To the editor:
In paroxysmal nocturnal hemoglobinuria (PNH), hemolysis is due to the absence on red blood cell (RBC) surface of the 2 complement regulators CD55 and CD59,1,2 which causes uncontrolled complement activation and consequent chronic intravascular hemolysis via the membrane attack complex (MAC). Eculizumab,3 a monoclonal antibody against complement fraction 5, has provided clinical benefit in several trials in PNH patients.4-7
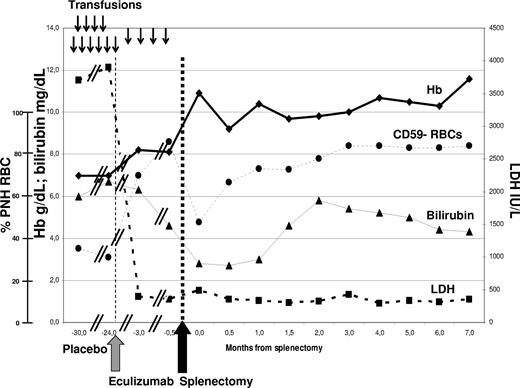
A young woman was referred to our department in 2004 for PNH, having received transfusion every 5 to 7 weeks since diagnosis in 1992. Preexisting hemoglobinopathies were excluded by standard laboratory tests. The patient presented severe anemia, normal white blood cell (WBC) and platelet counts, very high serum lactate dehydrogenase (LDH), and unconjugated bilirubin. She entered the double-blind placebo-controlled 26-week phase 3 efficacy and safety study (TRIUMPH) blinded trial5 in February 2005, showing no change in laboratory or clinical parameters throughout the 6-month study period (Figure 1); when randomization was disclosed, she was seen to have been assigned to the placebo arm. She entered the subsequent open-label Extension study in September 2005, receiving 600 mg eculizumab weekly for 4 weeks (loading dose) followed by 900 mg fortnightly (maintenance). Approval was obtained from the Federico II University Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki. Intravascular hemolysis was soon blocked, as shown by LDH normalization and hemosiderinuria disappearance; as expected, PNH type III RBCs increased from 40% to 70% (Figure 1). But despite preserved marrow function (testified by reticulocytosis and normal WBC and platelets) the patient still required transfusions every 3 to 4 months, even after introduction of recombinant erythropoietin. She showed persistent hyperbilirubinemia, partially due to a Gilbert syndrome (genetically confirmed). While on eculizumab, in vivo study of RBC survival by 51Cr labeling showed markedly reduced RBC half-life (10 days, normal range 25-35 days) with high spleen and liver counts. In addition, the Coomb test was positive for C3d only, and dual color flow cytometry of the PNH RBCs revealed significant C3b coating (19%).8 We hypothesized that eculizumab had blocked intravascular hemolysis, but could not oppose C3b accumulation on RBCs (uncontrolled by the absence of CD55), leading to hemolysis in the reticulo-endothelial system. To control extravascular hemolysis, we decided to remove the spleen, which was slightly enlarged (500 mL, by ultrasound scan). The patient underwent video-laparoscopic splenectomy, without complications; she received standard antiinfectious prophylaxis measures, including anti–haemophilus and anti–pneumococcal vaccination. Eight months after surgery hemoglobin was stable at more than 110 g/L (11 g/dL) without transfusion, bilirubin was only slightly elevated, and flow cytometry revealed a further increase of PNH RBC to 85% (Figure 1), and 41% of C3+ PNH RBCs.
Laboratory parameters in relation to anticomplement treatment and splenectomy. ↓: Two units of packed red cells transfused. Eculizumab (Soliris, Alexion Pharmaceuticals) was started in September 2005 (gray arrow), according to the standard schedule (600 mg weekly ×4, then 900 mg fortnightly). Splenectomy was performed in May 2006 (black arrow), supported by vaccinations and antibiotics. No infections or other clinical complications have been recorded to date. The need for transfusion (17 units in the previous 12 months) remained stable during the TRIUMPH study (placebo arm), and was significantly reduced after introduction of eculizumab, which resolved intravascular hemolysis with LDH normalization; however, transfusion independence with hemoglobin stabilization was achieved only after splenectomy. Bilirubinemia was persistently elevated (almost entirely unconjugated) with some fluctuations, in part due to associated Gilbert syndrome (genetically confirmed by the presence of homozygous (TA)7 polymorphism of the UGT1A1 gene).
Laboratory parameters in relation to anticomplement treatment and splenectomy. ↓: Two units of packed red cells transfused. Eculizumab (Soliris, Alexion Pharmaceuticals) was started in September 2005 (gray arrow), according to the standard schedule (600 mg weekly ×4, then 900 mg fortnightly). Splenectomy was performed in May 2006 (black arrow), supported by vaccinations and antibiotics. No infections or other clinical complications have been recorded to date. The need for transfusion (17 units in the previous 12 months) remained stable during the TRIUMPH study (placebo arm), and was significantly reduced after introduction of eculizumab, which resolved intravascular hemolysis with LDH normalization; however, transfusion independence with hemoglobin stabilization was achieved only after splenectomy. Bilirubinemia was persistently elevated (almost entirely unconjugated) with some fluctuations, in part due to associated Gilbert syndrome (genetically confirmed by the presence of homozygous (TA)7 polymorphism of the UGT1A1 gene).
Splenectomy has been anecdotally reported in PNH patients not receiving anticomplement therapy, with limited benefit.1 This is the first case of splenectomy in a PNH patient on eculizumab to resolve extravascular hemolysis. Our report suggests that extravascular hemolysis (mainly in the spleen), that is unapparent in untreated PNH patients due to the rapid clearance of circulating PNH RBCs, may play a pathogenic role in PNH when MAC-mediated lysis is blocked by the complement inhibitor eculizumab. The role of specific C3 split products in this process has not yet been pinpointed in PNH patients, but in other settings (red cell aging, cold agglutinin disease)9,10 C3b has been reported to lead to extravascular hemolysis, while the conversion to iC3b and C3d may be protective. Different rates of extravascular hemolysis in different PNH patients may stem from the pattern of C3 split products and/or polymorphisms of complement receptor and complement regulator molecules. A better understanding of the disease mechanism in PNH in the eculizumab era may pave the way to additional strategies to optimize the clinical efficacy of complement inhibitors in this disease.
Authorship
We thank the colleagues of the “Casa Sollievo della Sofferenza” Hospital (S. Giovanni Rotondo, FG) who referred the patient to us (Pellegrino Musto, Severio Mantuano, Angelo Michele Carella), and who performed the 51Cr study (Filippo Barbano) and splenectomy (Angelo Ambrosio); Alexion Pharmaceuticals (Cheshire, CT) was the promoter of the eculizumab clinical trials.
Conflict-of-interest disclosure: A.M.R. and B.R. have received lecture fees and a research grant and have served as consultants on the Advisory Committee for Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Antonio Maria Risitano, Hematology Section, Department of Biochemistry and Medical Biotechnologies, Federico II University, Via Pansini 5, Naples, Italy 80131; e-mail: amrisita@unina.it.