning regimen . | OS . | DFS/EFS . | Relapse . | NRM . | Median follow up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| City of Hope6 (1987) | 10 | 28 (23-45) | CR1; >CR1 | MRD | TBI/VP, TBI/VP/Cy | 60% | 60% | 0% | 40% | 19 |
| CIBMTR19 (1992) | 67 | 28 (5-49) | CR1; >CR1 | MRD | TBI-based Bu/Cy | NR | CR1, 38%; >CR1, 41%; ref, 25% | CR1, 34%; >CR1, 32%; ref, 57% | CR1, 42%; >CR1, 40%; ref, 42% | 36 |
| FHCRC20 (1997) | 18 | 25 (17-51) | CR1; >CR1 | URD | TBI/Cy | NR | 49% | NR | 22% | 17 |
| City of Hope/Stanford7 (1999) | 23 | 30 (6-42) | CR1 | MRD | TBI/VP | NR | All, 65%; < 1992, 45%; > 1992, 81% | 12% | 30% | 40 |
| French LALA21 (2002) | 60 | 42 (17-56) | CR1 | MRD; URD | TBI/VP/CY | 37% | NR | 50% | NR | 54 |
| MRC/ECOG22 (2003) | 87 | < 50 | CR1; >CR1 | MRD; URD | TBI/VP | MRD 42%; URD 36% | MRD 41%; URD 32% | MRD 37%; URD 25% | MRD 35%; URD 57% | NR1 |
| French SGFMTC4 (2003) | 121 | 35 (1-53) | CR1; >CR1 | MRD; URD; UCB | Most TBI/Cy-based | All, 37%; CR1, 50%; >CR1, 17% | NR | All, 44%; CR1, 37%; >CR1, 62% | 36% | 29 |
| Japanese3 (2005) | 197 | 37 (16-59) | CR1; >CR1 | MRD; URD | Most TBI/Cy-based | CR1, 34%; >CR1, 21%; ref, 9% | NR | NR | NR | 58 |
| This study (2008) | 79 | 36 (2-57) | CR1; >CR1 | MRD | TBI/VP; TBI/VP/Cy; TB1/UP/Bu | CR1, 54%; >CR1, 29% | CR1, 48%; >CR1, 26% | CR1, 28%; >CR1, 41% | CR1, 31%; >CR1, 54% | CR1, 75; >CR1, 127 |
| Group (year) . | n . | Median age, y . | Remission status . | Donor . | Conditioning regimen . | OS . | DFS/EFS . | Relapse . | NRM . | Median follow up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| City of Hope6 (1987) | 10 | 28 (23-45) | CR1; >CR1 | MRD | TBI/VP, TBI/VP/Cy | 60% | 60% | 0% | 40% | 19 |
| CIBMTR19 (1992) | 67 | 28 (5-49) | CR1; >CR1 | MRD | TBI-based Bu/Cy | NR | CR1, 38%; >CR1, 41%; ref, 25% | CR1, 34%; >CR1, 32%; ref, 57% | CR1, 42%; >CR1, 40%; ref, 42% | 36 |
| FHCRC20 (1997) | 18 | 25 (17-51) | CR1; >CR1 | URD | TBI/Cy | NR | 49% | NR | 22% | 17 |
| City of Hope/Stanford7 (1999) | 23 | 30 (6-42) | CR1 | MRD | TBI/VP | NR | All, 65%; < 1992, 45%; > 1992, 81% | 12% | 30% | 40 |
| French LALA21 (2002) | 60 | 42 (17-56) | CR1 | MRD; URD | TBI/VP/CY | 37% | NR | 50% | NR | 54 |
| MRC/ECOG22 (2003) | 87 | < 50 | CR1; >CR1 | MRD; URD | TBI/VP | MRD 42%; URD 36% | MRD 41%; URD 32% | MRD 37%; URD 25% | MRD 35%; URD 57% | NR1 |
| French SGFMTC4 (2003) | 121 | 35 (1-53) | CR1; >CR1 | MRD; URD; UCB | Most TBI/Cy-based | All, 37%; CR1, 50%; >CR1, 17% | NR | All, 44%; CR1, 37%; >CR1, 62% | 36% | 29 |
| Japanese3 (2005) | 197 | 37 (16-59) | CR1; >CR1 | MRD; URD | Most TBI/Cy-based | CR1, 34%; >CR1, 21%; ref, 9% | NR | NR | NR | 58 |
| This study (2008) | 79 | 36 (2-57) | CR1; >CR1 | MRD | TBI/VP; TBI/VP/Cy; TB1/UP/Bu | CR1, 54%; >CR1, 29% | CR1, 48%; >CR1, 26% | CR1, 28%; >CR1, 41% | CR1, 31%; >CR1, 54% | CR1, 75; >CR1, 127 |
OS indicates overall survival; DFS/EFS, disease-free survival/event-free survival; NRM, nonrelapse mortality; CR1, first complete remission; MRD, matched related donor; TBI: total body irradiation; VP, VP16; Cy, cyclophosphamide; mo, months; NR, not reported; ref, refractory; FHCRC, Fred Hutchinson Cancer Research Center; URD, unrelated donor; LALA, Leucemie Aigue Lymphoblastique de l'Adulte; MRC/ECOG, Medical Research Council/Eastern Cooperative Oncology Group; NR1, median follow up not given; survival data reported at 5-year time point; SGFM-TC, Societe Francaise de Greffe de Moelle–Therapie Cellulaire; and UCB, umbilical cord blood.
The Medical Research Council (MRC) from the United Kingdom and the Eastern Cooperative Oncology Group (ECOG) conducted a joint prospective study for newly diagnosed ALL patients (n = 1389) in which patients with identified donors were assigned to undergo allogeneic HCT with either matched related or unrelated donors. Patients without available donors received either autologous HCT or further standard chemotherapy. An interim analysis of this large trial in 2003 reported the outcomes of 87 patients with Ph+ ALL who underwent myeloablative allogeneic HCT.22 Fifty-seven patients received allografts from matched related donors, and 30 patients received allografts from matched unrelated donors. The 5-year OSs were 42% and 36% (P = .6), respectively, whereas NRMs were 35% and 57% (P = not significant), respectively. In an unbiased analysis, the Ph+ ALL patients who received a matched related donor graft had a 10% improvement in 5-year OS compared with the patients who underwent autologous HCT or standard chemotherapy. A recent update of the MRC/ECOG trial reported the impact of karyotype on 1373 ALL patients with adequate cytogenetic or molecular data.23 Nineteen percent of patients (n = 267) had the Ph+ chromosome at diagnosis. The OS of 22% and EFS of 16% for the Ph+ group was significantly inferior compared with the rest of the cohort who did not have the Philadelphia chromosome (OS and EFS of 41% and 36%, respectively, P < .001 for both). These outcomes included patients treated with either transplantation or conventional chemotherapy.
Similar to our study, registry data from the French Société Française de Greffe de Moelle–Thérapie Cellulaire (SFGM-TC) of 121 Ph+ ALL patients who underwent allogeneic HCT with either matched related or unrelated donors also confirmed that remission status was a highly significant favorable prognostic factor for survival.4 For patients in CR1 and beyond CR1, the 2-year OS was 50% and 17% (P < .001), respectively. The CR1 patients also experienced a significantly lower relapse rate (P = .02) that was not observed in our study. But similar to our results, the SFGM-TC study revealed a trend for improved OS for patients with a lower WBC (< 25 × 109/L vs > 25 × 109/L) at the time of diagnosis (P = .07). We also found that a high WBC (> 30 × 109/L [> 30 000/μL]) at diagnosis was an adverse factor for both OS (P = .03) and EFS (P = .04) in univariate analysis, with results remaining significant in a multivariate test. Also observed in our series, the development of grades 2 to 4 acute GVHD was associated with a significantly higher NRM that negatively impacted OS and DFS. However, in contrast to our study, the SGFM-TC report found that acute GVHD was a favorable factor in terms of decreasing relapse risk (P = .02). When separated by disease status in our study, NRM was notably higher in the beyond CR1 compared with CR1 patients (54% vs 31%, respectively, P = .03). As the relapse rate did not significantly differ by remission status in our series, the higher NRM in the beyond CR1 group accounted for the inferior OS and EFS.
Fifty-four percent of patients in the present study received BM as the source of HCs, which primarily reflects the change in practice within the field of HCT as virtually all grafts from 1999 consisted of peripheral blood as the HC source. The incidence of chronic GVHD was nearly twice as high in the PB than in the BM recipients (57% vs 29%, respectively, P = .01), although acute GVHD was not different, confirming the findings seen in randomized studies comparing recipient outcomes after PB and BM HCT.24,25 Interestingly, the higher incidence of chronic GVHD among the PB recipients did not decrease relapse risk as reported in other studies.
Relapse incidence did not vary by remission status, which may speak for the potent antileukemic effect of high-dose VP16 combined with FTBI. Our relapse incidence of 20% among 79 patients compares favorably or is superior compared with similar published studies, especially in light of our long follow-up time. The median time to relapse was less than 12 months, with the latest relapse observed at 27 months, indicating that most patients who remain in remission for approximately 1 to 2 years after HCT are most likely cured.
Approximately 85% of our patients received the conditioning regimen of FTBI with high-dose VP16, which we first introduced as a new preparative regimen in 1987.6 This regimen and the FTBI/high-dose cyclophosphamide regimen are currently the 2 most commonly offered regimens in the high-dose allogeneic HCT setting for acute leukemia patients. Interestingly, a recently published registry report compared the efficacy between the conditioning regimens of FTBI/Cy with FTBI/VP in 298 patients with ALL including patients with Ph+ ALL receiving allogeneic hematopoietic stem cells from matched related donors.11 No differences in survival or relapse were observed in the CR1 patients, but a significant advantage was seen in substituting etoposide for cyclophosphamide in the CR2 patients in terms of survival, relapse, and mortality, which again emphasizes the antileukemic efficacy of high-dose VP16.
Recent reports have detailed the feasibility and efficacy of adding imatinib to conventional chemotherapy to increase the rate of CR for newly diagnosed Ph+ ALL patients.26-29 Imatinib, which inhibits the breakpoint cluster region-Abelson (bcr-abl) tyrosine kinase, has been shown to induce responses in Ph+ ALL patients including patients who have previously failed allogeneic HCT.30-32 Achievement of CR after upfront combination chemotherapy is of tantamount importance because outcomes after allogeneic HCT are strongly affected by disease status at the time of HCT. When added to combination chemotherapy, imatinib has been shown to increase and prolong CR rates and to confer a survival advantage whether or not patients proceeded to allogeneic HCT compared with historical controls.26-29 Increasing the rate and duration of CR also improves the patient's candidacy for HCT. Our analysis commenced over a 20-year time period beginning in 1985, and thus only 17 patients (22%) in our series received imatinib with combination chemotherapy before HCT. Fifteen of these 17 patients obtained a complete remission before HCT. However, due to this small sample size and short median follow-up of this subset of patients, it is not possible to accurately discern the true impact of pre-HCT imatinib in our study. The use of imatinib after HCT as maintenance therapy is also under investigation and perhaps will also increase the efficacy of HCT.33
In summary, our results show that for Ph+ ALL patients with a matched sibling donor, remission status at the time of HCT is one of the most important prognostic factors for outcome, with approximately half of patients cured if they undergo transplantation in CR1. This cure rate falls nearly in half for patients who proceed to allogeneic HCT with advanced disease. Published results show no difference in outcome by donor type and, thus, a search for an unrelated donor should commence immediately if a matched related donor is not available. Fortunately, with the increasing availability of allogeneic HCT due to the rising number of alternative donors and with the increasing use of tyrosine kinase inhibitors before and after HCT, the cure rate for patients with Ph+ ALL will hopefully improve.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants PO1-CA 30206 and PO1-CA 49605 from the National Institutes of Health, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: G.G.L. performed the research, analyzed the data, and wrote the paper; J.M.P. analyzed the data; J.C.A., D.S.S., R.M.W., and R.S.N. critically reviewed the paper; and M.L.S. and A.M.C. contributed data and critically reviewed the paper; and K.G.B. and S.J.F. designed the original study and wrote sections of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ginna G. Laport, Division of Blood and Marrow Transplantation, Stanford University School of Medicine, 300 Pasteur Dr, Rm H3249, Stanford, CA 94305-5623; e-mail: glaport@stanford.edu.

![Figure 1. Overall survival. (A) Overall survival by remission status at transplantation. (B) Overall survival by decade of transplantation (1985-1995 [n = 35]; 1996-2005 [n = 44]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/3/10.1182_blood-2008-03-143115/4/m_zh80160822390001.jpeg?Expires=1769150254&Signature=JcaYsyrjGqVG826t-l0JGK0YsbuAC-LNB~ZPIVS0K084YjEzBFPQ-FBOREKX7dR6xfinJ9R~E8vJqxAbEJ8yqQPlU1GzRXSzsnCxwdId0nGb~a0U~8rdEA6FKL3YXFbjRKe6fGLZTc7fxTpmHXMg-yQ4SOZ3hR6ta-UvV0D5bTlVEwhAhaGmzhFVCjZsQH3nRHoTh67GKSbqB4Ow1MwihRhoRzHNTyQdPRvKjkZkvEZrpRvT0FoTf4bWD1RO0LUtiZDKldsO~yei16aNNyu0d2Oi~JfSlQe5cGWxsQ24yPeqjfn1TJ2Ob-cK6d47Mu47jj4i9okiK8g6TAxZkuHmlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal