Abstract
Natural killer (NK) cells were discovered more than 30 years ago. NK cells are large granular lymphocytes that belong to the innate immune system because unlike T or B lymphocytes of the adaptive or antigen-specific immune system, NK cells do not rearrange T-cell receptor or immunoglobulin genes from their germline configuration. During the past 2 decades there has been a substantial gain in our understanding of what and how NK-cells “see,” lending important insights into their functions and purpose in normal immune surveillance. The most recent discoveries in NK-cell receptor biology have fueled translational research that has led to remarkable results in treating human malignancy.
Introduction
Mother Nature is a highly efficient force with a “take-no-prisoners” approach toward biologic remnants that no longer support the survival of the species in the ever-changing world of pathogens. Cytolytic effector cells that resemble natural killer (NK) cells have been part of the innate immune defense system long before the arrival of the seemingly more sophisticated T and B cells of the adaptive immune system approximately 500 million years ago.1 Yet today, all 3 of these lymphocyte lineages survive with NK cells outnumbering B cells in the circulation by a 3-to-1 ratio and with newly discovered functional complexity that rivals their antigen-specific memory-bearing counterparts. Clearly, NK cells must serve a very important role in host defense or they would not be here. The few reports of complete NK-cell deficiencies in humans, each resulting in overwhelming fatal infection during childhood, lend further support to this notion.2 This review attempts to bring the interested readership of Blood up to date on some of the advances in our understanding of human NK-cell biology over the past 2 decades and their relevance to human host defense against infection and malignant disease. The review draws on experimental animal systems as may be necessary in instances where discoveries can thus far be interpreted only in that context.
What is an NK cell?
Years ago, the histologic and functional definition of an NK cell was that of a large granular lymphocyte that could kill a target cell “naturally,” that is, in a spontaneous fashion that did not require any priming and was not restricted by the target cell's expression of major histocompatibility complex (MHC) molecules.3-5 Experiments in mouse models of bone marrow graft rejection6,7 led to the proposal that NK cells would kill any target that lacked self–major histocompatibility complex (MHC) class I molecules (the “missing self” hypothesis).8 This extraordinary idea was developed before anyone knew what the NK cell was using to “see” its targets. It is now clear that NK cells have a multitude of inhibitory and activating receptors that engage MHC class I molecules, MHC class I–like molecules, and molecules unrelated to MHC. Thus, NK cells are indeed restricted in what target cells they can engage by the expression of the target's MHC ligands, but in a very complex fashion that remains incompletely understood. Notably, orthologs of more recently discovered NK-cell receptor families cannot be found beyond mammals,9,10 suggesting that the composite modern day NK cell emerged well after T and B cells appeared to define the vertebrate adaptive immune system. Furthermore, the complementary roles that NK and cytolytic T cells have in target recognition and host defense, and their similar mechanisms of cytolysis, suggest that these 2 cell types may have each evolved from a common ancestral cytolytic effector cell. Finally, a subset of human NK cells produce abundant cytokines with modest or no ability to lyse target cells. Thus, the older idea of an NK cell as an ancestral forerunner11 or as a cell defined by a simple function no longer applies.
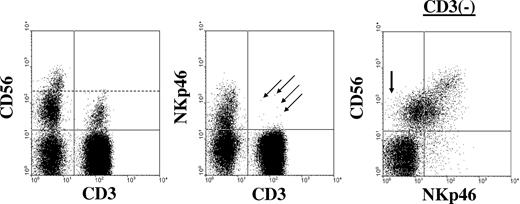
The traditional cell surface phenotype defining human NK cells within the lymphocyte gate on the flow cytometric analyzer shows an absence of CD3 (thereby excluding T cells) and expression of CD56, the 140-kDa isoform of neural cell adhesion molecule (NCAM) found on NK cells and a minority of T cells (Figure 1).12,13 In contrast, murine NK cells do not express CD56, and Vivier and colleagues have recently suggested that NKp46, a member of the highly conserved natural cytotoxicity receptor (NCR) family of NK-activating receptors,14 best defines NK cells across species (Walzer et al10 ). Nevertheless, under close examination NKp46 can be found on a small subset of human cytolytic T lymphocytes.15,16 Conversely, some CD56+CD3− cells have low-density expression or may even lack expression of NKp46 (Figure 1); it will be interesting to determine the precise nature of these minority of cells. The search will no doubt continue for a sensitive and truly specific pan–NK-cell marker. Until this is found, the phenotypic definition of NK cells will continue to be determined by their expression of a unique combination of non–NK-restricted surface antigens.
Human NK cells are CD3−CD56+NKp46+. Shown is a representative example of immunophenotypic analysis from a healthy donor when gating on peripheral blood lymphocytes. NK cells lack expression of CD3 and coexpress CD56 and NKp46. The left and middle dot plots were gated on total lymphocytes using forward scatter versus side scatter parameters. The dashed line in the left dot plot separates CD56dim from CD56bright NK cells. The arrows in the middle plot indicate a few CD3+ cells that coexpress NKp46. The right dot plot is gated on CD3− lymphocytes and demonstrates the 2 major NK-cell populations in the blood: CD56brightNKp46bright and CD56dimNKp46dim. The arrow in the first quadrant of the right dot plot highlights a few CD56dim NK cells that likely lack expression of NKp46.
Human NK cells are CD3−CD56+NKp46+. Shown is a representative example of immunophenotypic analysis from a healthy donor when gating on peripheral blood lymphocytes. NK cells lack expression of CD3 and coexpress CD56 and NKp46. The left and middle dot plots were gated on total lymphocytes using forward scatter versus side scatter parameters. The dashed line in the left dot plot separates CD56dim from CD56bright NK cells. The arrows in the middle plot indicate a few CD3+ cells that coexpress NKp46. The right dot plot is gated on CD3− lymphocytes and demonstrates the 2 major NK-cell populations in the blood: CD56brightNKp46bright and CD56dimNKp46dim. The arrow in the first quadrant of the right dot plot highlights a few CD56dim NK cells that likely lack expression of NKp46.
Where and how do human NK cells develop?
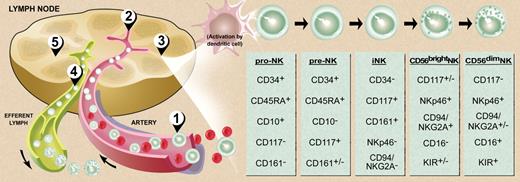
NK cells are believed to be relatively short-lived, and at any one time there are likely more than 2 billion circulating in an adult.17 Thus, one would think that the site of human NK-cell development producing such vast numbers of cells to maintain homeostasis would be obvious. While it is clear that NK cells are part of the hematopoietic system and are derived from CD34+ hematopoietic progenitor cells (HPCs),18-20 the site(s) of maturation and details of the process are only now beginning to emerge. Human T cells develop in the thymus and human B cells develop in the bone marrow, and the developmental intermediates for each of these 2 lymphocytes populations can be isolated in situ from their respective maturational sites. In contrast, attempts to characterize the full NK-cell developmental pathway from CD34+ HPCs within the bone marrow (or in the thymus) have not been successful. A first clue that NK development might not occur wholly in the bone marrow came from the observation that a subset of NK cells, termed CD56bright for their high-density surface expression of CD56, could be isolated from lymph nodes and tonsils (ie, secondary lymphoid tissue or SLT). CD56bright NK cells are relatively dominant in SLT compared with their more abundant CD56dim NK counterpart found in bone marrow, blood, and spleen.21 It had been postulated that this CD56bright NK population was less mature than the CD56dim subset,22 and activation of SLT CD56bright NK cells increased expression of NK receptors that are characteristic of the CD56dim subset.23 A unique population of CD34+CD45RA+ pre-NK cells was next discovered at low frequencies in bone marrow and in blood (< 1% and < 10% of the total resident CD34+ populations, respectively), but was found to be highly and selectively enriched in SLT, making this the first CD34+ subset described in these tissues.24 Similar cells have subsequently been described in mucosa-associated lymphoid tissue in the gut.25,26 This selective enrichment of both CD34+CD45RA+ pre-NK cells and CD56bright NK cells within SLT relative to BM or blood, along with an abundance of dendritic cells (DCs) and other antigen presenting cells (APCs) that express membrane-bound IL-15,27 which is required for NK-cell maturation,28-30 suggested that SLT may be a site for NK-cell development in vivo. Indeed, a search in SLT for phenotypically and functionally distinct cell populations that represent stages along the NK- cell developmental pathway from CD34+CD45RA+ HPCs to CD3−CD56bright NK cells in situ was successfully undertaken (Figure 2).31,35 The findings for human NK-cell development in SLT are strikingly similar to those for T-cell development in the thymus in terms of stages, activation antigens, and the dynamics of precursor migration into and out of the primary site of development.31 The results of these developmental studies further suggest that the CD56dim NK-cell subset is derived directly from the CD56bright NK subset, supporting Lanier et al's original proposal22 and consistent with more recent definitive studies.32,36-38 Interestingly, one of these recent studies also revealed an important role for the CD56 molecule itself in promoting this terminal maturational step. Using an in vitro coculture system consisting of purified CD56bright NK cells and human fibroblasts, Chan et al demonstrated that antibody blockade of the interaction between CD56 and fibroblast growth factor receptor-1 significantly inhibited the generation of CD56dim NK cells.36
Model of human NK-cell development. (1) Bone marrow–derived CD34+CD45RA+ HPCs circulate in the blood and (2) extravasate across lymph node high endothelial venules to enter the parafollicular space. There, (3) pro-NK cells are activated to progress through distinct stages of maturation (far right) to create both CD56bright and CD56dim NK cells.31 Maturing CD56dim NK cells return to the circulation via the efferent lymph (4),32 whereas some CD56bright NK cells remain within the secondary lymphoid tissue to interact with DCs (5).21,23,33,34 Illustration by Debra T. Dartez.
Model of human NK-cell development. (1) Bone marrow–derived CD34+CD45RA+ HPCs circulate in the blood and (2) extravasate across lymph node high endothelial venules to enter the parafollicular space. There, (3) pro-NK cells are activated to progress through distinct stages of maturation (far right) to create both CD56bright and CD56dim NK cells.31 Maturing CD56dim NK cells return to the circulation via the efferent lymph (4),32 whereas some CD56bright NK cells remain within the secondary lymphoid tissue to interact with DCs (5).21,23,33,34 Illustration by Debra T. Dartez.
Are all NK cells the same?
Similar to T cells, the total population of human CD3−CD56+NKp46+ NK cells is phenotypically and functionally heterogeneous. Approximately 10% of NK cells found in blood (and nearly 100% in SLT) have high surface density expression of CD56 and can produce abundant amounts of cytokines and chemokines within minutes of activation, yet these CD3−CD56brightNKp46bright cells (Figure 1) have little or no ability to spontaneously kill tumor cell targets.39 In contrast, the majority of circulating human NK cells with low surface density or “dim” expression of CD56 and NKp46 have relatively lesser ability to produce cytokines in response to activation, while some but not all can spontaneously lyse susceptible tumor cell targets.10 Despite accumulating evidence to support the notion that progression from CD56bright to CD56dim NK cells is likely part of a continuum in their development,32,36-38 it also seems likely that CD56bright and CD56dim NK cells have relatively distinct and important roles during the human immune response (discussed in the following 3 sections).
What do NK cells do?
Thus far it has been fully appreciated that NK cells can secrete cytokines and chemokines that influence the host's immune response, and/or kill certain infected or transformed cells via perforin/granzyme or death receptor (eg, Fas, TRAIL)–related pathways.2,40-44 Interferon gamma (IFN-γ) is considered the prototypic NK-cell cytokine, and its production by NK cells is known to shape the Th1 immune response,42 activate APCs to further up-regulate MHC class I expression,45 activate macrophage killing of obligate intracellular pathogens,46 and have antiproliferative effects on viral- and malignant-transformed cells.47 For many of these functions, it would make sense for NK cells to be in close proximity to APCs and T cells. Indeed, the subset of NK cells that is the most potent producer of IFN-γ (ie, CD56bright NK) is primarily located in the parafollicular T cell– and APC-rich region of SLT.21
How is IFN-γ production by CD56bright NK cells regulated?
The CD56bright NK subset generally needs 2 signals to produce IFN-γ, and one of these almost always includes IL-12. The second can be IL-1, IL-2, IL-15 or IL-18, or engagement of an NK-activating receptor such as CD16 (FcγRIIIa) or NKG2D.39,48,49 Each of these cytokines may be released from monocytes, macrophages, and/or DCs along with IL-12, suggesting that the interaction between APCs and NK cells as may occur during early immune activation in the lymph node is likely important.33,50 Interestingly, NK-cell production of IFN-γ and other proinflammatory cytokines and chemokines, such as TNF-α and MIP-1α, respectively, can occur within minutes of monokine costimulation,51 distinguishing it from the more delayed response of T cells. The release of IFN-γ by NK cells can in turn further activate the APCs to up-regulate MHC class I and increase APC cytokine secretion. At the same time, these APCs also release IL-10 and TGF-β, which can temper and even diminish the NK cell's IFN-γ secretion.44,52
The CD56brightCD16+/− NK-cell subset constitutively and uniquely expresses the high-affinity heterotrimeric IL-2 receptor complex53,54 and as such, can successfully compete for picomolar concentrations of IL-2 that are released by activated T cells in SLT. Costimulation of CD56bright NK cells with T cell–derived IL-2 and monocyte or DC-derived IL-12 results in the NK-cell production of relatively large amounts of IFN-γ,21 which in turn can further activate APCs and shape the antigen-driven T-cell response.42 Hence, as a result of their localization near APCs and T cells in SLT, and their ability to produce IFN-γ, CD56bright NK cells have the capability to link the human innate- and antigen-specific immune response.
Recent advances in NK signaling have provided a molecular mechanism for the observation that the CD56bright NK cell is a relatively more potent producer of cytokines compared with the seemingly more mature CD56dim NK cell. For example, constitutive expression of the phosphatase SHIP1, which dampens cell signaling, is absent or distinctly lower in CD56bright NK cells compared with CD56dim NK cells. Monokine costimulation of CD56bright NK cells with forced overexpression of SHIP1 produces substantially lower IFN-γ.55 It is also notable that the secretion of cytokines does not appear to result exclusively from activation, but rather from reciprocal antagonism of activating and suppressing signals whose shift in balance depends on the circumstance. For example, when IL-12 and IL-18 or IL-15 coactivate NK-cell production of IFN-γ, they also deactivate the immunosuppressive TGF-β signaling cascade, and vice versa.52 In contrast, when exogenously administered IL-2 expands and activates human NK cells, it also expands T regulatory cells that have an immunosuppressive effect on NK cells.56 As is detailed later, this alteration in balance of activating and inhibitory signaling also applies when the CD56dim cytolytic NK cell recognizes its target as friend or foe.
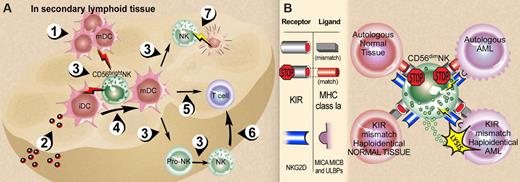
The proximity of CD56bright NK cells to DCs in human SLT and the immunoregulatory role of each cell type via their cytokine secretion have led to increasing interest in the potential interactions between NK cells and DCs.33,34 It seems quite plausible that it is the membrane-bound IL-15 expression on DCs that is important for NK-cell development, survival, activation, and expansion in SLT, while NK cells appear to have a role in regulating DC maturation via TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion,34 as well as a negative regulatory role. Human NK cells use their NKp30 activating receptor to kill autologous immature DCs, likely in cooperation with other activating NK receptors whose ligands are expressed on immature DCs within lymph nodes.33,57 It has been postulated that this NK killing of immature DCs might be an important editing process to restrict DC maturation, which in turn would limit their migration and priming of T cells in SLT.33 Some NK cell–DC interactions in SLT are summarized in Figure 3A.
CD56bright and CD56dim NK-cell interactions. (A) NK-DC interactions in secondary lymphoid tissue (SLT). (1) Activated mature DCs (mDCs) enter SLT from periphery or (2) immature DCs (iDCs) receive pathogens within SLT. Each express and/or secrete a variety of cytokines (3) that are required for NK-cell maturation and survival (eg, DC IL-15) and NK cell proinflammatory cytokine production (eg, DC IL-12 in combination with DC IL-1, IL-15, IL-18). Activated CD56bright NK cells in turn secrete TNF-α and GM-CSF that contribute to DC maturation, (4) and IFN-γ that contributes to DC activation and thus indirectly to antigen-specific T-cell priming (5). NK-cell IFN-γ also contributes directly to T-cell priming (6). NK cells can kill immature autologous DCs (7) via NKp30, which may assist in editing out hyporesponsive DCs or by limiting T-cell priming.33,34 (B) Summary of NK-cell recognition. The functional consequences of NK-cell receptor recognition depend on the integration of both inhibition and activation signals received in response to engagement of target cell ligands.58,59 Upper left: normal autologous tissues are not attacked because the predominant signal is recognition of self-MHC class Ia ligands by inhibitory KIRs (and other inhibitory receptors such as NKG2A/CD94 recognizing their ligands, not shown) in the absence of ligands for activating NK receptors. Upper right: Malignant autologous tumors such as acute myeloid leukemia (AML) have high-density surface expression of classical MHC class Ia and nonclassical MHC class I that that bind to KIR and NKG2A/CD94, respectively, and dominate over engagement of NK-cell activation receptors with their cognate ligands. Lower left: Normal allogeneic host tissue presumably lacks ligands that engage dominant activating NK receptors such as NKG2D and NCR, despite a mismatch of donor NK KIR with host MHC class Ia as well as donor NKG2A/CD94 and host HLA-E (not shown). Lower right: A mismatch of donor NK KIR and host MHC class Ia in the presence of ligand-engaged NKG2D, NCR, and other NK activation receptors60 likely contributes the dominant NK response of target cell lysis.48,61,62 Illustration by Debra T. Dartez.
CD56bright and CD56dim NK-cell interactions. (A) NK-DC interactions in secondary lymphoid tissue (SLT). (1) Activated mature DCs (mDCs) enter SLT from periphery or (2) immature DCs (iDCs) receive pathogens within SLT. Each express and/or secrete a variety of cytokines (3) that are required for NK-cell maturation and survival (eg, DC IL-15) and NK cell proinflammatory cytokine production (eg, DC IL-12 in combination with DC IL-1, IL-15, IL-18). Activated CD56bright NK cells in turn secrete TNF-α and GM-CSF that contribute to DC maturation, (4) and IFN-γ that contributes to DC activation and thus indirectly to antigen-specific T-cell priming (5). NK-cell IFN-γ also contributes directly to T-cell priming (6). NK cells can kill immature autologous DCs (7) via NKp30, which may assist in editing out hyporesponsive DCs or by limiting T-cell priming.33,34 (B) Summary of NK-cell recognition. The functional consequences of NK-cell receptor recognition depend on the integration of both inhibition and activation signals received in response to engagement of target cell ligands.58,59 Upper left: normal autologous tissues are not attacked because the predominant signal is recognition of self-MHC class Ia ligands by inhibitory KIRs (and other inhibitory receptors such as NKG2A/CD94 recognizing their ligands, not shown) in the absence of ligands for activating NK receptors. Upper right: Malignant autologous tumors such as acute myeloid leukemia (AML) have high-density surface expression of classical MHC class Ia and nonclassical MHC class I that that bind to KIR and NKG2A/CD94, respectively, and dominate over engagement of NK-cell activation receptors with their cognate ligands. Lower left: Normal allogeneic host tissue presumably lacks ligands that engage dominant activating NK receptors such as NKG2D and NCR, despite a mismatch of donor NK KIR with host MHC class Ia as well as donor NKG2A/CD94 and host HLA-E (not shown). Lower right: A mismatch of donor NK KIR and host MHC class Ia in the presence of ligand-engaged NKG2D, NCR, and other NK activation receptors60 likely contributes the dominant NK response of target cell lysis.48,61,62 Illustration by Debra T. Dartez.
Functional characterization of circulating NK cells
In contrast to the prevalence of CD56bright NK cells in SLT, the majority of circulating NK cells are CD56dim cells that display significantly higher cytolytic capacity against tumor target cells. CD56dim NK cells also abundantly express CD16 or the low-affinity Fcγ receptor IIIA, which can bind to the constant (Fc) region of immunoglobulin when immobilized on a cell surface. This receptor-ligand binding is followed by a CD16-mediated activation signal that results in NK-cell degranulation and perforin-dependent target cell lysis called antibody-dependent cellular cytotoxicity (ADCC). An experimental mouse model has lent strong support to the notion that this NK-mediated ADCC is a dominant component of effective antitumor activity seen when rituximab and trastuzumab are used for treatment of lymphoma and breast cancer, respectively.63 Similar to both NK cytokine release and NK killing, CD16-mediated ADCC is tempered by inhibitory Fc receptors expressed on APCs, as the absence of these inhibitory Fc receptors improves the efficacy of ADCC in vivo.63 This provides another example where striking a balance between activation and inhibitory signaling is important in regulating the innate immune response.
Perhaps the most compelling evidence for the importance of the CD56dimCD16+ human NK-cell subset is an experiment performed in collaboration with Mother Nature. It has been noted and confirmed that patients with CD20+ follicular lymphoma fared better when treated with the humanized anti-CD20 monoclonal antibody rituximab if their FcγRIIIa gene had a polymorphism that resulted in higher affinity for rituximab and enhanced ADCC activity in vitro.64,65 A phase 2 clinical trial was therefore undertaken to expand and activate CD16+ NK cells in vivo using IL-2 during and after rituximab infusion for follicular lymphoma patients who had relapsed shortly after receiving rituximab alone or had rituximab-refractory disease.66 The study failed to show any evidence of efficacy, but this could have been related to the fact that similar doses of IL-2 have been shown to substantially expand CD4+ regulatory T cells that have an immunosuppressive effect on NK-cell function.56,67,68
How is natural cytotoxicity by CD56dim NK cells accomplished and regulated?
CD56dim NK cells can efficiently lyse target cells in the absence of prior stimulation and without the need for antibody recognition. As noted in the second section of this review, the missing self hypothesis predicts that NK cells will become uninhibited and lyse target cells when expression of MHC class I is lost or deficient on the target cells.8 We now know that while inhibition may be lost, activation and consequent killing are not guaranteed. Rather, target cell recognition by NK cells is complex and involves alteration in the balance between activating and inhibitory signals that are simultaneously delivered to NK cells following the engagement of ligands by several distinct families of NK receptors.48,58,59
A detailed review of the inhibitory and activating families of NK receptors and their respective ligands was recently published by Long and colleagues (Bryceson et al48 ); this topic is reviewed briefly here. It may be enough to say that under normal circumstances of immune surveillance of self, human NK cells have inhibitory receptors that recognize MHC class I molecules as their cognate ligands on virtually every cell in the body. These receptors include the inhibitory killer immunoglobulin-like receptors (KIRs) that bind to classical MHC class Ia ligands (HLA-A, B, and C), and the inhibitory CD94-NKG2A heterodimeric receptors that bind the nonclassical MHC class Ib (HLA-E, discussed in the next paragraph). In this system, every NK cell with killing capacity must have an inhibitory receptor that recognizes as least one of the MHC class I gene products on the cell's surface, and this does indeed appear to be the case.69 Once engaged with classical MHC class I, the inhibitory KIR sends a signal to the NK cell that dominates over any activating signal and prevents the NK cell from killing the MHC class I–expressing cell (Figure 3B).48,58,59 Interestingly, although more than a dozen individual KIR molecules have been identified in the human genome, each individual NK cell expresses only a fraction of the available KIRs.69 Thus, there is a varied pattern of KIR expression that allows for a diverse NK-cell repertoire capable of sensing minute changes in MHC class I expression,9 at least in part due to KIR gene silencing by DNA methylation.70,71 Indeed, the regulation of this receptor system is currently a major focus of ongoing research.72 It would suffice to say that recognition of MHC class I ligands by inhibitory NK receptors is a critical part of NK-cell education or maturation and is required for NK cells to recognize missing self-MHC class I.73
If NK recognition of host pathogens was as simple as noting the absence or mutations of self-MHC class I molecules, one would expect class I–deficient people to have NK cell–mediated autoimmune disease, but they do not.74-76 Further, one would expect severe graft-versus-host disease (GVHD) when stem cell transplant patients are given T cell–depleted grafts with donor NK cells that do not recognize host MHC class I ligands, yet GVHD is not seen.61 Three reasons for this are already apparent. First, as discussed in the preceding paragraph, it seems that if an individual lacks MHC class I, their matching inhibitory receptors have never been engaged, leaving the NK cell in a virgin or uneducated state, unable to effectively recognize and kill target cells that lack MHC class I.74-76 Second, in addition to KIRs, there are other inhibitory receptors expressed on the surface of NK cells that, when engaged by their cognate ligands, also dampen killing and cytokine responses.69,75,77 One mentioned in the previous paragraph is the CD94-NKG2A heterodimeric C-type lectin-like receptor that recognizes the nonclassical MHC class Ib HLA E molecules. The groove within the HLA-E molecule actually holds leader peptides that are derived from both classical and nonclassical HLA types and is recognized by CD94/NKG2A. In this way, the NK cell surveys its HLA-E–expressing target using its inhibitory CD94/NKG2A as a sensor to assess the net overall target cell expression of total MHC class I, something that can be down-modulated by viral infection or malignant transformation.78 Another inhibitory NK receptor is the C-type lectin-like receptor NKR-P1A (CD161) that interacts with lectin-like transcript-1, a host encoded non-MHC ligand.79,80 Thus, while classical MHC class Ia expression is important for KIR-mediated self-tolerance, its absence still leaves a backup of other inhibitory receptor-ligand systems to help the NK cells determine whether tolerance of the host tissue is appropriate. Interestingly, each of the NK inhibitory receptors share the same signaling mechanism, and this involves the recruitment of the phosphatase Src homology 2 domain-containing phosphatase 1 (SHP-1) to the receptors' phosphorylated, cytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs).81,82 The relevance of these inhibitory receptor systems in host immunity can be appreciated in the mechanistic study of successful viral immune evasion. For example, RCTL is a lectin encoded by rat cytomegalovirus (CMV), and is a ligand for the rat NK cell's CD161. By engaging CD161, the host's NK responsiveness to the viral infection is diminished.83 A comparable scenario in human CMV infection has also been described.84
The third reason why MHC class I–deficient cells or normal tissues of allogeneic transplant patients receiving donor KIR-MHC class I–mismatched NK cells are not susceptible to NK- cell lysis relates to the dynamic equilibrium between inhibitory and activating processes that exists for cytolysis.58,59 As mentioned in the previous paragraph, the simple absence of MHC class I is not necessarily sufficient to confer cytolysis, implying that for killing to occur there must be activating receptors on NK cells whose affinity for target cell ligands dominates over the NK cell's inhibitory signals. Indeed, a number of activating receptors have now been characterized on NK cells.48 These receptors include NKG2D,85 the NCR,86 the nectin and nectin-like receptors,87 and NKp80.88 In addition, NK cells are also equipped with KIR and CD94/NKG2 receptors that have intracytoplasmic tails that can confer activation rather than inhibitory signals.48 Under normal circumstances, receptor affinity for their respective classical and nonclassical MHC class I ligands are lower than their inhibitory receptor counterparts, and this allows for the inhibitory signal to dominate.89-91
NKG2D is perhaps one of the best characterized among the activating NK-cell receptors. It is a C-type lectin-like receptor that is expressed on the surface of all human NK cells and recognizes at least 6 ligands, each of which has MHC class I homology. Three of these are transmembrane proteins (MICA, MICB, and ULBP4),85,92 and 3 are glycophosphatidylinositol (GPI)–anchored proteins (ULBP1-3).93 Upon receptor-ligand interaction, NKG2D phosphorylates an adaptor protein that recruits and activates phosphatidylinositol 3 (PI-3) kinase, which in turn results in perforin-dependent cytotoxicity.94,95 Importantly, these NKG2D ligands are not expressed on normal tissues, but rather are induced during times of genotoxic or cellular stress as is seen with viral and malignant transformation.96 Thus, normal tissues expressing self-MHC class I ligands that are matched with at least one of their NK cells' inhibitory NK receptors lack expression of NKG2D ligands and are therefore not susceptible to autologous NK-mediated killing (Figure 3B). Likewise, normal host tissues that have a mismatch between self-MHC class I ligands and donor NK cells' inhibitory KIRs also lack NKG2D ligands and thus are not susceptible to NK killing. The latter likely provides at least a part of the explanation for the absence of GVHD in the scenario of mismatched T cell–depleted transplantation described in the third paragraph of this section and detailed in the next section (Figure 3B).
There are good in vivo experimental data demonstrating that NKG2D is critical for effective containment of primary tumor and tumor metastasis, and Smyth et al have elegantly shown a function for this recognition pathway in host protection from de novo chemical-induced tumorigenesis.97 Perhaps the most impressive data regarding the importance of the NKG2D receptor in human immune surveillance come from the examination of epithelial cancers that shed MICA/B ligands into the serum; these soluble ligands down-modulate NKG2D expression on NK cells and cytolytic T cells with consequent reduction in their cytolytic effectiveness,98-101 while costimulating the expansion of NKG2D+ immunosuppressive T cells.102 Similar modulation of the NKG2D receptor-ligand system to promote tumor evasion can be induced by regulatory T cell–associated or tumor-associated TGF-β expression.103,104 It is interesting to note that while NK cells survey for the absence or down-regulation of MHC class I on host cells, and cytolytic T cells seem to complement this by surveying for the alteration of peptide presentation on host cells with abundant self-MHC class I,105 successful tumors exploit their common vulnerability and disarm both of these cytolytic effector systems by targeting NKG2D.
How can all this knowledge be used in the clinical setting?
The most striking evidence to date in support of NK cells as true antitumor effector cells in humans comes from the full haploidentical T cell–depleted stem cell transplantation studies conducted by Velardi and colleagues at the University of Perugia, Italy (Ruggeri et al61 ). It has been known for more than 35 years that NK cells have a predilection for killing hematopoietic cells in the experimental settings of mismatched bone marrow transplantation (then termed hybrid resistance),6,7 and the evidence for human NK alloreactivity was first put forth by Moretta and colleagues in 1988 (Ciccone et al106 ). It was subsequently determined that the alloreactivity in the murine model of hybrid resistance resulted from a mismatch between NK inhibitory receptors and their MHC class I ligands (ie, missing self),107 as well as the presence of activating ligands such as NKG2D on the hematopoietic cells being rejected.108 When the inhibitory KIRs were identified on human NK cells,109,110 it was realized that donor cell recognition of missing self on a recipient's tumor cell could result from purposefully mismatching the donor's NK inhibitory KIR and its cognate self-MHC class I ligand on the recipient.111 The challenge and opportunity to exploit this new biology for clinical purposes could now be realized.
The patient and donor selection criteria required to ensure missing self and consequent NK alloreactivity have been reviewed in detail elsewhere62,112 and are based upon the original observation that polymorphisms at MHC class-1a genetic loci influence NK- cell alloreactivity.109 The greatest likelihood of generating an NK KIR-MHC class I mismatch occurs in the setting of full HLA haploidentical-mismatched T cell–depleted hematopoietic stem cell transplantation, and the malignant disease most likely to be susceptible to NK-mediated graft-versus-leukemia is one derived from the hematopoietic system, acute myeloid leukemia (AML). As noted above, the presence of a mismatch between the donor NK cell KIR repertoire and MHC class I expression by recipient AML blasts is not necessarily sufficient to ensure NK-cell activation and tumor cell lysis, but rather is likely required in combination with an activation signal processed through NKG2D and/or any of the NCRs.48 Even prior to a transplantation preparative regimen, AML blasts have weak expression of ligands that bind to and activate NKG2D, and are susceptible to killing via the NCR and other activating NK receptors.60,113 Importantly, the activating ligands for the NCR remain incompletely defined.
The Perugia Bone Marrow Transplant Center recently reported on the results of 112 haploidentical T cell–depleted transplants for high-risk AML. The data clearly show a highly statistically significant survival advantage for patients receiving T cell–depleted full haploidentical transplants where the NK alloreactivity exists in the graft-versus-leukemia direction compared with transplants without such alloreactivity.61,112 Further, the event-free survival of the NK alloreactive cases are comparable with the best results using HLA-matched unrelated and cord blood donors, options that were likely not available to the majority of these patients. The near complete absence of morbidity and mortality from GVHD is likely multifactorial and explained by (1) the low burden of donor T cells infused with the graft; (2) the absence or low-level expression of activating NK receptor ligands (eg, MICA/B and ULBPs) on the cell surface of host skin, gut, and liver; and (3) the experimental evidence showing that donor haploidentical NK cells kill recipient DCs, thus preventing the priming of donor T cells that is required for GVHD.114 These results provide a wonderful example of how understanding basic receptor biology can lead to new therapeutic strategies with results that improve peoples' lives. Given the major role that the NCRs appear to have in NK-mediated lysis of human leukemic blasts and human immature DCs,33,57,60 it is all but certain that the discovery of their ligands will further improve our understanding of viral and tumor immune evasion as well as the strategy for curing these diseases.
Concluding remarks
Since their discovery more than 3 decades ago, our knowledge about the function of human NK cells has grown exponentially. Once considered a forerunner of the seemingly more sophisticated antigen-specific, memory-bearing adaptive immune system,11 it is now clear that NK cells are highly sophisticated players of the innate immune system with certain recognition features that arrived on the evolutionary scene with primates approximately 400 million years after the birth of adaptive immunity.9 Their maturational pathway is strikingly similar to that of T cells, yet the discovery of developmental NK cell intermediates in SLT suggests this is a unique site for NK ontogeny in humans. The phenotypic heterogeneity of NK cells is accompanied by relatively distinct functional attributes, and it now appears that NK cells bridge innate- and antigen-specific immunity via their secretion of cytokines in SLT, while complimenting cytolytic effector T-cell immune surveillance by recognizing pathogens with absent or diminished MHC class I expression. Whether it is cytokine secretion or cytotoxicity, NK- cell function appears to result from the absolute sum of simultaneous activation and inhibition signals. Although we have gained only a partial understanding of how NK cells recognize a target cell as friend or foe, the principles have already enhanced our understanding of how pathogens evade the immune system and how the immune system can be engineered to cure some forms of cancer.
Acknowledgments
I apologize for my failure to provide original citations of many discoveries discussed in this review as well as omitting several fascinating aspects of human NK-cell biology (eg, uterine NK cells,115 NK neoplasms,116 parasitic infections117 ) that would have been included without strict space limitations. Special thanks to Aharon G. Freud and Bradley W. Blaser for their assistance with figure sketches, discussion, and editing, and to Debra Tyler for final illustrations. This work is dedicated to Jerry Ritz and Christine Canning (Dana-Farber Cancer Institute, Harvard Medical School) with sincere appreciation for their guidance, patience, and enthusiasm during my training in human immunology from 1986 through 1989.
M.A.C. is supported by the National Cancer Institute (CA101140, CA95426, CA89341, CA68458, CA16058) and the Leukemia & Lymphoma Society of America.
National Institutes of Health
Authorship
Contribution: M.A.C. wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Michael A. Caligiuri, Director and CEO, The Ohio State University Comprehensive Cancer Center, The James Cancer Hospital & Solove Research Institute, 300 West 10th St, Rm 517, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal