Abstract
The thymus constitutes the primary lymphoid organ responsible for the generation of naive T cells. Its stromal compartment is largely composed of a scaffold of different subsets of epithelial cells that provide soluble and membrane-bound molecules essential for thymocyte maturation and selection. With senescence, a steady decline in the thymic output of T cells has been observed. Numeric and qualitative changes in the stromal compartment of the thymus resulting in reduced thymopoietic capacity have been suggested to account for this physiologic process. The precise cellular and molecular mechanisms underlying thymic senescence are, however, only incompletely understood. Here, we demonstrate that TGF-β signaling in thymic epithelial cells exerts a direct influence on the cell's capacity to support thymopoiesis in the aged mouse as the physiologic process of thymic senescence is mitigated in mice deficient for the expression of TGF-βRII on thymic epithelial cells. Moreover, TGF-β signaling in these stromal cells transiently hinders the early phase of thymic reconstitution after myeloablative conditioning and hematopoietic stem cell transplantation. Hence, inhibition of TGF-β signaling decelerates the process of age-related thymic involution and may hasten the reconstitution of regular thymopoiesis after hematopoietic stem cell transplantation.
Introduction
The thymus represents the primary lymphoid organ in which bone marrow–derived precursors mature to T cells as a consequence of close physical and functional interactions with specific stromal cells.1,2 Thymic epithelial cells (TECs; a progeny of the third pharyngeal pouch endoderm) constitute an indispensable part of this stromal compartment as they supply soluble and cell-bound stimuli required for thymocyte proliferation, differentiation, and selection.3 Throughout life, the thymus provides a permanent output of naive T cells with a broad repertoire of antigen receptor specificities. The recent thymic emigrants replace those peripheral T cells that are continuously lost as a consequence of activation or senescence. Yet, size and function of the thymus gradually diminish in the aging individual.4-6 The decrease in thymic productivity is initially rapid and only later followed by a less pronounced decline.6 Known as thymic involution, this process is morphologically characterized by a change in the composition and organization of the stromal network, a gradual loss of thymic epithelial cells and thymocytes, and alterations in intrathymic T-cell subsets.7,8 Concurrently, there is a steady decline in the output of newly developed naive T cells. The prevailing view is that puberty-associated sex steroids play a key role in initiating and promoting thymic involution.9,10 Several observations suggest, however, that a physiologic decline in thymus function may already be in progress at the onset of puberty.5 Indeed, endogenous and exogenous factors other than sex steroids, including glucocorticoids and inflammatory cytokines, have also been identified to influence thymic function and output.6
The molecular mechanisms operational in the physiologic process of thymic involution remain largely elusive but are likely to affect hematopoietic precursor cells at a prethymic stage as well as the thymic stromal compartments.8,11 Exploring knockout and transgenic mice, genetic factors necessary to sustain thymic function have been identified.12-14 However, a specific role for any of these candidate factors in the physiologic senescence of the thymus has yet to be unequivocally proven. Efforts to map quantitative trait loci that influence the size of the thymus and the rate of its involution have suggested that the transforming growth factor β2 (TGF-β2) plays an age-dependent negative role in controlling thymic weight and cellularity.15
Three TGF-β isoforms have been identified that are encoded by distinct loci but share a high level of sequence and structural homology.16 It is thought that the individual TGF-β isoforms effect unique sets of physiologic functions due to their different tissue distributions and temporal expression patterns. TGF-β induces a heteromeric complex of type I and type II serine/threonine kinase receptors after ligand binding.17 Subsequent signal transduction occurs as the TGF-β type II receptor (TGFβRII) phosphorylates the type I receptor chain that in turn initiates the activation of second messengers designated R-Smads, ultimately leading to the transcriptional activation of several target genes.17
TGF-βs play a regulatory role in most processes linked to the control of somatic tissue development and renewal, exerting either a positive or a negative effect on proliferation, differentiation, and cell death. Within the lymphohematopoietic system, TGF-β1 not only acts as one of the most potent negative regulators of hematopoiesis18 but also directly affects T-cell development. For example, TGF-β1 has been shown to inhibit the IL-1–, IL-2–, and IL-7–dependent proliferation of thymocytes,19,20 the differentiation of CD4−CD8low to CD4+CD8+ (double-positive) thymocytes,21-24 and the differentiation of CD4+ cells into type 1 and type 2 T helper cells.25,26 High concentrations of TGF-β2 inhibit the proliferation of lineage-negative, Sca++, c-kit+ (LSK) progenitor cells that give rise to thymic T-cell precursors.27 It is, however, unknown whether the changes in T-cell development are caused by TGF-β signaling directly to thymocytes or, alternatively, whether the observed alterations are the consequence of TGF-β–mediated effects on the thymic stromal environment.
The expression of TGF-β and its receptors can be detected during thymic organogenesis as early as day 10.5 of embryonic development (E10.5) (Schmid et al28 and G.A.H., unpublished data, December 2006), which suggests an important role for the TGF-β pathway in TEC differentiation and function. Disruption of the TGFβRII, the TGF-β2, or the TGF-β3 locus results in embryonic and perinatal lethality.29-31 In contrast, mice deficient for TGF-β1 can be viable but display early in life multiorgan inflammatory lesions and a lethal wasting syndrome, hence precluding a detailed analysis of thymic function and postnatal development.32
Here, we demonstrate that mice deficient for TGFβRII expression on TECs but not on thymocytes display a regular T-cell development but demonstrate a mitigated thymic involution, changes in both TEC phenotype and composition, and an accumulation of mature T cells. Moreover, these studies reveal that TGF-β signaling in TECs hinders efficient thymus-dependent T-cell reconstitution after myeloablative irradiation and hematopoietic stem cell transplantation.
Methods
Mice
Mouse strains Rosa26LacZ (B6;129S-Gtrosa26tm1Sor) and Z/EGLobe21(B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J), B6.SJL-PtprcaPep3b/BoyJ (B6.CD45.1), and B6.Foxn1nu/nu were obtained from The Jackson Laboratory (Bar Harbor, ME). The generation of Foxn1-Cre, TGFβRIIlox/lox, and Hoxa3-Cre mice has been previously reported.33-35 For embryonic staging, the day of the vaginal plug was designated as embryonic day 0.5 (E0.5). Mice were 5 to 10 weeks of age at the time of experiments unless stated otherwise and housed at the Center's Animal Facility in accordance with Institutional and Cantonal Review Boards.
Flow cytometry and thymic stromal cell sorting
Thymi, spleens, and lymph nodes were dissected from mice that had been killed; organs were minced through a nylon mesh, washed in Hanks buffered saline solution (HBSS), and used for flow cytometry. For the analysis of thymic stromal cells, isolated thymic lobes were prepared as previously reported.35,36 The resulting fractions were washed, filtered, and individually prepared for counting and flow cytometric analysis. CD45− MHCII+ stromal cells were considered of epithelial origin.
Single thymocyte suspensions were stained using directly labeled antibodies specific for CD3 (clone KT3), CD4 (RM4-5), CD8a (53-6.7), CD19 (1D3), CD24 (30-F1), CD25 (PC61), CD44 (IM7CD45.1), CD62L (MEL-14), CD69 (H1.2F3), pan-NK (DX5), B220 (RA3-6B2), TCRβ (H57-597), TCRγδ (GL-3), c-kit (2B8), and Sca-1 (D7) (eBioscience, San Diego, CA, and BD Biosciences PharMingen, San Diego, CA). Stromal cell preparations were stained using antibodies to CD45 (30-F11), MHC class I (AF6-88.5) and MHC class II (AF6-120.1), ICAM-1 (3E2; BD Biosciences, Mountain View, CA), and BrdU (3D4; BD Biosciences) and analyzed with a FACSCalibur flow cytometer (BD Biosciences) using the CellQuestPro software (BD Biosciences).
Immunofluorescence
Immunohistology was performed on thymic sections (6-8 μm) as previously reported35,36 using moAb to K18 (Ks18.04; Progene, Heidelberg, Germany), polyclonal Ab to K5 (Covance, Princeton, NJ), and biotinylated UEA1 lectin (Vector Laboratories, Burlingame, CA). Images were captured on a Zeiss LSM 510 confocal microscope (20×/0.5 NA objective; Carl Zeiss, Feldbach, Switzerland) at room temperature, and overlays were performed using the Zeiss LSM 510 software version 3.2 (Carl Zeiss).
Fetal thymic organ culture and lymphocyte depletion
Fetal thymi were cultured as described previously.37 Lymphocyte depletion was performed before transplantation of the thymic grafts into nude recipients for a 5-day culture period using 2-deoxy-guanosine (2dGuo; Sigma-Aldrich, Buchs, Switzerland).
In vivo BrdU and FITC labeling
To measure TEC proliferation, mice (12-18 weeks of age) were first injected with 1 mg BrdU (Sigma-Aldrich) and thereafter fed with BrdU-containing drinking water (0.8 mg/mL). On day 14, thymic stromal cells were isolated as detailed in “Flow cytometry and thymic stromal cell sorting,” stained for the expression of CD45 and MHCII, and analyzed for BrdU incorporation as described.35
Intrathymic FITC injection for the detection of recent thymic emigrants was performed as previously described.35
Hematopoietic stem cell transplantation
Eight-week-old TGFβRIIlox/lox and TGFβRIIlox/lox::Foxn1-Cre mice were lethally irradiated (9.5 Gy) using a 137Cs source (0.81 Gy/min) and were subsequently intravenously infused with 107 T cell–depleted bone marrow cells derived from B6.CD45.1 mice.
Statistical analysis
Values are means plus or minus SD. A 2-sided t test or a nonparametric U test was used for 2-group comparisons as applicable. The statistical significance level was set to 5%. Analysis was performed using STATA version 9.2 (StataCorp, College Station, TX).
Results
Thymic stroma deficient for TGFβRII expression support regular T-cell development
Mice deficient for the expression of TGFβRII receptor chain die around E11.5 due to severe defects in hematopoiesis and blood vessel formation.31 It has thus been impossible to study the role of TGFβRII for thymus formation, homeostasis, and function. To identify therefore the importance of TGF-β signaling in thymic epithelial cells (TECs), the deletion of the TGFβRII gene was specifically targeted to this cell type using the Cre/lox recombination system. For this purpose, mice expressing the Cre recombinase under the transcriptional control of the Foxn1 locus (designated Foxn1-Cre35 ) were crossed to mice in which exon 3 of the TGFβRII locus is flanked by loxP sites (designated TGFβRIIlox/lox). The successful recombination within the TGFβRII locus results in a complete loss of TGF-β signaling.33
The thymic lobes of newborn and adult TGFβRIIlox/lox::Foxn1-Cre mice were correctly positioned in the anterior mediastinum and displayed a regular separation into distinct cortical and medullary compartments (Figure 1Ai-x and data not shown). Regardless of a lack of TGF-β signaling in TECs, the number of early thymocyte precursors (ETPs), defined as lin− CD25− c-kit+ CD44+ thymocytes,38 and their further differentiation were unaffected (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Staining thymocytes for the cell surface expression of several markers typical for thymocytes at a later stage of development, including CD4 and CD8, revealed initially a normal relative distribution of the different subpopulations (Figure 1Axi,xii and data not shown). However, both absolute and relative numbers of T cells with a typically postselection, mature phenotype (ie, CD3+CD4+ or CD3+CD8+) were increased in older TGFβRIIlox/lox::Foxn1-Cre mice (≥ 5 weeks) compared with Cre-deficient littermates and aged-matched controls (“T cells with a mature phenotype accumulate in the thymus of mice deficient for TGFβII expression by TECs”). To ascertain that the observed phenotype was indeed caused by a successful Cre-mediated recombination of the TGFβRII locus, the deletion of TGFβRII exon 3 was verified in 4-week-old TGFβRIIlox/lox::Foxn1-Cre mice. As demonstrated in Figure 1B, the pool of TECs (purity ≥ 95%) displayed a close tocomplete deletion within the TGFβRII locus, whereas hematopoietic stromal cells of the thymus exhibited a regular genomic sequence.
Thymic development and lymphopoietic function in the absence of TGF-β signaling in thymic stromal cells. (A) Gross anatomic, histologic, and flow cytometric analysis of thymic tissue from 2-week-old TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (i,ii) Thymus in situ (T indicates thymus; H, heart; and L, lung). (iii,iv) H&E staining (magnification 4×). (v-x) Immunofluorescence microscopy; magnification, 20×. Cortical (v,vi) and medullary (vii-x) regions, stained for cytokeratin 8 (v-viii), cytokeratin 5 (v-x), ERTR7 (v-viii), and UEA-1 (ix,x). (xi,xii: representative fluorescence-activated cell sorting [FACS] profile for the cell surface expression of CD4 and CD8 [mean ± SD of 6 mice analyzed per group]). Percentages on plots are of total cells within gate. (B) Genomic deletion analysis of sorted thymic dendritic cells (CD45+ MHCII+, left lane) and epithelial cells (CD45− MHCII+, right lane, purity ≥ 95) isolated from 4-week-old TGFβRIIlox/lox::Foxn1-Cre mice. The upper band denotes the undeleted allele, whereas the lower band represents the deleted allele. (C) Hoxa-controlled Cre expression pattern. Top: Sagittal section of an E10.25 Rosa26lacZ::Hoxa3-Cre embryo, assayed for β-galactosidase expression and counterstained with nuclear fast red; PA indicates pharyngeal arch. Bottom: FACS analysis of E13.5 epithelial (left panel) and nonepithelial (right panel) thymic stromal cells from Z/EGLobe21 and Z/EGLobe21::Hoxa3-Cre mice for GFP expression. Percentages on graphs are of total cells within gate. (D) Thymocyte development in E12.5 FTOC of thymic lobes isolated from TGFβRIIlox/lox::Hoxa3-Cre and TGFβRIIlox/lox E12.5 embryos after a 12-day culture period. Representative dot blots of flow cytometric analysis for the cell surface expression of CD4 and CD8 (n = 3 for TGFβRIIlox/lox::Hoxa3-Cre; n = 4 for TGFβRIIlox/lox). Percentages on plots are of total cells within gate.
Thymic development and lymphopoietic function in the absence of TGF-β signaling in thymic stromal cells. (A) Gross anatomic, histologic, and flow cytometric analysis of thymic tissue from 2-week-old TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (i,ii) Thymus in situ (T indicates thymus; H, heart; and L, lung). (iii,iv) H&E staining (magnification 4×). (v-x) Immunofluorescence microscopy; magnification, 20×. Cortical (v,vi) and medullary (vii-x) regions, stained for cytokeratin 8 (v-viii), cytokeratin 5 (v-x), ERTR7 (v-viii), and UEA-1 (ix,x). (xi,xii: representative fluorescence-activated cell sorting [FACS] profile for the cell surface expression of CD4 and CD8 [mean ± SD of 6 mice analyzed per group]). Percentages on plots are of total cells within gate. (B) Genomic deletion analysis of sorted thymic dendritic cells (CD45+ MHCII+, left lane) and epithelial cells (CD45− MHCII+, right lane, purity ≥ 95) isolated from 4-week-old TGFβRIIlox/lox::Foxn1-Cre mice. The upper band denotes the undeleted allele, whereas the lower band represents the deleted allele. (C) Hoxa-controlled Cre expression pattern. Top: Sagittal section of an E10.25 Rosa26lacZ::Hoxa3-Cre embryo, assayed for β-galactosidase expression and counterstained with nuclear fast red; PA indicates pharyngeal arch. Bottom: FACS analysis of E13.5 epithelial (left panel) and nonepithelial (right panel) thymic stromal cells from Z/EGLobe21 and Z/EGLobe21::Hoxa3-Cre mice for GFP expression. Percentages on graphs are of total cells within gate. (D) Thymocyte development in E12.5 FTOC of thymic lobes isolated from TGFβRIIlox/lox::Hoxa3-Cre and TGFβRIIlox/lox E12.5 embryos after a 12-day culture period. Representative dot blots of flow cytometric analysis for the cell surface expression of CD4 and CD8 (n = 3 for TGFβRIIlox/lox::Hoxa3-Cre; n = 4 for TGFβRIIlox/lox). Percentages on plots are of total cells within gate.
In TGFβRIIlox/lox::Foxn1-Cre mice, the Cre recombinase is expressed the earliest at E11.5 (S.Z., M.P.K., L.T.J., G.A.M., “Stabilized β-catenin in thymic epithelial cells blocks thymus development and function,” submitted December 2007). Hence recombination occurs both after the commitment of endodermal cells to a thymus fate (as marked at E10.5 by Foxn1 expression) and after the formation of a separate thymus anlage.39 Relatively late Cre-mediated deletion of TGFβRII exon 3 in TECs could thus explain the absence of a more pronounced phenotype. To achieve Cre-mediated recombination at an earlier time point during thymic organogenesis, TGFβRIIlox/lox mice were crossed to Hoxa3-Cre mice, where Cre expression not only occurs before E10.2534 but can also be detected at E13.5 in the vast majority of TECs and a minor subpopulation of nonepithelial, nonhematopoietic stromal cells (Figure 1C). Because TGFβRIIlox/lox::Hoxa3-Cre mice die at E14.5, possibly secondary to cardiovascular malformation (data not shown), the thymic development of these mice was investigated using E12.5 thymic organ cultures (FTOCs). Under these conditions, thymocyte maturation was comparable for lobes isolated from Hoxa3-Cre::TGFβRIIlox/fox and TGFβRIIlox/fox mice, revealing after 12 days in culture a normal progression from immature CD4−CD8− to either CD4+ or CD8+ (ie, single positive, SP) mature T cells (Figure 1D). Taken together, these results demonstrate that the lack of TGFβRII-mediated signaling in TECs neither affects the formation of a thymus anlage nor does it preclude the establishment of a normally structured thymic microenvironment able to support regular T-cell maturation.
Diminished age-related thymic involution in the absence of TGFβRII expression by TECs
TGFβ mRNA levels are increased in the aging human thymus, suggesting a role for TGFβ in the older thymus.40 Furthermore, physiologic doses of androgens not only induce increased thymic levels of TGF-β1 mRNA but also reverse thymus enlargement in mice after castration.41,42 To identify the contribution of TGFβ to age-related changes of the thymus, TGFβ mRNA and protein levels were determined in thymi of young, middle-aged, and aged naive mice (Figure S2A). A gradual increase in TGFβ2 mRNA levels was noted to correspond to advanced age, whereas mRNA levels for TGF-β1 showed only a limited and transient increase over time. In keeping with this finding, increased protein levels were detected by Western blotting in total thymic tissue lysates taken from middle-aged and aged mice (Figure S2B). It is furthermore of note, that TECs constitutively express TGFβRII (Figure S2C). To investigate whether the changes in TGF-β expression affect thymic cellularity in a TEC-dependent manner, the number of thymocytes was measured in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (Figure 2A,B). In the first few weeks of life, the number of thymocytes did not significantly differ between these 2 mouse strains, demonstrating that a lack of TGF-β signaling did not impact on thymic cellularity. With the onset of puberty, the number of thymocytes was elevated in adolescent TGFβRIIlox/lox::Foxn1-Cre mice (4-5 weeks of age). This difference became more prominent in older (12-28 weeks of age: 34% ± 12%, P = .001) and aged (44-64 weeks of age, 64% ± 38%, P = .002) mice (Figure 2A,B). Concurrent with this increase in thymic cellularity and indicative of a higher export of mature T cells in 28-week-old TGFβRIIlox/lox::Foxn1-Cre mice, a higher frequency of naive T cells (CD44low, CD62Lhigh) was noted in the spleen of these mice compared with control littermates (Figure S3A). Whereas the TCRVβ repertoire was similar for peripheral T cells from TGFβRIIlox/lox::Foxn1-Cre mice and littermate controls (Figure S3B) at one year of age, the frequency of recent thymic emigrants (RTEs, as determined by the number of sjTREC copies) was consistently increased, albeit without statistical significance (Figure S3C)
Mitigated thymic involution in TGFβRIIlox/lox::Foxn1 Cre mice. (A) Thymic cellularity in prepubescent, adolescent, and adult mice either deficient (▵) or proficient (●) for TGF-β signaling in TECs. Each symbol denotes the average of 2 or more mice of the respective genotype of the same litter at the indicated age. A logarithmic regression curve was calculated for TGFβRIIlox/lox::Foxn1-Cre (broken line) and TGFβRIIlox/lox (solid line). Using a paired t test, a significant difference in thymic cellularity between the 2 mouse strains is represented by the horizontal bar in the upper part of the graph. (B) Ratio of thymic cellularity between TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox littermates displayed for 5 separate age groups; n denotes the number of litters per age group analyzed (with ≥ 2 animals per litter and genotype). The broken horizontal line represents the values of TGFβRIIlox/lox mice normalized to 1. The white line in each bar represents the mean, the bar represents the 25th percentile, and the whiskers represent the 75th percentile. P values were calculated using a paired t test. (C) Total thymic cellularity of 2dGuo-depleted thymic grafts from E12.5 TGFβRIIlox/lox::Hoxa3Cre (□, n = 6) and TGFβRIIlox/lox (■, n = 10) embryos grafted into syngeneic nu/nu mice and analyzed 12 weeks later (P value obtained using a nonparametric U test). This experiment was independently performed 4 times with similar results. Error bars represent SD.
Mitigated thymic involution in TGFβRIIlox/lox::Foxn1 Cre mice. (A) Thymic cellularity in prepubescent, adolescent, and adult mice either deficient (▵) or proficient (●) for TGF-β signaling in TECs. Each symbol denotes the average of 2 or more mice of the respective genotype of the same litter at the indicated age. A logarithmic regression curve was calculated for TGFβRIIlox/lox::Foxn1-Cre (broken line) and TGFβRIIlox/lox (solid line). Using a paired t test, a significant difference in thymic cellularity between the 2 mouse strains is represented by the horizontal bar in the upper part of the graph. (B) Ratio of thymic cellularity between TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox littermates displayed for 5 separate age groups; n denotes the number of litters per age group analyzed (with ≥ 2 animals per litter and genotype). The broken horizontal line represents the values of TGFβRIIlox/lox mice normalized to 1. The white line in each bar represents the mean, the bar represents the 25th percentile, and the whiskers represent the 75th percentile. P values were calculated using a paired t test. (C) Total thymic cellularity of 2dGuo-depleted thymic grafts from E12.5 TGFβRIIlox/lox::Hoxa3Cre (□, n = 6) and TGFβRIIlox/lox (■, n = 10) embryos grafted into syngeneic nu/nu mice and analyzed 12 weeks later (P value obtained using a nonparametric U test). This experiment was independently performed 4 times with similar results. Error bars represent SD.
An increase in total thymocytes by 2.6-fold was also observed in transfer experiments using thymic lobes isolated from E12.5 TGFβRIIlox/lox::Hoxa3-Cre embryos that had first been depleted of lymphocytes by 2dGuo and then grafted for 12 weeks under the kidney capsule of syngeneic, nude recipients (Figure 2C). Hence, 2 independently generated mouse lines in which TGF-β signaling in TECs was blocked revealed the negative and indirect influence of TGF-β on thymocyte cellularity in adult mice.
Changes in the composition of the thymic epithelial stroma in TGFβRIIlox/lox::Foxn1-Cre mice
We next investigated whether the loss of TGF-β signaling in TECs also affected the number and phenotype of thymic epithelia in newborn and older TGFβRIIlox/lox::Foxn1-Cre mice. MHC class II expression was chosen as a marker to differentiate TEC subpopulations because it has been demonstrated that the ratio of TECs with a high surface expression of MHCII (designated MHCIIhigh) to TECs with an intermediate MHCII cell surface expression (MHCIIint) gradually decreases with age.8 Total TEC cellularity was comparable for newborn (0.13 ± 0.03 × 106 cells in TGFβRIIlox/lox::Foxn1-Cre vs 0.14 ± 0.01 × 106 cells in TGFβRIIlox/lox mice) and 8-week-old TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (Figure 3A). However, the fraction of MHCIIhigh TECs was significantly elevated in 8-week-old mutant mice in comparison with controls (Figure 3B left side). Similar differences were obtained comparing MHCIIhigh TEC cellularity in TGFβRIIlox/lox::Hoxa3-Cre and TGFβRIIlox/lox thymic lobes that had been transplanted as E12.5 grafts under the kidney capsule of syngeneic nude hosts and analyzed 10 to 12 weeks later (Figure 3B right side). In contrast to TGFβRIIlox/lox mice, mutant littermates had maintained their TEC cellularity at 28 weeks of age compared with younger mice (Figure 3A).
The composition, phenotype, and proliferation of the thymic epithelial compartment are altered in TGFβRIIlox/lox::Foxn1-Cre mice. (A) TEC cellularity in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox littermates at the indicated ages. Data are given for TECs expressing either high ( ) or intermediate (
) or intermediate ( ) MHC cell surface concentrations. Total TEC cellularity at the age of 8 weeks: 0.39 (± 0.06) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.35 (± 0.07) × 106 cells (TGFβRIIlox/lox, P = .36 derived from a t test, with 6 and 4 mice per group, respectively); at the age of 28 weeks: 0.389 (± 0.100) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.192 (± 0.031) × 106 cells (TGFβRIIlox/lox, P = .02 derived from a t test, with 4 and 3 mice per group, respectively); and at the age of 64 weeks: 0.166 (± 0.073) × 106 versus 0.080 (± 0.029) × 106 (P < .015 derived from a t test, with 4 and 6 mice per group, respectively). This analysis was performed at least twice with similar results. (B) Fraction of MHCIIhigh TECs in comparison with total TECs (CD45+MHCII+). TECs derived from TGFβRIIlox/lox::Foxn1-Cre (8 weeks of age) and TGFβRIIlox/lox::Hoxa3-Cre (10-12 weeks after transplantation) mice show similar increases in the MHCIIhigh fraction in comparison with Cre-negative littermate controls. (C) Spontaneous proliferation of TECs isolated from TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (16 weeks of age) as measured by the fraction of BrdU-incorporating TECs. One representative experiment of 2 with 4 or more animals in each group is shown. P value was obtained using a t test. Error bars represent SD.
) MHC cell surface concentrations. Total TEC cellularity at the age of 8 weeks: 0.39 (± 0.06) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.35 (± 0.07) × 106 cells (TGFβRIIlox/lox, P = .36 derived from a t test, with 6 and 4 mice per group, respectively); at the age of 28 weeks: 0.389 (± 0.100) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.192 (± 0.031) × 106 cells (TGFβRIIlox/lox, P = .02 derived from a t test, with 4 and 3 mice per group, respectively); and at the age of 64 weeks: 0.166 (± 0.073) × 106 versus 0.080 (± 0.029) × 106 (P < .015 derived from a t test, with 4 and 6 mice per group, respectively). This analysis was performed at least twice with similar results. (B) Fraction of MHCIIhigh TECs in comparison with total TECs (CD45+MHCII+). TECs derived from TGFβRIIlox/lox::Foxn1-Cre (8 weeks of age) and TGFβRIIlox/lox::Hoxa3-Cre (10-12 weeks after transplantation) mice show similar increases in the MHCIIhigh fraction in comparison with Cre-negative littermate controls. (C) Spontaneous proliferation of TECs isolated from TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (16 weeks of age) as measured by the fraction of BrdU-incorporating TECs. One representative experiment of 2 with 4 or more animals in each group is shown. P value was obtained using a t test. Error bars represent SD.
The composition, phenotype, and proliferation of the thymic epithelial compartment are altered in TGFβRIIlox/lox::Foxn1-Cre mice. (A) TEC cellularity in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox littermates at the indicated ages. Data are given for TECs expressing either high ( ) or intermediate (
) or intermediate ( ) MHC cell surface concentrations. Total TEC cellularity at the age of 8 weeks: 0.39 (± 0.06) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.35 (± 0.07) × 106 cells (TGFβRIIlox/lox, P = .36 derived from a t test, with 6 and 4 mice per group, respectively); at the age of 28 weeks: 0.389 (± 0.100) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.192 (± 0.031) × 106 cells (TGFβRIIlox/lox, P = .02 derived from a t test, with 4 and 3 mice per group, respectively); and at the age of 64 weeks: 0.166 (± 0.073) × 106 versus 0.080 (± 0.029) × 106 (P < .015 derived from a t test, with 4 and 6 mice per group, respectively). This analysis was performed at least twice with similar results. (B) Fraction of MHCIIhigh TECs in comparison with total TECs (CD45+MHCII+). TECs derived from TGFβRIIlox/lox::Foxn1-Cre (8 weeks of age) and TGFβRIIlox/lox::Hoxa3-Cre (10-12 weeks after transplantation) mice show similar increases in the MHCIIhigh fraction in comparison with Cre-negative littermate controls. (C) Spontaneous proliferation of TECs isolated from TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (16 weeks of age) as measured by the fraction of BrdU-incorporating TECs. One representative experiment of 2 with 4 or more animals in each group is shown. P value was obtained using a t test. Error bars represent SD.
) MHC cell surface concentrations. Total TEC cellularity at the age of 8 weeks: 0.39 (± 0.06) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.35 (± 0.07) × 106 cells (TGFβRIIlox/lox, P = .36 derived from a t test, with 6 and 4 mice per group, respectively); at the age of 28 weeks: 0.389 (± 0.100) × 106 cells (TGFβRIIlox/lox::Foxn1-Cre) versus 0.192 (± 0.031) × 106 cells (TGFβRIIlox/lox, P = .02 derived from a t test, with 4 and 3 mice per group, respectively); and at the age of 64 weeks: 0.166 (± 0.073) × 106 versus 0.080 (± 0.029) × 106 (P < .015 derived from a t test, with 4 and 6 mice per group, respectively). This analysis was performed at least twice with similar results. (B) Fraction of MHCIIhigh TECs in comparison with total TECs (CD45+MHCII+). TECs derived from TGFβRIIlox/lox::Foxn1-Cre (8 weeks of age) and TGFβRIIlox/lox::Hoxa3-Cre (10-12 weeks after transplantation) mice show similar increases in the MHCIIhigh fraction in comparison with Cre-negative littermate controls. (C) Spontaneous proliferation of TECs isolated from TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (16 weeks of age) as measured by the fraction of BrdU-incorporating TECs. One representative experiment of 2 with 4 or more animals in each group is shown. P value was obtained using a t test. Error bars represent SD.
The number of MHCIIhigh TECs decreased slightly in the TGFβRIIlox/lox::Foxn1-Cre mice between 8 and 28 weeks of age, whereas the amount of MHCIIint TECs remained unchanged (Figure 3A). In contrast, both subpopulations of TECs (ie, MHCIIhigh and MHCIIint) progressively diminished in wild-type mice during the same time period (Figure 3A). The unchanged TEC cellularity of 28-week-old TGFβRIIlox/lox::Foxn1-Cre mice correlated with a higher fraction of proliferating TECs in comparison with TGFβRIIlox/lox mice (Figure 3C). This correlation was in keeping with observations of a reduced in vitro proliferation by both medullary and cortical TEC lines exposed to TGF-β (Figure S4A,B).43 Programmed cell death among primary TECs was, however, unaffected in the absence of TGFβRII-mediatedsignaling (data not shown). In old mice (64 weeks of age), the absolute number of MHChigh TECs and MHCint TECs was decreased in comparison with younger animals, but nonetheless TGFβRIIlox/lox::Foxn1-Cre mice retained a significantly higher TEC cellularity in comparison with control littermates (Figure 3A). Independent of these numeric differences, the architecture of the stromal microenvironment was comparable for TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (Figure S5).
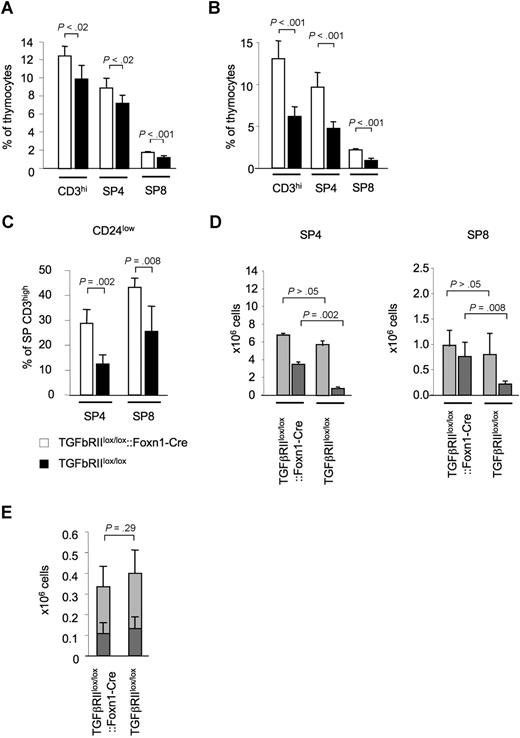
T cells with a mature phenotype accumulate in the thymus of mice deficient for TGFβRII expression by TECs
The lack of TGF-β–mediated signaling in TECs correlated both with an absolute and a relative increase of single-positive (SP) mature (ie, CD3high) thymocytes. This finding was apparent as early as day 5 after birth and was most prominent in young animals (5-12 weeks of age) but could also be observed in older mice deficient in TGF-β signaling in TECs (12-28 weeks of age; Figure 4A; Table S1). Mature SP thymocytes also accumulated in TGFβRIIlox/lox::Hoxa3-Cre thymic grafts placed under the kidney capsule of syngeneic nude recipients (Figure 4B). An extended phenotypic analysis of these mature thymocytes from TGFβRIIlox/lox::Foxn1-Cre mice revealed a higher frequency of CD24low cells (Figure 4C,D) and CD44low CD62Lhigh cells (data not shown), consistent with the notion that these cells have not entered the thymus from the periphery as recirculating mature T cells.45
The absence of TGFβ signaling in TECs results in an increase in mature, single-positive thymocytes. (A) Relative frequency of phenotypically mature (ie, CD3high) thymocytes in 8-week-old TGFβRIIlox/lox::Foxn1-Cre (□) and TGFβRIIlox/lox (■) mice. (B) Relative frequency of CD3high SP thymocytes in E12.5 thymic lobes of phenotypically mature thymocytes in TGFβRIIlox/lox::Hoxa3-Cre (□) and TGFβRIIlox/lox (■) embryos grafted under the kidney capsule of syngeneic nude recipients and analyzed 12 weeks later. (C) Relative frequency of CD24low and (D) absolute numbers of CD24low ( ) and CD24high (
) and CD24high ( ) thymocytes among single-positive, CD3high thymocytes in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (E) Thymic export measured by intrathymic FITC injection of 5-week-old mice and quantification of FITC+ cells 24 hours later: absolute number of FITC+ T cells detected in spleen and lymph nodes (
) thymocytes among single-positive, CD3high thymocytes in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (E) Thymic export measured by intrathymic FITC injection of 5-week-old mice and quantification of FITC+ cells 24 hours later: absolute number of FITC+ T cells detected in spleen and lymph nodes ( CD4+ T cells,
CD4+ T cells,  CD8+ T cells). One of 3 experiments with similar results shown. P values were obtained using a t test. Error bars represent SD.
CD8+ T cells). One of 3 experiments with similar results shown. P values were obtained using a t test. Error bars represent SD.
The absence of TGFβ signaling in TECs results in an increase in mature, single-positive thymocytes. (A) Relative frequency of phenotypically mature (ie, CD3high) thymocytes in 8-week-old TGFβRIIlox/lox::Foxn1-Cre (□) and TGFβRIIlox/lox (■) mice. (B) Relative frequency of CD3high SP thymocytes in E12.5 thymic lobes of phenotypically mature thymocytes in TGFβRIIlox/lox::Hoxa3-Cre (□) and TGFβRIIlox/lox (■) embryos grafted under the kidney capsule of syngeneic nude recipients and analyzed 12 weeks later. (C) Relative frequency of CD24low and (D) absolute numbers of CD24low ( ) and CD24high (
) and CD24high ( ) thymocytes among single-positive, CD3high thymocytes in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (E) Thymic export measured by intrathymic FITC injection of 5-week-old mice and quantification of FITC+ cells 24 hours later: absolute number of FITC+ T cells detected in spleen and lymph nodes (
) thymocytes among single-positive, CD3high thymocytes in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (E) Thymic export measured by intrathymic FITC injection of 5-week-old mice and quantification of FITC+ cells 24 hours later: absolute number of FITC+ T cells detected in spleen and lymph nodes ( CD4+ T cells,
CD4+ T cells,  CD8+ T cells). One of 3 experiments with similar results shown. P values were obtained using a t test. Error bars represent SD.
CD8+ T cells). One of 3 experiments with similar results shown. P values were obtained using a t test. Error bars represent SD.
We next determined cell proliferation in situ and thymic export of SP thymocytes as 2 possible, mutually not exclusive explanations for the accumulation of these cells in TGFβRIIlox/lox::Foxn1-Cre mice. There was no difference in either BrdU incorporation (Figure S6) or DNA content among CD24low thymic T cells comparing mutant and control animals (data not shown). These results suggested equal proliferation rates for these mature thymocytes. To assess alterations in thymic T-cell export, mutant and control mice were intrathymically injected at 4 weeks of age with FITC. Twenty-four hours later, recent thymic emigrants were identified in lymph nodes and spleen as labeled T cells. Although the fraction of FITC-marked peripheral T cells in relation to all labeled cells (ie, thymocytes and recent thymic emigrants) was (statistically significantly) decreased in TGFβRIIlox/lox::Foxn1-Cre mice compared with TGFβRIIlox/lox littermates (data not shown), the absolute number of recent thymic emigrants did not differ between the 2 mouse strains (Figure 4E). In keeping with this result, transcription levels for the chemokine receptor S1P45 among mature thymocytes were comparable for TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice (data not shown). These findings in 4-week-old mice indicate that aberrant TGF-β signaling in TECs influences the pool size of single-positive, mature thymocytes but not their export.
TGF-β in TECs affects thymic reconstitution after irradiation
In thymocytes and other cells, γ-irradiation leads to the release and availability of bioactive TGF-β leading to cell-cycle arrest, induction of apoptosis, and/or immunosuppression.24,46-48 We therefore determined in irradiated recipients of hematopoietic stem cell transplants (HSCTs) the consequences of TGF-β signaling on TEC support of thymopoiesis early after transplantation. To this end, 7- to 8-week-old TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice were lethally irradiated, received transplants of T cell–depleted, syngeneic bone marrow cells, and were analyzed for thymopoiesis at different time points within the first 4 weeks after grafting. In the first 2 weeks, thymus cellularity was significantly increased in TGFβRIIlox/lox::Foxn1-Cre mice, yet displayed a normal frequency of the different subpopulations compared with control mice that underwent transplantation (Figure 5C,D). In parallel, a significant increase in TEC cellularity was noted in TGFβRIIlox/lox::Foxn1-Cre mice 4 and 9 days after transplantation (Figure 5B). Four weeks after irradiation and bone marrow transplantation, total thymic cellularity and the frequency of the different thymocyte subpopulations were identical for both groups of recipient mice and comparable with that of naive animals (Figure 5A and data not shown). These results suggested that a lack of TGF-β signaling protected from radiation-induced cell injury. Despite the accelerated kinetics in thymopoiesis, TGFβRIIlox/lox::Foxn1-Cre mice displayed 4 weeks after transplantation a mild yet statistically not significant increase in splenic T cells compared with TGFβRIIlox/lox controls (9.9 ± 0.6 × 106 vs 8.9 ± 0.5 × 106 CD4+ T cells and 2.4 ± 0.6 × 106 vs 2.0 ± 0.3 × 106).
Enhanced thymopoiesis in TGFβRIIlox/lox::Foxn1-Cre and recipient mice of HSCT early after irradiation. (A) Total thymic cellularity and (B) TEC cellularity in lethally irradiated recipients of T-cell–depleted bone marrow cells at different time points after transplantation (TEC cellularity of unconditioned mice that did not undergo transplantation was 0.42 × 106 ± 0.07 × 106 vs 0.41 × 106 ± 0.07 × 106, P > .05, for TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox, respectively). (C) The relative distribution of thymocytes according to CD4 and CD8 expression and (D) the absolute numbers of the 4 CD3−CD4−CD8− subpopulations (DN1-4) were determined in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice 2 weeks after lethal irradiation (950 cGy) and transfer of 107 T cell–depleted bone marrow cells. Error bars represent SD, and percentages on plots in panel C are of total cells within gate.
Enhanced thymopoiesis in TGFβRIIlox/lox::Foxn1-Cre and recipient mice of HSCT early after irradiation. (A) Total thymic cellularity and (B) TEC cellularity in lethally irradiated recipients of T-cell–depleted bone marrow cells at different time points after transplantation (TEC cellularity of unconditioned mice that did not undergo transplantation was 0.42 × 106 ± 0.07 × 106 vs 0.41 × 106 ± 0.07 × 106, P > .05, for TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox, respectively). (C) The relative distribution of thymocytes according to CD4 and CD8 expression and (D) the absolute numbers of the 4 CD3−CD4−CD8− subpopulations (DN1-4) were determined in TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice 2 weeks after lethal irradiation (950 cGy) and transfer of 107 T cell–depleted bone marrow cells. Error bars represent SD, and percentages on plots in panel C are of total cells within gate.
Discussion
Although there is a wealth of information concerning the effect of TGF-β signaling on T-cell development, only relatively little is known regarding the in vivo role of TGF-β for TEC development and function. The present study presents insight into the contributions of TGF-β signaling under both steady-state conditions and after lethal irradiation. Here, we demonstrate that the loss of TGF-β signaling in TECs is not essential for thymus organogenesis but impacts (1) on total thymocyte cellularity as a function of age, (2) on the pool of mature thymocytes, (3) on the phenotype, proliferation, and cellular composition of the thymic epithelial compartment, and (4) on the TECs' capacity to support early reconstitution of thymopoiesis after irradiation.
An important and complex role has been assigned to TGF-β signaling for regulating the expansion, activation, and effector functions of mature T cells in peripheral lymphoid organs and target tissues during an adaptive immune response.49 Although the profile of thymic TGF-β and TGFβRII expression during organogenesis and in the adult mouse would also have suggested a central role of this ligand-receptor pair for the formation and function of the thymic microenvironment, TGF-β signaling is largely dispensable for TEC differentiation and thymopoiesis (Chen et al24 and this report). The formation of the pharyngeal apparatus involves coordinated actions between neural crest cells and cells of ectodermal, endodermal, and mesodermal origin.50 Moreover, TGF-β signaling may be required in the third pharyngeal arch for nonepithelial cells that influence TEC development and function. Indeed, neural crest stem cells are responsive to TGF-β signaling and, consequently, adopt different cell fates.51-53 Ablation of TGFβRII-mediated signaling in neural crest cells alters their postmigratory differentiation, consequently reducing the perithymic condensation of mesenchyme and diminishing the detection of neural crest–derived cells in the established thymic cortex.54 Interestingly, these morphologic changes correlate with a significant decrease in thymus size and other defects and are consistent with a proposed role of neural crest cells in thymus organogenesis.54,55 Thus, the requirement for TGF-β during thymus organogenesis is independent of direct signaling to TECs but appears to be indirect via the involvement of neural crest cells and mesenchymal-epithelial cross-talk during thymus organogenesis.2
Whereas previous studies could not exclude the possibility that functional loss of a single isoform of TGF-β is replaced by other isoforms or placental transfer,56 the conditional ablation of TGFβRII in TECs excluded such a redundancy and resulted in newborns in a phenotype identical to that previously described for young TGF-β1–deficient mice.24 Moreover, the high efficiency in deleting exon 3 excludes the possibility that a high frequency of TECs from TGF-βRIIlox/lox::Foxn1-Cre and TGF-β RIIlox/lox::Hoxa3-Cre mice maintain TGFβRII expression and hence account for the observed phenotype.
The molecular mechanisms operational in the process of senescence-related thymic involution are not precisely known despite their relevance for the loss of immunologic competence in the aged. Indications that TGF-β signaling may be involved in this process have been largely circumstantial, such as increased TGF-β steady-state mRNA levels in the aging human thymus.40 More recently, haploinsufficiency for TGF-β2 was shown to be associated with a decreased rate of thymic involution. Although this observation has been attributed to TGF-β's action on early thymopoiesis, bone marrow transplantation experiments suggest the possibility that the noted decrease in thymic involution was at least in part the consequence of altered functions of the thymic stroma.57 The data presented here are clearly in support of the view that TGF-β signaling in TECs is directly associated with the phenomenon of thymic involution. Indeed, TECs deficient in TGF-β signaling retain a higher proliferative capacity that correlates with an increased representation of these cells in TGFβRIIlox/lox::Foxn1-Cre mice. Yet, the overall structure of the thymic microenvironment was unaffected despite the lack of TGF-β signaling in TECs (Figure S5). Therefore, the observation of a mitigated thymic involution in the mutant mice suggests that the enlarged stromal compartment is able to host an increased number of thymocytes. However, besides its role in regulating cell cycle and apoptosis,44 TGFβ also affects the expression of factors controlling growth, differentiation, and chemotaxis.58-60 Studies are now under way to identify the molecular consequences of abolished TGF-β signaling in TECs.
The thymocyte-to-TEC ratio decreased with age in both mouse strains (data not shown), arguing for the fact that TGF-β is not uniquely responsible for the phenomenon of thymic involution. The corollary between the loss of TGF-β signaling in TECs and thymocyte development was subtle but yet caused a consistently significant accumulation of mature single-positive thymocytes. This increase is unlikely to be caused by T cells recirculating from the periphery to the thymus but is rather reflective of an accumulation of mature postselection thymocytes, because these cells are low in their expression of CD24, CD69, and CD44 but display high surface concentrations of CD62L.61 A higher proliferative rate of mature thymocytes, which would have accounted for their increased frequency, could also be excluded, suggesting a local retention of these mature cells within the thymic microenvironment. In support of this conclusion are 2 additional observations: the increased detection of these phenotypically mature thymocytes in TGFβRIIlox/lox::Foxn1-Cre mice as young as 5 days of life, and the significantly smaller fraction of mature thymo-cytes that is exported to the periphery in the young adult TGFβRIIlox/lox::Foxn1-Cre mice. The observed alterations in the composition and size of the thymic microenvironment provide an additional explanation for changes in thymopoiesis in TGFβRIIlox/lox::Foxn1-Cre mice because an enlarged (and possibly functionally altered) stromal scaffold is likely to accommodate more and possibly different thymocytes.
After irradiation, thymocytes release high concentrations of TGF-β resulting in an immunosuppressive milieu,47 which may not only affect thymocyte differentiation directly but may also influence stromal cell types. Indeed, exposure of thymic epithelia to TGF-β modulates the production of cytokines important for T-cell development.59 Our findings in TGFβRIIlox/lox::Foxn1-Cre mice are in keeping with such a mechanism. Furthermore, they suggest that the pharmacologic inhibition of TGF-β activity could constitute a novel component of a conditioning regimen to hasten thymopoietic activity after hematopoietic stem cell transplantation. This assertion is additionally supported by our preliminary observations that a systemic inhibition of TGF-β enhances thymic reconstitution.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank K. Hafen, A. Peter, and E. Christen for expert technical help, and U. Schaller for excellent secretarial assistance.
This work was supported by the Swiss National Science Foundation (Berne, Switzerland) grants 3100-68310.02 (G.A.H.), by a grant from the European Community 6th Framework Programs Euro-Thymaide Integrated Project (Liege, Belgium; G.A.H.), by National Institutes of Health (Bethesda, MD) grant ROI-A1057477-01 (G.A.H), and by a grant from the Roche Research Foundation (Basel, Switzerland; M.M.H.-H.).
National Institutes of Health
Authorship
Contribution: M.M.H.-H. and G.A.H. designed the work and wrote the paper; M.M.H.-H., S.Z., M.P.K., L.T.J., and T.B. performed work; and A.M.M. and J.R. provided materials.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Georg A. Holländer, Laboratory of Pediatric Immunology, Center for Biomedicine, University of Basel and The University Children's Hospital (UKBB), Mattenstrasse 28, 4058 Basel, Switzerland; e-mail: georg-a.hollaender@unibas.ch.

![Figure 1. Thymic development and lymphopoietic function in the absence of TGF-β signaling in thymic stromal cells. (A) Gross anatomic, histologic, and flow cytometric analysis of thymic tissue from 2-week-old TGFβRIIlox/lox::Foxn1-Cre and TGFβRIIlox/lox mice. (i,ii) Thymus in situ (T indicates thymus; H, heart; and L, lung). (iii,iv) H&E staining (magnification 4×). (v-x) Immunofluorescence microscopy; magnification, 20×. Cortical (v,vi) and medullary (vii-x) regions, stained for cytokeratin 8 (v-viii), cytokeratin 5 (v-x), ERTR7 (v-viii), and UEA-1 (ix,x). (xi,xii: representative fluorescence-activated cell sorting [FACS] profile for the cell surface expression of CD4 and CD8 [mean ± SD of 6 mice analyzed per group]). Percentages on plots are of total cells within gate. (B) Genomic deletion analysis of sorted thymic dendritic cells (CD45+ MHCII+, left lane) and epithelial cells (CD45− MHCII+, right lane, purity ≥ 95) isolated from 4-week-old TGFβRIIlox/lox::Foxn1-Cre mice. The upper band denotes the undeleted allele, whereas the lower band represents the deleted allele. (C) Hoxa-controlled Cre expression pattern. Top: Sagittal section of an E10.25 Rosa26lacZ::Hoxa3-Cre embryo, assayed for β-galactosidase expression and counterstained with nuclear fast red; PA indicates pharyngeal arch. Bottom: FACS analysis of E13.5 epithelial (left panel) and nonepithelial (right panel) thymic stromal cells from Z/EGLobe21 and Z/EGLobe21::Hoxa3-Cre mice for GFP expression. Percentages on graphs are of total cells within gate. (D) Thymocyte development in E12.5 FTOC of thymic lobes isolated from TGFβRIIlox/lox::Hoxa3-Cre and TGFβRIIlox/lox E12.5 embryos after a 12-day culture period. Representative dot blots of flow cytometric analysis for the cell surface expression of CD4 and CD8 (n = 3 for TGFβRIIlox/lox::Hoxa3-Cre; n = 4 for TGFβRIIlox/lox). Percentages on plots are of total cells within gate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/3/10.1182_blood-2007-10-115618/4/m_zh80150821260001.jpeg?Expires=1766005100&Signature=FJ49HzR74Wo4eIsd3FR1jWjKKr6lRYTqJFOXGh9s3p~Z4X5dwvLf4~B1HcwbMcJ-SQQuv-Fs5-TmyYVAeC7eJYUveoTG4XnnSAfNAund8-zS982dO9HgtdAYjkTpHg2BBnr6SDAVdNfAXPQIGC3frEc6~pY8PXEjHTj-peOzlguAk~xnsau7qGp1d4XbSXCCHJb3v7Q1bQBOqxr68hyp16WfX4GC9irDs3fRFNi5X1YuTKh2trTioFTqZ~9EwbQrKWg6wyEYSCBOdwmNyg1Juu-vTCj6h5grTMif7HEkdQhEjKG5uMZ3sQavpbk9XDbKZQ5Mt2y8NjstRVFCTwz2AQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal