In this issue of Blood, Wimmer-Kleikamp and colleagues report that high levels of cellular PTPases hold activities of EphA3 kinase in check and support stable cell-cell adhesion, rather than default repulsion.
Intense studies on Eph receptor tyrosine kinases and their membrane-anchored ligands, called ephrins, have revealed a large array of essential and versatile functions of Eph/ephrin systems in many developmental and disease processes.1 Dynamic regulation of cell-cell and cell–extracellular-matrix adhesion is one key mechanism underlying Eph functions. Upon engagement of Ephs with ephrins on 2 opposing cells, the initially adhesive event frequently becomes repulsive, leading to the separation of 2 cells. Indeed, this is a default outcome in most developmental and physiological settings. For EphA/ephrin-A systems, the repulsion is preceded by the cleavage of ephrin-A by ADAM10 protease to allow the 2 cells to untangle2 (see figure). For EphB- and ephrin-B-bearing cells, endocytosis of receptor/ligand complexes enables the 2 cells to disengage.3,4
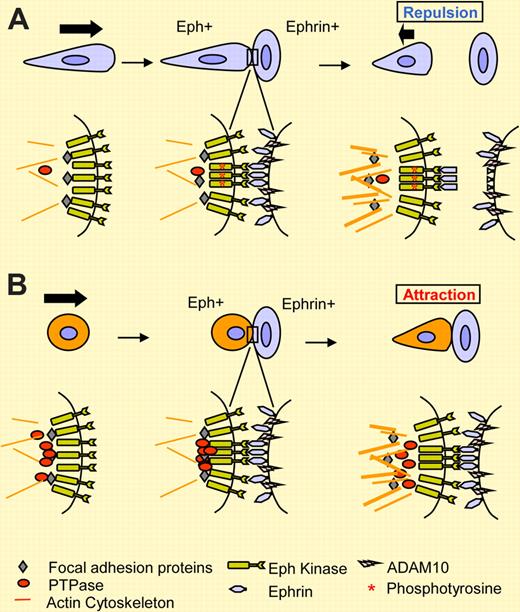
Repulsive (A) versus attractive (B) outcome upon cell-contact–induced EphA/ephrin-A interactions. The studies by Wimmer-Kleikamp and coworkers, as reported in this issue of Blood, support a role for PTPases in promoting adhesion of LK63 leukemia cells to ephrin-A5 via EphA3. In a more typical (default) response such as that observed in HEK293/EphA3 cells, EphA3 activation by autophosphorylation (denoted by an asterisk) was followed by cleavage of ephrin-A5, enabling cell separation. In LK63 cells, high levels of endogenous PTPases (red circle) prevent EphA3 activation by ephrin-A5. This, together with the lack of ephrin-A5 cleavage, supports cell adhesion.
Repulsive (A) versus attractive (B) outcome upon cell-contact–induced EphA/ephrin-A interactions. The studies by Wimmer-Kleikamp and coworkers, as reported in this issue of Blood, support a role for PTPases in promoting adhesion of LK63 leukemia cells to ephrin-A5 via EphA3. In a more typical (default) response such as that observed in HEK293/EphA3 cells, EphA3 activation by autophosphorylation (denoted by an asterisk) was followed by cleavage of ephrin-A5, enabling cell separation. In LK63 cells, high levels of endogenous PTPases (red circle) prevent EphA3 activation by ephrin-A5. This, together with the lack of ephrin-A5 cleavage, supports cell adhesion.
Paradoxically, Eph/ephrin interactions are also known to induce cell-cell adhesion under some circumstances.5,6 In this issue of Blood, Wimmer-Kleikamp et al uncover an interesting new mechanism underlying the attractive response of LK63 leukemia cells that express high level of endogenous EphA3. LK63 cells were grown in suspension and did not adhere to fibronectin (FN). However, they rapidly adhered to ephrin-A5 immobilized on platelets or expressed on the cell surface. This was followed by actin-rich filopodia protrusions that were decorated with focal adhesion proteins. Despite the presence of ADAM10 protease, LK63 cells failed to cleave ephrin-A5. These attractive responses contrasted with those from adherent HEK293-expressing exogenous EphA3. The latter adhered to FN, but were repulsed by immobilized ephrin-A5 in a dose-dependent manner, and cleaved ephrin-A5. It turns out that the lack of ephrin-A5 cleavage is only part of the reason for the attraction of LK63 cells: high levels of endogenous protein tyrosine phosphatases (PTPases) in LK63 cells also appear to play a major role. Thus, EphA3 was held in an unphosphorylated and presumably inactive state even after ligand stimulation. In an elegant reverse immunoprecipitation experiment, lysates from LK63 cells caused dephosphorylation of ligand-activated and phosphorylated EphA3 from HEK293/EphA3 cells, suggesting that the presence of PTPase activities in LK63 cells suppressed EphA3 activation. Conversely, the inactive and unphosphorylated EphA3 from LK63 cells was phosphorylated after incubation with lysates from ligand-stimulated HEK293/EphA3 cells. Inhibition of PTPases with vanadate in LK63 cells led to EphA3 phosphorylation, which was correlated with defective adhesion and filopodia extension, supporting a role for PTPases in attraction of LK63 cells toward ephrin-A5 (see figure panel B).
In addition to PTPases, 2 other mechanisms have been reported previously that shed light on how initial Eph/ephrin interaction may turn into stable cell-cell adhesion or repulsion. Whereas full-length EphA7 mediates repulsive responses, 2 splice variants of EphA7 cause truncation of the kinase domain. The truncated variants inhibit ephrin-A5–induced phosphorylation of full-length EphA7 in a dominant-negative manner, and turn repulsion into adhesion.6 Another reported mechanism involves cis inactivation of Eph by ephrins expressed on the same cells. It is reported that the cis interaction site on EphA3 is independent of the ligand-binding domain, and effectively prevents EphA3 phosphorylation by ephrins acting in trans.7 Therefore, all 3 mechanisms, including high PTPases, appear to lead to inhibition of Eph kinase activation in order to support cell-cell adhesion rather than repulsion. In so doing, the Eph/ephrin systems provide cells with a highly adaptable set of tools for selective cell-cell interactions, which are likely to play critical roles in normal development and physiology as well as disease processes including immune surveillance and tumor metastasis.
Although PTPases do appear to contribute to cell decision-making regarding adhesion or repulsion, the identities of the PTPases involved remain elusive, which hampers a more rigorous and conclusive illustration that they can indeed serve as arbitrators of attraction in certain cell types, such as LK63 cells. In addition, interpretations of data generated from vanadate treatment are complicated by the expected pleiotropic effects on many other tyrosine-phosphorylated proteins. However, the multitude of well-performed cellular and biochemical studies by Wimmer-Kleikamp et al add a new and interesting direction to our pursuit of understanding how a cell decides to retract or engage when coming in contact with an ephrin-presenting cell.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal