Abstract
Increasing studies suggest that SALL4 may play vital roles in leukemogenesis and stem cell phenotypes. We have mapped the global gene targets of SALL4 using chromatin immunoprecipitation followed by microarray hybridization and identified more than 2000 high-confidence, SALL4-binding genes in the human acute promyelocytic leukemic cell line, NB4. Analysis of SALL4-binding sites reveals that genes involved in cell death, cancer, DNA replication/repair, and cell cycle were highly enriched (P < .05). These genes include 38 important apoptosis-inducing genes (TNF, TP53, PTEN, CARD9, CARD11, CYCS, LTA) and apoptosis-inhibiting genes (Bmi-1, BCL2, XIAP, DAD1, TEGT). Real-time polymerase chain reaction has shown that expression levels of these genes changed significantly after SALL4 knockdown, which ubiquitously led to cell apoptosis. Flow cytometry revealed that reduction of SALL4 expression in NB4 and other leukemia cell lines dramatically increased caspase-3, annexin V, and DNA fragmentation activity. Bromodeoxyuridine-incorporation assays showed decreased numbers of S-phase cells and increased numbers of G1- and G2-phase cells indicating reduced DNA synthesis, consistent with results from cell proliferation assays. In addition, NB4 cells that express low levels of SALL4 have significantly decreased tumorigenecity in immunodeficient mice. Our studies provide a foundation in the development of leukemia stem cell–specific therapy by targeting SALL4.
Introduction
SALL4 is a zinc-finger transcription factor and a member of the SALL gene family, which was originally cloned based on sequence homology to Drosophila spalt (sal).1-3 In Drosophila, sal is a homeotic gene and essential in the development of posterior head and anterior tail segments.4
Human SALL4 mutations are associated with the Duane-radial ray syndrome (DRRS; also called Okihiro syndrome), a human autosomal-dominant syndrome involving multiple organ defects.3,5,6 Our group and others have shown that murine SALL4 plays an essential role in maintaining the properties of embryonic stem (ES) cells and governing the fate of the primitive inner cell mass by interacting with Oct4 and Nanog.7-11 Interestingly, SALL4 protein expression is correlated with stem and progenitor cell populations in various organ systems including bone marrow.8,12,13 We have demonstrated previously that SALL4 is expressed constitutively in human leukemia cell lines and primary acute myeloid leukemia (AML) cells derived from patient samples.12 Transgenic mice that overexpress SALL4B, one of the SALL4 isoforms, exhibit myelodysplastic syndrome (MDS)–like symptoms and subsequently develop AML that is transplantable.12
The polycomb gene Bmi-1 has been identified recently as a major downstream target of SALL4.14 Bmi-1 is an apoptosis regulator that has been well studied in many models including leukemia, the epidermis, neural lineages, and recently prostate cancer.15-21 Bmi-1 plays an essential role in regulating adult, self-renewing, hematopoietic stem cells (HSCs) and leukemic stem cells (LSCs).18,22-27 Bmi-1 is highly expressed in purified HSCs, and this expression declines with differentiation.23 Knockout of Bmi-1 in mice results in a progressive loss of all hematopoietic lineages. This loss results from the inability of the Bmi-1−/− stem cells to self-renew.18 The presence of Bmi-1 appears to be important in the maintenance of LSC identity. Recently, Bmi-1 expression has been used as an important marker for predicting progression of MDS to AML.28
Despite the prominent roles of SALL4 in ES cells and leukemic cells, the mechanisms controlling cell growth, proliferation, and apoptosis through the downstream targets of SALL4 remain largely unexplored. We undertook a genome-wide analysis of gene promoters directly or indirectly bound by SALL4 using chromatin immunoprecipitation followed by microarray hybridization (ChIP-chip) to determine potential mechanism whereby SALL4 regulates proliferation and apoptosis. Our results indicate that SALL4 acts as a key regulator of cell growth and apoptosis in leukemic cells. Massive apoptosis and significant growth arrest occurs when SALL4 expression is down-regulated in various leukemic cells. In addition, reduction of SALL4 markedly diminishes tumorigenicity of leukemic cells in immunodeficient mice.
Methods
Mice
Nonobese diabetic–severe combined immunodeficient (NOD.SCID)/NCr mice (6-8 weeks old) were maintained under specific pathogen-free conditions. All animal experiments were preapproved by the Office of Laboratory Animal Welfare, Institutional Animal Care and Use Committee.
Cell culture and ChIP
Human acute promyelocytic leukemia NB4 cells were maintained in RPMI-1640 supplemented with 10% (vol/vol) fetal bovine serum (FBS), and ChIP assays were performed as previously described.14
ChIP-chip
A complete protocol was provided by NimbleGen Systems (Madison, WI). In brief, NB4 cells were grown, cross-linked with formaldehyde, and sheared by sonication. An anti-SALL4 antibody and nonimmune rabbit serum were used for immunoprecipitation.12,29 ChIP-purified DNA was blunt-ended using T4 polymerase, ligated to linkers, and subjected to low-cycle polymerase chain reaction (PCR) amplification. Promoter tiling arrays were produced by NimbleGen Systems. The RefSeq human promoter array is a single array containing 2.7 kb sequence with 2.2 kb representative of the proximal promoter region and 500 bp of the 5′ terminal coding sequence (from National Center for Biotechnology Information [NCBI, Bethesda, MD] Build 36; HG18). The promoter region is covered by 50- to 75-mer probes at roughly 100-bp spacing depending on the sequence composition of the region. The arrays were hybridized, and the data were extracted according to standard procedures by NimbleGen Systems.
Data extraction was done using NimbleScan by NimbleGen Systems. NimbleScan searches for 4 or more probes above a cutoff value ranging from 90% to 15% using a 500-bp sliding window. The cutoff value is a percentage of the hypothetical maximum determined using the mean plus 6 standard deviations, and under default scanning conditions decreases in 1% increments from 90% to 15%. The data are then randomized 20 times to evaluate the possibility of false positives, and each peak is assigned a false discovery rate (FDR) based on this randomization. Predicted binding sites were confirmed using quantitative real-time (Q-RT)–PCR. Negative control primers were designed flanking predicted SALL4-binding sites.
Retrovirus production and SALL4 knockdown
Three short-hairpin RNA-expressing plasmids, 1 control (pRS) and 2 SALL4 specific (no. 7410, no. 7412; all 3 from Origen, Rockville, MD), were transfected into Phoenix packaging cells (Orbigen, San Diego, CA) using Lipofectamine 2000 (Invitrogen, Frederick, MD). Shed virus was harvested 48 hours after transfection, and control or stable SALL4 knockdown NB4 clones were obtained under puromycin (1.2 μg/mL) selection after 7 days.
Quantitative real-time PCR
Total RNA extraction and Q-RT-PCR assays were performed as previously described.12
Microarray-based mRNA profiling
A catalog human microarray (NCBI Build 36; HG18) was manufactured by NimbleGen Systems. The human genes in this design (n = 47 633) were selected from the Homo sapiens entries in the RefSeq collection, full-length human mRNAs, and experimental gene models from NCBI. Nine 60-mer probes were positioned 1.5-kb upstream from the 3′ end of each target. Probes were spaced evenly over the length of the target region (≤ 1.5 kb) where possible so that the exact spacing is dependent on the length of the target sequence. Redundant genes were removed by identifying identical target probe sets and placing only one exemplar sequence on the array for each probe set. Signal intensities were calculated as the mean 3 × 3-grid pixel comprising each feature. Gene expression analysis was done using robust multichip average.
Expression microarrays (NimbleGen Systems) were used to examine gene expression patterns before and after RNA interference in NB4 cells. After virus infection, up to 75% of endogenous SALL4 expression was reduced as observed using Q-RT-PCR analysis. The experiment was repeated 3 times, and the total RNA isolated from each experiment was pooled (both control and knockdown groups) for expression assays. Ingenuity Systems (version 2.01; Redwood City, CA) software was used to analyze the hybridization results, and only genes whose expression levels were altered more than 2-fold were considered differentially expressed and singled out for further analysis. All microarray data for this paper have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE10734.30
Apoptosis, cell proliferation, and cell-cycle arrest assays
Cultured cells (approximately 106 cells) were treated with control or SALL4 shRNA retroviruses. Cell viability was evaluated and quantified by cell counting following trypan blue exclusion. To quantify apoptosis, cells were harvested for flow cytometry-based caspase-3, annexin V/propidium iodide (Pharmingen, BD Biosciences, San Diego, CA), and DNA fragmentation assays (ApoAlert DNA Fragmentation Assay Kit; Clontech, Palo Alto, CA) through flow cytometry. For DNA synthesis and cell-cycle arrest assays, a portion of the harvested cells were used to incorporate BrdU (Pharmingen, BD Biosciences) following the manufacturer's instructions.
Statistics
Ingenuity Pathway Analysis (Ingenuity Systems, http://www.ingenuity.com) tools were used for ChIP-chip data. Preprocessed data files from NimbleGen Systems were filtered to remove duplicate genes according to the NCBI Entrez Gene ID setting.31 The list of genes was then inputted into Ingenuity Pathway Analysis with the NCBI Entrez Gene ID as the observed gene identifier. A more stringent FDR less than 0.10 was used for pathway analysis. Genes were analyzed according to relevant biologic functions and pathways.
Preprocessed gene expression data from NimbleGen Systems was used to convert to the log2 ratio. Two controls (C) and 2 treated (T) data sets then were used to generate 4 ratios according to the following expression: log2 (T/C) = log2 (T) − log2 (C). It is necessary to do this to obtain more values for this sample t test. A one-sample t test was performed using Tigr MultiExperiment Viewer (TIGR) with P less than .05.32 The significant gene list was inputted into the Ingenuity Pathway Analysis tool.
Results
SALL4 regulatory networks in leukemic cells
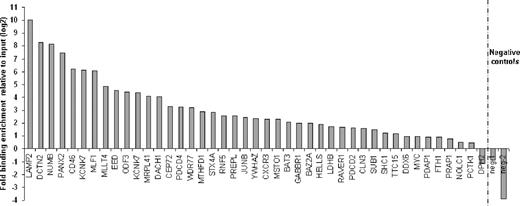
Previously we have demonstrated that SALL4 plays an important role in leukemogenesis. To identify the molecular pathway(s) that regulate SALL4-mediated leukemogenesis, we used ChIP-chip as a screening tool in the AML-derived cell line NB4, which expresses relatively high levels of SALL4. Using highly specific anti-SALL4 antibodies, we identified more than 2500 target genes bound by SALL4 within promoter regions spanning 2.2-kb upstream and 500-bp downstream of the transcription start site (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We chose a promoter array for the ChIP-chip because it has been shown to contain approximately 90% of transcription factor–binding sites.33 To verify that the SALL4-binding sites identified with the RefSeq array were authentic, we performed independent ChIP experiments coupled with a quantitative real-time PCR (ChIP-Q-RT-PCR) in NB4 cells. Through this strategy, 81 promoter regions were subjected to ChIP-enrichment validation. We successfully validated the binding of SALL4 to 39 randomly chosen promoter regions (39/43) in addition to 35 apoptosis genes (35/38, 91% of the selected targets total; Figure 1; Figure S2). Thus, using ChIP-chip assays, roughly 91% of identified promoters were considered genuine SALL4-binding loci in NB4 cells.
Validation of ChIP-chip–identified SALL4-binding sites by Q-RT-PCR. Chromatin immunoprecipitation experiments were performed in NB4 cells with antibodies raised against SALL4. Forty-three randomly selected promoters identified as SALL4 targets with ChIP-chip arrays were analyzed by Q-RT-PCR. ChIP-Q-RT-PCR identified 39 targets having more than 2-fold enrichment versus the input control. The fold change of SALL4 immunoprecipitated DNA over the input is presented on a log2 scale. Negative control primers were designed based on the genomic regions surrounding the peak as shown in Figure S2.
Validation of ChIP-chip–identified SALL4-binding sites by Q-RT-PCR. Chromatin immunoprecipitation experiments were performed in NB4 cells with antibodies raised against SALL4. Forty-three randomly selected promoters identified as SALL4 targets with ChIP-chip arrays were analyzed by Q-RT-PCR. ChIP-Q-RT-PCR identified 39 targets having more than 2-fold enrichment versus the input control. The fold change of SALL4 immunoprecipitated DNA over the input is presented on a log2 scale. Negative control primers were designed based on the genomic regions surrounding the peak as shown in Figure S2.
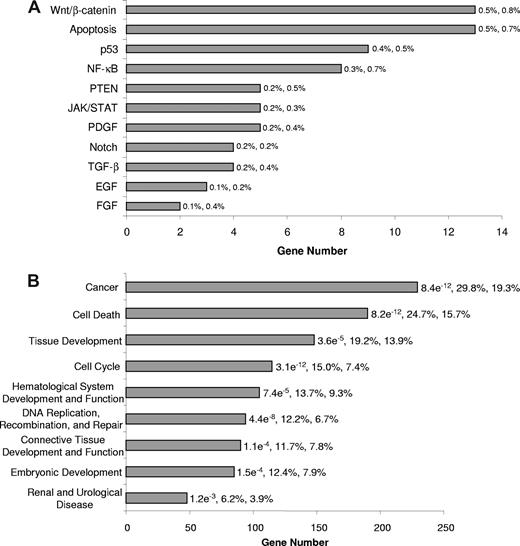
We next categorized the signaling pathways associated with the SALL4-binding sites identified through ChIP-chip assay. Using Ingenuity Pathway Analysis, we determined that SALL4-binding genes are involved in more than 30 different signaling pathways. The most prominent of these pathways was the Wnt, apoptosis, PTEN, NF-κB, and p53 signaling pathways, each of which play important roles in apoptosis (Figure 2A). We also assessed pathway distributions of ChIP-chip data for SALL4-binding genes in primary AML (French-American-British classification: M0) and chronic acute leukemic transformation leukemic cells (data not shown). These results demonstrated similar pathway distributions as the NB4 cells. These observations suggest that targets of SALL4 identified in NB4 cells will likely regulate a broader spectrum of AML cell types and is consistent with our previous suggestion that SALL4 plays a role in the transformation of different types of AML.12
Classification of SALL4-bound genes in NB4 leukemia cells. SALL4 target genes were classified based on annotations important to cell growth and cell death extracted from Ingenuity Pathway Analysis. It should be noted that specific genes may be represented in more than one classification group. (A) Classification of SALL4 target genes is based on signaling pathways associated apoptosis and cell growth properties according to Ingenuity Pathway Analysis knowledge base. P values for this analysis are not presented due to the low number of genes within each pathway. The first and second percentages represent the number of genes bound within each pathway relevant to the total SALL4 binding and the total number of genes within each pathway relevant to the genes on the array, respectively. (B) Classification of molecular functions associated with SALL4 target genes. The P values presented for each category are calculated using Fisher exact test against the genes represented on the promoter tiling array. The first and second percentages are the total number of genes bound by SALL4 in each function relevant to the number of genes bound by SALL4 and the number of genes on the array that are classified in each function compared with the total number of genes represented on the array, respectively. Unannotated genes were removed from this analysis, and the analysis was done using the top tier of SALL4-bound genes. The genes bound by SALL4 and the genes within each classification are presented in Table S2.
Classification of SALL4-bound genes in NB4 leukemia cells. SALL4 target genes were classified based on annotations important to cell growth and cell death extracted from Ingenuity Pathway Analysis. It should be noted that specific genes may be represented in more than one classification group. (A) Classification of SALL4 target genes is based on signaling pathways associated apoptosis and cell growth properties according to Ingenuity Pathway Analysis knowledge base. P values for this analysis are not presented due to the low number of genes within each pathway. The first and second percentages represent the number of genes bound within each pathway relevant to the total SALL4 binding and the total number of genes within each pathway relevant to the genes on the array, respectively. (B) Classification of molecular functions associated with SALL4 target genes. The P values presented for each category are calculated using Fisher exact test against the genes represented on the promoter tiling array. The first and second percentages are the total number of genes bound by SALL4 in each function relevant to the number of genes bound by SALL4 and the number of genes on the array that are classified in each function compared with the total number of genes represented on the array, respectively. Unannotated genes were removed from this analysis, and the analysis was done using the top tier of SALL4-bound genes. The genes bound by SALL4 and the genes within each classification are presented in Table S2.
We further classified the SALL4-binding genes based on their molecular and cellular functions. Interestingly, cell death and cancer genes were among the highly enriched groups with P values less than 10−11. Other classifications that were enriched included cell cycle, and hematologic-related genes (Figure 2B; Table S2). These functional distributions suggest that SALL4 is tightly associated with leukemic cell growth.
SALL4 directly regulates expression of apoptosis genes
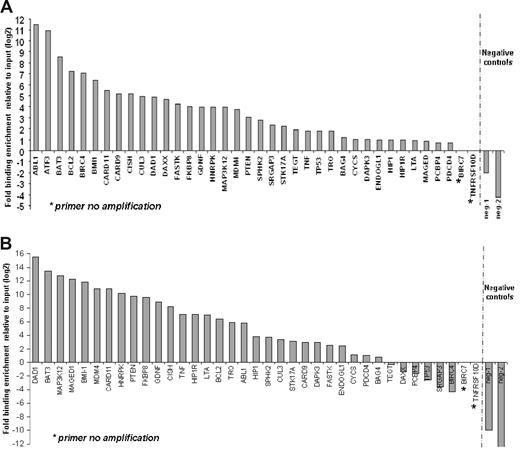
Substantial numbers of genes that are regulators of apoptosis were found in the top tier of genes bound by SALL4 in the ChIP-chip assays. These genes include apoptosis-inducing genes, such as TNF, LTA, TNFRSF10D, TP53, PTEN, CARD9, CARD11, CASP6, and ATF3; apoptosis-inhibiting genes, such as BCL2, TEGT, Bmi-1, BIRC4, and BIRC7; and apoptotic process related genes such as CYCS and BAT3 (Figure 3). A detailed list of these genes is provided in Table 1. These genes were of primary importance in regulating cell survival and may be responsible for the role that SALL4 plays in leukemogenesis. For this reason, we sought to confirm SALL4 binding to promoter regions of these genes using a replicate preparation of ChIP DNA. Primers were designed based on locations of peaks from the ChIP-chip data and were used for Q-RT-PCR verification of immunoprecipitation. The resulting data established that 35 of 38 apoptosis genes were indeed targets of SALL4 in NB4 cells (Figure 3A). We also sought to determine whether these apoptosis genes were targets of SALL4 in another cell line. The CD34+ cell population derived from human primary bone marrow cells expresses high levels of SALL4. Using this cell population, we demonstrate that 28 of 37 apoptosis genes are indeed targets of SALL4 in the CD34+ fraction (Figure 3B). These data strongly suggest that SALL4 binding to the promoters of a subset of apoptosis genes may account for the malignant phenotype in NB4 cells (as well as other leukemic cell types) and that aberrant expression of these apoptosis genes may lead to this phenotype.
ChIP-Q-RT-PCR of apoptosis genes asso-ciated with SALL4 binding. Thirty-eight apoptosis genes identified by ChIP-chip were subjected to ChIP-Q-RT-PCR to confirm SALL4 binding by an alternative method. These genes are candidates for SALL4 involvement in leukemogenesis. (A) In NB4 cells, 35 of the 38 genes identified by ChIP-chip were confirmed as bound by SALL4 using ChIP-PCR. (B) Identification of SALL4 binding to apoptosis genes in CD34+ cells sorted from human bone marrow samples. CD34+ cells are likely progenitor cells to the promyelocytic cell line NB4 within these lineages. Identified SALL4 target genes had more than 2-fold enrichment compared with the input control. The position of negative control primers is presented in Figure S1. In Figure 2B, primer neg-2 failed to amplify the experimental DNA but did amplify the input DNA, indicating the absence of DNA template in the experimental group. This is represented by a fold change of less than 0.001.
ChIP-Q-RT-PCR of apoptosis genes asso-ciated with SALL4 binding. Thirty-eight apoptosis genes identified by ChIP-chip were subjected to ChIP-Q-RT-PCR to confirm SALL4 binding by an alternative method. These genes are candidates for SALL4 involvement in leukemogenesis. (A) In NB4 cells, 35 of the 38 genes identified by ChIP-chip were confirmed as bound by SALL4 using ChIP-PCR. (B) Identification of SALL4 binding to apoptosis genes in CD34+ cells sorted from human bone marrow samples. CD34+ cells are likely progenitor cells to the promyelocytic cell line NB4 within these lineages. Identified SALL4 target genes had more than 2-fold enrichment compared with the input control. The position of negative control primers is presented in Figure S1. In Figure 2B, primer neg-2 failed to amplify the experimental DNA but did amplify the input DNA, indicating the absence of DNA template in the experimental group. This is represented by a fold change of less than 0.001.
SALL4-bound genes related to apoptosis
| Name . | Description . |
|---|---|
| ABL1 | v-abl Abelson murine leukemia viral oncogene homolog 1 |
| ATF3 | Activating transcription factor 3 |
| BAG4 | BCL2-associated athanogene 4 |
| BAT3 | HLA-B associated transcript 3 |
| BCL2 | B-cell CLL/lymphoma 2 |
| BIRC4 | Baculoviral IAP repeat-containing 4 |
| BIRC7 | Baculoviral IAP repeat-containing 7 (livin) |
| BMI1 | B lymphoma Mo-MLV insertion region (mouse) |
| CARD11 | Caspase recruitment domain family, member 11 |
| CARD9 | Caspase recruitment domain family, member 9 |
| CISH | Cytokine inducible SH2-containing protein |
| CUL3 | Cullin 3 |
| CYCS | Cytochrome c, somatic |
| DAD1 | Defender against cell death 1 |
| DAPK3 | Death-associated protein kinase 3 |
| DAXX | Death-associated protein 6 |
| ENDOGL1 | Endonuclease G-like 1 |
| FASTK | Fas-activated serine/threonine kinase |
| FGFRL1 | Fibroblast growth factor receptor-like 1 |
| FKBP8 | FK506 binding protein 8, 38kDa |
| GDNF | Glial cell derived neurotrophic factor |
| HIP1 | Huntingtin interacting protein 1 |
| HIP1R | Huntingtin interacting protein 1 related |
| HNRPK | Heterogeneous nuclear ribonucleoprotein K |
| LTA | Lymphotoxin alpha (TNF superfamily, member 1) |
| MAGED1 | Melanoma antigen family D, 1 |
| MAP3K12 | Mitogen-activated protein kinase kinase kinase 12 |
| MDM4 | Mdm4 transformed 3T3 cell double minute 4, p53 binding protein (mouse) |
| PCBP4 | Poly(rC) binding protein 4 |
| PDCD4 | Programmed cell death 4 (neoplastic transformation inhibitor) |
| PTEN | Phosphatase and tensin homolog (mutated in multiple advanced cancers 1) |
| SPHK2 | Sphingosine kinase 2 |
| STK17A | Serine/threonine kinase 17a (apoptosis-inducing) |
| TEGT | Testis enhanced gene transcript (BAX inhibitor 1) |
| TNF | Tumor necrosis factor (TNF superfamily, member 2) |
| TNFRSF10D | Tumor necrosis factor receptor superfamily, member 10d |
| TP53 | Tumor protein p53 (Li-Fraumeni syndrome) |
| TRO | Trophinin |
| Name . | Description . |
|---|---|
| ABL1 | v-abl Abelson murine leukemia viral oncogene homolog 1 |
| ATF3 | Activating transcription factor 3 |
| BAG4 | BCL2-associated athanogene 4 |
| BAT3 | HLA-B associated transcript 3 |
| BCL2 | B-cell CLL/lymphoma 2 |
| BIRC4 | Baculoviral IAP repeat-containing 4 |
| BIRC7 | Baculoviral IAP repeat-containing 7 (livin) |
| BMI1 | B lymphoma Mo-MLV insertion region (mouse) |
| CARD11 | Caspase recruitment domain family, member 11 |
| CARD9 | Caspase recruitment domain family, member 9 |
| CISH | Cytokine inducible SH2-containing protein |
| CUL3 | Cullin 3 |
| CYCS | Cytochrome c, somatic |
| DAD1 | Defender against cell death 1 |
| DAPK3 | Death-associated protein kinase 3 |
| DAXX | Death-associated protein 6 |
| ENDOGL1 | Endonuclease G-like 1 |
| FASTK | Fas-activated serine/threonine kinase |
| FGFRL1 | Fibroblast growth factor receptor-like 1 |
| FKBP8 | FK506 binding protein 8, 38kDa |
| GDNF | Glial cell derived neurotrophic factor |
| HIP1 | Huntingtin interacting protein 1 |
| HIP1R | Huntingtin interacting protein 1 related |
| HNRPK | Heterogeneous nuclear ribonucleoprotein K |
| LTA | Lymphotoxin alpha (TNF superfamily, member 1) |
| MAGED1 | Melanoma antigen family D, 1 |
| MAP3K12 | Mitogen-activated protein kinase kinase kinase 12 |
| MDM4 | Mdm4 transformed 3T3 cell double minute 4, p53 binding protein (mouse) |
| PCBP4 | Poly(rC) binding protein 4 |
| PDCD4 | Programmed cell death 4 (neoplastic transformation inhibitor) |
| PTEN | Phosphatase and tensin homolog (mutated in multiple advanced cancers 1) |
| SPHK2 | Sphingosine kinase 2 |
| STK17A | Serine/threonine kinase 17a (apoptosis-inducing) |
| TEGT | Testis enhanced gene transcript (BAX inhibitor 1) |
| TNF | Tumor necrosis factor (TNF superfamily, member 2) |
| TNFRSF10D | Tumor necrosis factor receptor superfamily, member 10d |
| TP53 | Tumor protein p53 (Li-Fraumeni syndrome) |
| TRO | Trophinin |
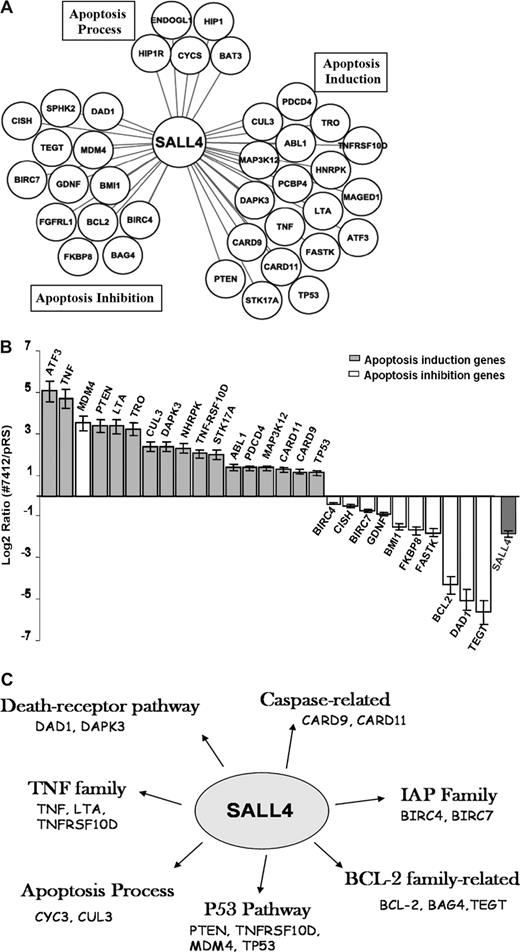
We next focused on interrogating the transcriptional effects of SALL4 on these genes in leukemic cells. We analyzed “direct target” genes of SALL4 associated with apoptosis using retrovirus-induced SALL4 reduction combined with Q-RT-PCR. The term “direct target” includes all promoters that are bound by SALL4, either by direct interaction with the DNA or indirectly as a component in DNA-protein–binding complexes. Interestingly, 17 apoptosis-inducing genes, including TNF, TP53, PTEN, CARD9, CARD11, ATF3, and LTA were consistently up-regulated in NB4 cells in which SALL4 expression was reduced by approximately 75% compared with control NB4 cells (Figure 4B). By contrast, 10 apoptosis-inhibiting genes, including Bmi-1, BCL2, DAD1, TEGT, BIRC7, and BIRC4 (XIAP) were consistently down-regulated in the same experiments. Notably, SALL4 appears to bind and regulate multiple target genes in all major apoptotic pathways (Figure 4C). The one notable outlier was the apoptosis inhibition gene MDM4, whose expression was up-regulated. However, the functional role of MDM4 acting in apoptosis inhibition is controversial.35 Nevertheless, these expression studies indicate that SALL4 is a key regulator of apoptosis and cell growth by up-regulating genes necessary for apoptosis and down-regulating genes that inhibit apoptosis.
SALL4 binds and transcriptionally regulates genes involving various apoptotic pathways. (A) SALL4 binds to apoptosis-inducing genes, apoptosis-inhibiting genes, as well as genes involved in other apoptosis processes based on the PANTHER classification system.34 (B) Q-RT-PCR shows differentially regulated expression levels of apoptotic genes in SALL4 knockdown NB4 cells. Error bars represent standard deviations for duplicate measurements (quantification replicas) normalized to GAPDH levels. Lowering levels of SALL4 expression in NB4 cells induces gene expression that favors apoptosis. (C) When comparing the SALL4 target gene set with the total analyzed gene pool, we found that SALL4 binds to genes involving various apoptotic pathways, including p53, BCL2, TNF, and PTEN. Data were processed using Ingenuity Pathway Analysis.
SALL4 binds and transcriptionally regulates genes involving various apoptotic pathways. (A) SALL4 binds to apoptosis-inducing genes, apoptosis-inhibiting genes, as well as genes involved in other apoptosis processes based on the PANTHER classification system.34 (B) Q-RT-PCR shows differentially regulated expression levels of apoptotic genes in SALL4 knockdown NB4 cells. Error bars represent standard deviations for duplicate measurements (quantification replicas) normalized to GAPDH levels. Lowering levels of SALL4 expression in NB4 cells induces gene expression that favors apoptosis. (C) When comparing the SALL4 target gene set with the total analyzed gene pool, we found that SALL4 binds to genes involving various apoptotic pathways, including p53, BCL2, TNF, and PTEN. Data were processed using Ingenuity Pathway Analysis.
Down-regulated SALL4 induces apoptosis and cell-cycle arrest in NB4 cells
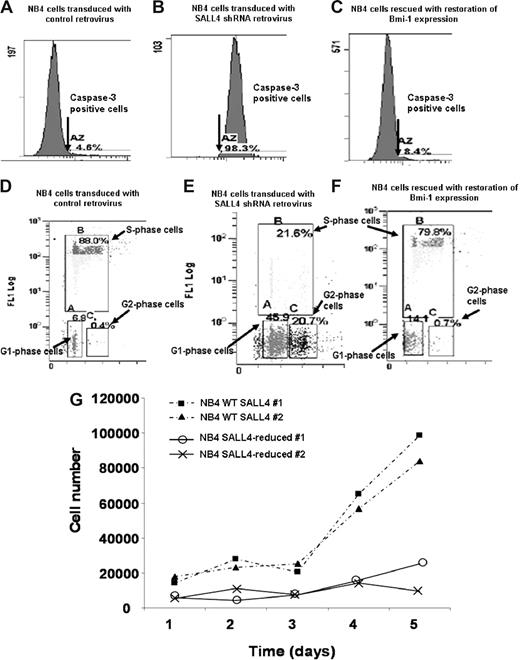
To further test our hypothesis that SALL4 acts as a key regulator in leukemic cell apoptosis and cell growth and determine possibly phenotypic consequences of this regulation, we adopted a RNA inference strategy to reduce SALL4 expression that was followed by flow cytometric assays in NB4 cells. Two short-hairpin (shRNA) retroviral constructs that target different regions of the SALL4 mRNA, termed no. 7410 and no. 7412, were shown to reduce SALL4 mRNA in NB4 cells by Q-RT-PCR (Figure S1A). The shRNA retroviral construct, no. 7412, was more effective in suppressing SALL4 expression and it was used in all further experiments. We first performed flow cytometric assays to monitor the activity of caspase-3 in different cell lines. Caspase-3 is one of the key protein markers for early stages of apoptosis. For virus-treated NB4 cells that express approximately 25% of wild-type (WT) SALL4 levels, there was a 21-fold increase in caspase-3 activity. The number of cells expressing caspase-3 went from 4.6% in WT cells to 98.3% in SALL4-deficient cells as measured by flow cytometry (Figure 5A,B). The apoptotic effect was further verified using data based on expression of the later-stage apoptosis marker annexin V (Figure S3). Annexin V revealed that approximately 75% of the SALL4-reduced cells were apoptotic, whereas only 12% of the controls cells were apoptotic (more than a 6-fold change). DNA fragmentation assays revealed an increase in fragmenting DNA from 1.8% in the control cell population to 39% in the SALL4-reduced population (more than a 21-fold increase [Figure S4]). Another shRNA (no. 7410) against the SALL4 mRNA was also able to produce the same apoptotic phenotype that was proportional to the degree of SALL4 reduction, suggesting that this apoptosis was not due to off-target effects (Figure 5; Figure S1B,C). Similar major increases in apoptosis were observed in other cell lines including KBM5 and NTERA2 when SALL4 expression was decreased (data not shown). This observation strongly suggests that the specific reduction of SALL4 in leukemic cells induces massive cell apoptosis.
Flow cytometric assays of caspase-3 activity, cell-cycle changes, and cell proliferation assays in SALL4 knockdown NB4 cells. Two shRNA retroviral constructs that targeted different regions of the SALL4 were made, and their ability to reduce SALL4 mRNA in NB4 cells was confirmed by Q-RT-PCR (Figure S1). NB4 cells with approximately 75% reduction of SALL4 expression levels (via shRNA no. 7412) were used in these experiments. (A,D) NB4 cells transduced with the control retrovirus. (B,E) NB4 cells transduced with SALL4 shRNA retroviruses. (C,F) Bmi-1–expressing plasmid was transfected into SALL4 knockdown NB4 cells. Caspase-3 activity analysis shows shRNA-mediated reduction of SALL4 induces apoptosis in NB4 cells (A,B). SALL4 knockdown of NB4 cells can be rescued from apoptosis with ectopic expression of Bmi-1 (C). Cell-cycle changes and cellular DNA synthesis in control NB4 cells and SALL4 knockdown NB4 cells were monitored by BrdU-incorporation assay and analyzed by flow cytometry (3% background debris were excluded); data show knockdown of SALL4 induces cell-cycle arrest and decreased DNA synthesis (D,E). By ectopically expressing Bmi-1, SALL4 knockdown cells can be rescued from cell-cycle and DNA synthesis arrest (F). (G) Proliferation assay shows that SALL4 knockdown affects the growth rate of NB4 cells. Cells (5 × 104) from stable clones expressing a control or SALL4 shRNA retrovirus (experiment) were plated in RPMI with 10% FBS. This assay was performed in duplicate and cell numbers were counted daily using a trypan blue–based assay.
Flow cytometric assays of caspase-3 activity, cell-cycle changes, and cell proliferation assays in SALL4 knockdown NB4 cells. Two shRNA retroviral constructs that targeted different regions of the SALL4 were made, and their ability to reduce SALL4 mRNA in NB4 cells was confirmed by Q-RT-PCR (Figure S1). NB4 cells with approximately 75% reduction of SALL4 expression levels (via shRNA no. 7412) were used in these experiments. (A,D) NB4 cells transduced with the control retrovirus. (B,E) NB4 cells transduced with SALL4 shRNA retroviruses. (C,F) Bmi-1–expressing plasmid was transfected into SALL4 knockdown NB4 cells. Caspase-3 activity analysis shows shRNA-mediated reduction of SALL4 induces apoptosis in NB4 cells (A,B). SALL4 knockdown of NB4 cells can be rescued from apoptosis with ectopic expression of Bmi-1 (C). Cell-cycle changes and cellular DNA synthesis in control NB4 cells and SALL4 knockdown NB4 cells were monitored by BrdU-incorporation assay and analyzed by flow cytometry (3% background debris were excluded); data show knockdown of SALL4 induces cell-cycle arrest and decreased DNA synthesis (D,E). By ectopically expressing Bmi-1, SALL4 knockdown cells can be rescued from cell-cycle and DNA synthesis arrest (F). (G) Proliferation assay shows that SALL4 knockdown affects the growth rate of NB4 cells. Cells (5 × 104) from stable clones expressing a control or SALL4 shRNA retrovirus (experiment) were plated in RPMI with 10% FBS. This assay was performed in duplicate and cell numbers were counted daily using a trypan blue–based assay.
We further monitored cell-cycle changes and cellular DNA synthesis in SALL4-reduced and control NB4 cells through bromodeoxyuridine (BrdU)–incorporation assays and flow cytometry. The NB4 cells that expressed roughly 25% of WT SALL4 showed approximately a 4-fold decrease in S-phase cells and a significant increase in the G1- and G2-phase cells (6- and 50-fold increases, respectively), which paralleled the decrease in DNA synthesis as measured by BrdU incorporation (Figure 5D,E). We applied these same studies on other cancerous human cells, such as the embryonic carcinoma cell line NTERA2 and chronic myeloid leukemia cell line KBM5 (both purchased from ATCC, Manassas, VA), and similar results were observed (data not shown). Studies of cell-cycle changes and cellular DNA synthesis were also consistent with results from cell proliferation assays. As shown in Figure 5G, NB4 cells transduced with SALL4-specific shRNA retroviruses grew poorly during 5 days of virus infection. By comparison, growth of NB4 cells was not affected by infection with control viruses. Taken together, these data clearly demonstrate that SALL4 reduction can induce dramatic apoptosis and significant growth arrest in NB4 leukemia cells.
SALL4 knockdown-induced cell-cycle arrest and apoptosis reversed by Bmi-1
We have shown previously that Bmi-1 is a major downstream target of SALL4 in leukemic cells.14 Bmi-1−/− cells display altered expression of the cell-cycle inhibitor gene p16INK4a, resulting in the promotion of cell-cycle arrest and apoptosis. Given the apoptotic effect caused by a reduction in SALL4, overexpression of Bmi-1, a major SALL4 downstream target, may be able to rescue SALL4-induced apoptosis and cell-cycle arrest. SALL4-deficient NB4 cells were transfected with an expression vector containing Bmi-1 and the levels of caspase-3 activity, changes in cell cycle, and cellular DNA synthesis were measured by flow cytometry. As shown in Figure 5C, SALL4-induced caspase-3 activity was restored to a near-normal level by overexpression of Bmi-1 (compared with Figure 5A). Furthermore, reintroduction of Bmi-1 in SALL4-deficient NB4 cells resulted in an increase in the S-phase population and a decrease in the G1- and G2-phase populations (Figure 5F). SALL4-deficient cells, where Bmi-1 was restored to a relatively normal level, incorporated BrdU in a manner similar to the control NB4 cells. Interestingly, restoration of SALL4 functions occurred when the Bmi-1 expression level was corrected to approximately 75% of the normal level (data not shown). This also suggests that cell apoptosis is due exclusively to the specific reduction of SALL4 mRNA. Overexpression of Bmi-1 had little effect on caspase-3 activity, cell-cycle changes, and DNA synthesis in control NB4 cells (data not shown). This may be due to these cells having a high level of endogenous Bmi-1 expression.
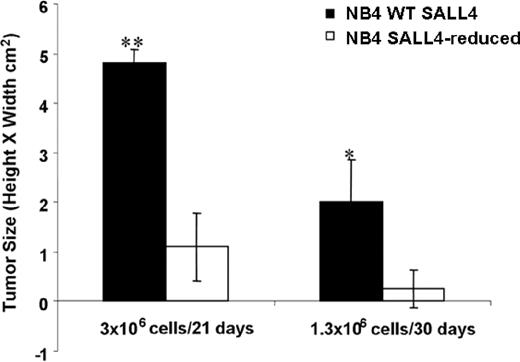
Reduction of SALL4 diminishes tumorigenicity in human leukemia cells
Decreased SALL4 expression in NB4 cells leading to massive cell apoptosis implies an essential role for SALL4 in the development of leukemia. To test this hypothesis in an animal model, we examined the effect of SALL4 knockdown on the ability of NB4 AML cells to induce tumors in immunodeficient mice. NB4 AML cells infected with either control (pRS) or SALL4 shRNA-expressing retroviruses (no. 7412) were injected subcutaneously into the flanks of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Injections were conducted using 3 × 106 or 1.3 × 106 cells. Mice injected with control cells (pRS) at both doses developed large tumor masses after 3 weeks. Two of 4 mice injected with SALL4-reduced cells at the lower dose developed tumors, whereas mice injected with the higher dose developed tumors in all 4 animals. Both dosages showed significantly diminished tumorigenic activity, producing tumors that were on average approximately 5 times smaller than tumors produced by the control cells (Figure 6; P < .01). The difference in tumor size is quite large; however, it is not proportional to the degree of apoptosis seen in SALL4-reduced cells. We hypothesize that because retrovirus infection is variable, tumors seen in mice injected with SALL4-reduced cells may have derived from a subpopulation of NB4 cells that express relatively normal levels of SALL4. To test this, Q-RT-PCR was performed to determine the status of SALL4 expression on tumors from cells infected with SALL4 shRNA retroviruses. Interestingly, tumor cells of animals injected with SALL4-reduced NB4 cells expressed a normal level of SALL4, similar to the level of SALL4 in the control cell tumors (data not shown). This study indicates that the tumors derived from NB4 cells that were not derived from the SALL4-reduced cells that were originally injected. Taken together, our results demonstrate that SALL4 is required for tumorigenicity in AML cells.
Knockdown of SALL4 inhibits the tumorigenicity of NB4 leukemia cells. Tumor growth in NOD/SCID mice injected with control NB4 cells or SALL4 reduction cells was estimated with caliper measurements. Cells were injected at 2 different dosages, and tumor size was determined on day 21 or 30 after injection. Error bars represent the standard deviation. *P < .05; **P < .01.
Knockdown of SALL4 inhibits the tumorigenicity of NB4 leukemia cells. Tumor growth in NOD/SCID mice injected with control NB4 cells or SALL4 reduction cells was estimated with caliper measurements. Cells were injected at 2 different dosages, and tumor size was determined on day 21 or 30 after injection. Error bars represent the standard deviation. *P < .05; **P < .01.
Discussion
One interesting finding of this study is the striking level of genes that bind SALL4 within NB4 cells. Many reported transcription factors do not have as extensive binding in genome-wide analyses, although transcription factors NANOG and E2F1 have been demonstrated to bind an equivalent number of gene promoters.36,37 In addition, ChIP-chip experiments in human and mouse ES cells have shown that SALL4 also binds to multiple thousands of gene promoters (Y.M., unpublished data, June 2007). In recent studies, SALL4 has been used as a part of a gene signature and marker for somatic cell reprogramming due to its vital role in ES cells.38,39
Inherent in the quality of ChIP-chip experiments is the quality of the antibody. We have used this antibody extensively for the previously described experiments and rigorously characterized it for use in ChIP. It is currently used for immunohistochemistry,12,29 and flow cytometry where it uniquely and specifically identifies the SALL4 protein. In addition, we used a commercially available anti-HA antibody to characterize the specificity of the SALL4 antibody. Using mouse ES cells, ChIP-enriched regions were identified using both ChIP-chip and ChIP-PCR with the anti-SALL4 antibody. Then, in SALL4+/− mouse ES cells transfected with exogenous SALL4-HA, we used the HA antibody to pull down ChIP-enriched fragments. The correlation between these 2 sets of ChIP DNA was greater than 88%. Finally, ChIP-chip data have been collected for multiple leukemia cell lines and reflect similar, but complex, binding patterns (data not shown).
The functional categories of SALL4 target genes in NB4 leukemic cells revealed a wide variety of cellular processes. Most striking, SALL4 frequently binds to target genes involving various apoptotic pathways, including p53, BCL2, TNF, and PTEN, when comparing the SALL4 target genes with those in the total analyzed gene pool. All of these apoptotic pathways experience a dramatic effect by the down-regulation of SALL4 and result in apoptosis. Similar results were observed in other cell lines including NTERA2 and KBM5 (unpublished data). These observations suggest that down-regulation of SALL4 could modulate the various apoptotic pathways disseminating apoptotic signals. In support of this interpretation, we have observed that reduction in SALL4 levels by 75% triggers massive apoptosis in the human leukemic cell line NB4.
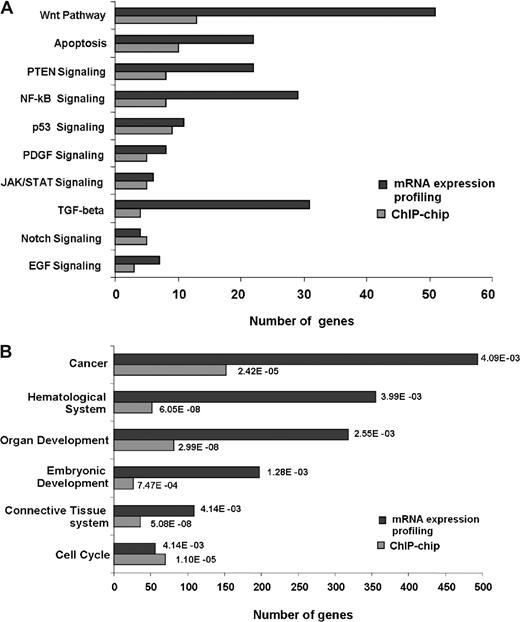
Our conclusion that SALL4 acts as a key regulator in leukemic cells was further strengthened by mRNA microarray profiling (NimbleGen Systems). Global mRNA changes in NB4 cells after SALL4 knockdown were analyzed, and affected genes (either significantly up-regulated or down-regulated) were classified using Ingenuity Pathway Analysis. As shown in Figure 7, SALL4 not only binds genes involved in important cell growth pathways such as the Wnt, apoptosis, PTEN, and NF-kB signaling pathways, but gene expression levels in these pathways are also affected by reduction of SALL4. Correlating promoter binding and affected pathway distributions (Pearson correlation coefficient of 0.7) provides insight into the mechanisms whereby SALL4 may regulate leukemic cell growth. Alternately, functional analyses of expression data consistently demonstrated that cancer-related genes are regulated by SALL4. These expression microarray data were considered reliable since approximately 50% to 75% SALL4 reduction was observed in duplicate experiments, which is consistent with Q-RT-PCR results.
SALL4 binds and transcriptionally regulates genes involving various pathways and biologic functions. Gene-expression changes derived from NB4 cell mRNA expression profiling following SALL4 reduction were classified and compared with ChIP-chip data.  represent the number of SALL4-bound promoters identified from the ChIP-chip assay;
represent the number of SALL4-bound promoters identified from the ChIP-chip assay;  represent the number of statistically significant (P < .05) altered mRNA transcripts in SALL4 knockdown NB4 cells. Their distribution in each signaling pathway (A) or biologic functional category (B) was determined by Ingenuity Pathway Analysis. The P values presented for each category are based on overrepresentation and calculated against the knowledge base provided by Ingenuity Pathway Analysis.
represent the number of statistically significant (P < .05) altered mRNA transcripts in SALL4 knockdown NB4 cells. Their distribution in each signaling pathway (A) or biologic functional category (B) was determined by Ingenuity Pathway Analysis. The P values presented for each category are based on overrepresentation and calculated against the knowledge base provided by Ingenuity Pathway Analysis.
SALL4 binds and transcriptionally regulates genes involving various pathways and biologic functions. Gene-expression changes derived from NB4 cell mRNA expression profiling following SALL4 reduction were classified and compared with ChIP-chip data.  represent the number of SALL4-bound promoters identified from the ChIP-chip assay;
represent the number of SALL4-bound promoters identified from the ChIP-chip assay;  represent the number of statistically significant (P < .05) altered mRNA transcripts in SALL4 knockdown NB4 cells. Their distribution in each signaling pathway (A) or biologic functional category (B) was determined by Ingenuity Pathway Analysis. The P values presented for each category are based on overrepresentation and calculated against the knowledge base provided by Ingenuity Pathway Analysis.
represent the number of statistically significant (P < .05) altered mRNA transcripts in SALL4 knockdown NB4 cells. Their distribution in each signaling pathway (A) or biologic functional category (B) was determined by Ingenuity Pathway Analysis. The P values presented for each category are based on overrepresentation and calculated against the knowledge base provided by Ingenuity Pathway Analysis.
Previously, we have demonstrated that Bmi-1 is a downstream target for SALL4 in leukemic cells. Here we demonstrate that induced apoptosis and cell-cycle arrest in leukemic cells expressing reduced levels of SALL4 can be reversed by ectopically expressing Bmi-1. This raises an interesting question: How does Bmi-1 do this? Perhaps SALL4 and Bmi-1 modulate common target genes in apoptotic and cell-cycle pathways that are interrelated. Therefore, forced expression of Bmi-1 would be capable of reversing the deleterious effects associated with SALL4 down-regulation in leukemic cells.
Recently, we have completed similar experiments on cancer stem cells and normal ES cells of both mouse and human origin (Y.M., unpublished data, June 2007). Preliminary results showed that reduction of SALL4 led to significant apoptosis in NTERA2, a human embryonal carcinoma cell line, as measured by caspase-3 activity (10-fold increase) and through morphologic examination. In contrast, no significant cell death or increased caspase-3 activity was observed in ES cells expressing normal levels of SALL4 (data not shown). In addition, in our conventional SALL4 knockout animal model, SALL4+/− bone marrows displayed little cell death compared with WT controls, and immature hematopoietic stem cell and hematopoietic progenitor cells in SALL4+/− mice were only mildly decreased compared with WT (data not shown). The molecular mechanism of these phenotypes in cancerous and normal cells is unknown; however, data presented here suggest SALL4 binds to some different target genes in the NB4 cell line, such as DAXX, BIRC4, and p53, compared with the CD34+ cell population of whole bone marrows. Since the reduction of SALL4 has dramatic effect on the survival of leukemic cells but not normal ES cells, it is tempting to speculate that the SALL4/Bmi-1 regulatory pathway might be an attractive target for therapeutic intervention by inducing cancer stem cells to undergo apoptosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH, Bethesda, MD) grants K08 CA097185 (Y.M.), NIH R01HL087948 (Y.M.), P20 RR016464 (Y.M.), and K08 DK063220 (L.C.) and by the National Blood Foundation (Bethesda, MD; L.C.).
National Institutes of Health
Authorship
Contribution: Y.M., J.Y., and L.C. designed research; J.Y., C.G., and Z.A. performed research; L.M.F., D.C.W., H.D., J.Y., D.X., Y.M., and T.C.F. analyzed data; and L.M.F., D.C.W., Y.M, J.Y., L.C., and T.C.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Y. Ma, Division of Laboratory Medicine, Nevada Cancer Institute, One Breakthrough Way, Las Vegas, NV 89135; e-mail: yma@nvcancer.org; or L. Chai, Department of Pathology, Joint Program in Transfusion Medicine, Brigham and Women's Hospital/Children's Hospital Boston, Harvard Medical School, 75 Francis St, Boston, MA 02115; e-mail: lchai@partners.org.