Abstract
Spectrin is the backbone of the erythroid cytoskeleton; sph/sph mice have severe hereditary spherocytosis (HS) because of a mutation in the murine erythroid α-spectrin gene. sph/sph mice have a high incidence of thrombosis and infarction in multiple tissues, suggesting significant vascular dysfunction. In the current study, we provide evidence for both pulmonary and systemic vascular dysfunction in sph/sph mice. We found increased levels of soluble cell adhesion molecules in sph/sph mice, suggesting activation of the vascular endothelium. We hypothesized that plasma hemoglobin released by intravascular hemolysis initiates endothelial injury through nitric oxide (NO) scavenging and oxidative damage. Likewise, electron paramagnetic resonance spectroscopy showed that plasma hemoglobin is much greater in sph/sph mice. Moreover, plasma from sph/sph mice had significantly higher oxidative potential. Finally, xanthine oxidase, a potent superoxide generator, is decreased in subpopulations of liver hepatocytes and increased on liver endothelium in sph/sph mice. These results indicate that vasoregulation is abnormal, and NO-based vasoregulatory mechanisms particularly impaired, in sph/sph mice. Together, these data indicate that sph/sph mice with severe HS have increased plasma hemoglobin and NO scavenging capacity, likely contributing to aberrant vasoregulation and initiating oxidative damage.
Introduction
The membrane skeleton, a multiprotein complex located just beneath the plasma membrane, provides the red blood cells (RBCs) with the mechanical strength and deformability required to withstand the high shear forces of the microcapillaries. Spectrin, a tetramer composed of α- and β-subunits, is the backbone of the erythroid membrane skeleton and is tethered to the membrane at 2 positions. Disruption of either spectrin or proteins involved in tethering spectrin to the RBC plasma membrane can result in the hemolytic anemia known as hereditary spherocytosis (HS) in both humans and mice.1-3
We have shown that sph/sph mice have severe autosomal recessive HS because of a spontaneous single-base deletion in the murine erythroid α-spectrin gene, Spna1.4 Although heterozygous (sph/+) mice are phenotypically normal, the sph/sph RBC is extremely fragile, resulting in an RBC life span of approximately 1 day (normal is 48 days).5 As a consequence, sph/sph mice have a very severe hemolytic anemia, with hematocrit values of 0.15 to 0.20 and compensatory reticulocytosis of 90% to 95%.5 As a result of the severe hemolysis, sph/sph mice with severe HS develop multiorgan pathology in kidney, heart, liver, and spleen.6,7 In addition, sph/sph mice develop thrombosis and infarction in multiple tissues,6-8 suggesting significant vascular dysfunction.
Recent studies of patients with hemolytic anemia have indicated that the incidence of pulmonary hypertension, as indicated by increased tricuspid regurgitant jet velocity (≥ 3.0 m/sec), is relatively high in patients with sickle cell disease (SCD), thalassemia, and paroxysmal nocturnal hemoglobinuria.9-18 Furthermore, it has been suggested that the pulmonary hypertension in SCD and thalassemia correlates with markers of hemolysis and that the hemolytic process may directly contribute to its pathophysiology.9,10,12,13,17 Plasma-free hemoglobin (Hb) is released during intravascular hemolysis and is associated with multiple clinical symptoms in addition to pulmonary hypertension, including hemoglobinuria, increased blood pressure and creatine kinase levels, platelet activation, and increased mortality.9 Furthermore, cell-free Hb is an efficient scavenger of nitric oxide (NO), a critical regulator of vascular homeostasis.10,19-23 The rapid reaction between NO and oxygenated Hb (oxyHb) generates nitrate and methemoglobin (metHb).19,20,24,25 Intravascular RBC hemolysis also results in the release of RBC arginase, which efficiently reacts with l-Arg to produce ornithine, thus scavenging the substrate for additional NO production by NO synthase.9 Thus, bioavailable NO may be greatly reduced by intravascular hemolysis, contributing significantly to vascular dysfunction.
Once in the plasma, Hb may release its heme group. This is particularly evident with metHb, in which the heme group is bound more loosely. Plasma free hemoglobin and heme both have proinflammatory and pro-oxidant effects on endothelial cells and are associated with increased expression of endothelial cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM-1), and E-selectin.9,12,26-29 NO, in contrast, inhibits the expression of the same endothelial cell adhesion molecules.9 Normally, the plasma proteins haptoglobin and hemopexin, which bind hemoglobin and heme, respectively, are protective against the potentially damaging effects of intravascular hemolysis.30,31 However, in hemolytic anemia, these pathways are often overwhelmed, leading to unmeasurable steady-state levels of these proteins and the constant presence of unbound cell-free Hb in the plasma.9,20,23,30 Furthermore, because haptoglobin is reported to have inhibitory effects on Th2 but not Th1 cytokine release, the absence of available plasma haptoglobin in hemolytic anemia could have modulatory effects on the inflammatory response to oxidative tissue damage.32,33 Thus, the effects of intravascular hemolysis on vascular and organ function may extend beyond loss of vasodilation due to reduced bioavailable NO; plasma free Hb may also directly cause endothelial injury and contribute to a proinflammatory phenotype.
In these studies, we document pulmonary hypertension in sph/sph mice as well as blunted vasorelaxation of systemic arteries in response to acetylcholine. In addition, we find evidence of injury and activation of the vascular endothelium as measured by increased levels of soluble cell adhesion molecules in the plasma of sph/sph mice compared with control mice. Increased plasma-free hemoglobin (Hb), NO scavenging capacity, and oxidizing potential in the plasma of sph/sph mice supports the hypothesis that intravascular hemolysis of sph/sph RBCs may initiate endothelial dysfunction through NO scavenging and oxidative damage. We also present data suggesting that xanthine oxidase (XO), a potent generator of superoxide, may also directly contribute to the endothelial injury observed in sph/sph mice. Our results indicate that, similar to mice and humans with SCD and humans with thalassemia or paroxysmal nocturnal hemoglobinuria, sph/sph mice with severe hereditary spherocytosis exhibit aberrant pulmonary and systemic vasoregulation that is likely related to NO scavenging and oxidative damage initiated by increased plasma free hemoglobin resulting from hemolysis.
Methods
Mice
The sph mutation is maintained in the heterozygous state on both the WB/ReJ (WB) and C57BL/6J (B6) backgrounds. F1 hybrid (WBB6F1) mutant (sph/sph) and normal (+/+, sph/+) mice were generated by mating WB and B6 heterozygotes and were genetically identical except at the mutated locus. F1 hybrid mice survive longer than mutant mice on inbred strain backgrounds. Mice were housed and cared for according to the specifications of the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal experiments were approved by the Institutional Animal Care and Use Committees of the Medical College of Wisconsin and the University of Colorado Health Sciences Center. Experimental groups contained similar numbers of male and female mice. Experimental mice were 6 weeks of age or older; this age was chosen to reflect “adult” mice, because mice of the WBB6F1 background attain sexual maturity at this age.
Blood preparation
Blood was obtained from deeply anesthetized mice by cardiac puncture and anticoagulated with sodium citrate. Blood cells were separated from plasma by centrifugation at 2000g for 10 minutes; plasma was further clarified by centrifugation at 8100g for 10 minutes, then aliquoted and stored at −80°C.
Immunohistochemistry
After phlebotomy, mice were perfused transcardially with 20 mL of room temperature phosphate-buffered saline. Lung and liver were then dissected free and fixed in 10% neutral buffered formalin. Fixed tissue samples were embedded in paraffin and sectioned. Immunohistochemistry on unstained tissue sections for detection of xanthine dehydrogenase/xanthine oxidase (XD/XO) was performed using primary antibodies (Ab) directed against XD/XO (rabbit polyclonal Ab Sc-20991; Santa Cruz Biotechnology, Santa Cruz, CA) or von Willebrand Factor (VWF) (positive control for endothelial cells; rabbit polyclonal Ab A0082; Dako North America, Carpinteria, CA). Paraffin-embedded liver sections (4 μm) were processed as follows before applying primary antibodies: (1) treatment for antigen-retrieval (BioGenex, San Ramon, CA); (2) endogenous peroxidase blocked (Dako North America); (3) avidin/biotin blocked (Vector Laboratories, Burlingame, CA); and (4) serum blocked (Dako North America). Sections were incubated with primary antibodies (8 μg/mL for XO; 5.6 μg/mL for VWF) at 37°C for 1 hour. Antibody-antigen interaction was amplified using a biotin-streptavidin system (avidin-biotin complex kit; Vector Laboratories) and developed with 3,3′-diaminobenzidine substrate according to the manufacturer's instructions (diaminobenzidene [DAB] stain kit, Vector Laboratories). Nuclei were counterstained with hematoxylin (Vector Laboratories) and coverslipped with PerMount (Thermo Fisher Scientific, Waltham, MA). Representative images were captured using a Nikon Eclipse E600-Spot camera system (Diagnostic Instruments, Sterling Heights, MI) with 40× or 100× (oil-immersion) objectives. Adjacent tissue sections were used for negative control (omitting the primary antibodies) and IgG isotype control (rabbit IgG, Jackson Immunoresearch Laboratories, West Grove, PA).
Facialis artery vasodilation studies
Vasodilation studies of pressurized facialis arteries (180-250 μm) preconstricted with U46619 (10−9 to 10−8 mol/L) in response to stimulation with acetylcholine (ACh; 10−7 to 10−4 mol/L) in the presence and absence of l-nitroarginine methyl ester (l-NAME, 100 μM) were performed as described previously.34,35
Assessment of pulmonary hypertension
Anesthetized mice were placed in a supine position while breathing room air. A 26-gauge needle was inserted percutaneously into the thorax via a subxyphoid approach. Right ventricular pressures were measured using a pressure transducer (Gulton-Statham, Costa Mesa, CA) and recorded on a multichannel recorder (Grass Institute, Quinen, MA).36 After measurement of right ventricular systolic pressure (RVSP), mice were euthanized, hearts resected, and atria removed to the plane of the atrial-ventricular valves. Right ventricle (RV) was then dissected free of left ventricle plus septum (LV + S), weighed, and weights were normalized to body weight (RV/BW).36,37
ELISA
Levels of soluble murine vascular cell adhesion molecule-1 (sVCAM-1), soluble P-selectin (sP-sel), and soluble E-selectin (sE-sel) were determined by enzyme-linked immunosorbent assay (ELISA) of plasma samples from normal and sph/sph mice. Separate sets of plasma samples from normal and sph/sph mice were used in ELISA assays where buffer was used in place of the secondary antibody in plasma-coated wells to test for nonspecific binding of plasma factors to the ELISA plates. Removal of microparticles from another set of plasma samples from normal and sph/sph mice was accomplished by ultracentrifugation of plasma at 200 000g for 2 hours at 4°C; plasma supernatant was assayed immediately after ultracentrifugation. For comparison of plasma levels of sVCAM-1, sP-sel, and sE-sel before and after removal of microparticles, an aliquot of thawed plasma was held on ice (unspun, “−UCS”) while the remaining volume of plasma was ultracentrifuged. Ultracentrifuged (“+UCS”) and plasma aliquot held on ice (“−UCS”) were assayed immediately after completion of ultracentrifuge spin. ELISA kits were from R&D Systems (Minneapolis, MN): sVCAM-1, kit MVC00; sE-sel, kit MES00; sP-sel, kit MPS00.
Electron paramagnetic resonance spectroscopy
Electron paramagnetic resonance (EPR) studies were performed on an Elexsys X-band EPR system (Bruker, Newark, DE) equipped with a liquid helium cryostat and a liquid nitrogen-based variable temperature unit. Plasma samples were placed in a 3-mm diameter quartz EPR tube and immediately frozen in liquid nitrogen before storage at −80°C. MetHb levels were determined at 4.5 K by EPR and quantified with reference to a standard curve generated from authentic metHb. Spectrophotometer conditions were: microwave power, 2 mW; modulation amplitude, 5 G; scan time, 43 seconds; scan width, 500 G. Total (metHb + oxyHb) hemoglobin levels were determined after treatment of plasma with the NO donor compound PROLI/NO (100 μM final concentration), which rapidly releases NO and converts all oxyHb to the metHb form.
Oxygen uptake assay for plasma oxidizing potential
Linoleic acid (10 mM) dissolved in 50 mM phosphate, pH 7.4, containing 2% cholate and 1 mM diethylenetriaminepentaacetic acid was equilibrated at 37°C in the chamber of a Clark-Type oxygen electrode (Rank Brothers) polarized at 0.6 V. After securing the lid on the electrode, 10 μL plasma was injected through the injection port using a Hamilton syringe. The time-course of oxygen consumption consists of a fast phase of oxygen uptake, which then slows to a background rate. Oxygen concentration was continuously monitored for at least 10 minutes until the fast phase of oxygen uptake tapered off. Oxidizing potential was calculated as the total amount of oxygen consumed in the fast phase of oxidation.
Statistics
All data are presented as mean (± SEM). Data were analyzed by unpaired Student t test assuming equal variances, with the exception of data for Figure 3B,D,F, which was analyzed by paired Student t test between values for the same plasma samples before and after ultracentrifugation to remove microparticles. A 2-tailed P value less than .05 was considered statistically significant.
Results
Pulmonary hypertension in sph/sph mice
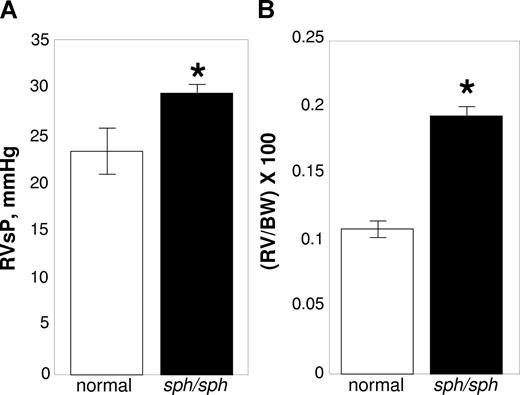
We investigated sph/sph mice with severe HS for signs of pulmonary hypertension. Right ventricular pressures were measured using a pressure transducer and recorded on a multichannel recorder.36 After measurement, the animal was euthanized and the heart was resected; the RV was dissected free of LV + S, weighed, and weights normalized to the BW of the animal.36,37 As shown in Figure 1A, RVSPs were significantly increased in sph/sph mice compared with their normal littermates (P < .03). In agreement, RV/BW was also increased in sph/sph mice compared with their normal littermates (Figure 1B; P < .0001). These results are indicative of the presence of pulmonary hypertension in sph/sph mice.
Evidence for pulmonary hypertension in sph/sph mice. (A) RVSP is increased in sph/sph mice compared with normal mice. (B) RV/BW in increased in sph/sph mice compared with normal mice. Anesthetized control (normal, □) and sph/sph (■) mice were placed in a supine position while breathing room air. RvSP (A) was measured as described under “Methods.” After measurement of RvSP, mice were euthanized, hearts were resected, and RV/BW (B) was determined as described under “Methods.” Data are presented as mean (± SEM) for n = 4 normal and n = 6 sph/sph mice. Normal mice were between 6 and 12 weeks of age; n = 3 sph/sph mice were 6 weeks of age, and n = 2 were 12 weeks of age and n = 1 was 22 weeks of age. No differences in RvSP or RV/BW were noted between the younger and older sph/sph mice used for these experiments. *P < .03 for RVSP (A) and P < .0001 for RV/BW (B) sph/sph compared with normal mice.
Evidence for pulmonary hypertension in sph/sph mice. (A) RVSP is increased in sph/sph mice compared with normal mice. (B) RV/BW in increased in sph/sph mice compared with normal mice. Anesthetized control (normal, □) and sph/sph (■) mice were placed in a supine position while breathing room air. RvSP (A) was measured as described under “Methods.” After measurement of RvSP, mice were euthanized, hearts were resected, and RV/BW (B) was determined as described under “Methods.” Data are presented as mean (± SEM) for n = 4 normal and n = 6 sph/sph mice. Normal mice were between 6 and 12 weeks of age; n = 3 sph/sph mice were 6 weeks of age, and n = 2 were 12 weeks of age and n = 1 was 22 weeks of age. No differences in RvSP or RV/BW were noted between the younger and older sph/sph mice used for these experiments. *P < .03 for RVSP (A) and P < .0001 for RV/BW (B) sph/sph compared with normal mice.
Evidence for systemic vascular dysfunction in sph/sph mice
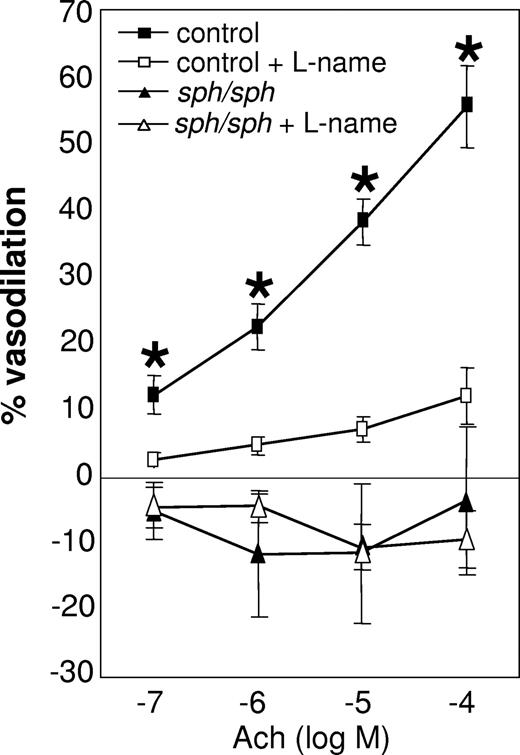
Systemic vascular function was evaluated using the facialis artery, a branch off the carotid artery (diameter 180; 200 μm); dilation of this vessel is known to be endothelial nitric-oxide synthase (eNOS)-dependent in healthy C57BL/6 mice.34,35,38,39 Measurements of facialis arteries from normal and sph/sph mice revealed severely impaired endothelial-dependent vasodilation in response to acetylcholine stimulation in sph/sph mice (Figure 2; P < .005). Acetylcholine-induced vasodilation was inhibited in vessels from normal mice by the eNOS antagonist l-NAME, confirming that the vasodilation studied in these experiments occurred by an eNOS-dependent mechanism. The lack of similar response to l-NAME inhibition of eNOS in vessels from sph/sph mice reflects the lack of NO-dependent vasodilation in response to acetylcholine. In addition, it is possible that the vessels from the mutant mice have adapted to dilate by non-eNOS dependent mechanisms. This phenomenon is also seen in vessels from subjects with coronary artery disease, which exhibit elevated levels of inflammation and oxidative stress.40,41
Impaired dilation of the facialis artery in sph/sph mice in response to stimulation with acetylcholine. Facialis arteries (180-250 μm) were isolated from control (■,□) and sph/sph (▴,▵) mice as described under “Methods.” Pressurized arteries were preconstricted with the thromboxane A2 agonist U46619 (10−9-10−8 mol/L; 15-hydroxy-11α,9α-(epoxymethano)prosta-5,13-dienoic acid). Vessel dilation in response to stimulation with acetylcholine (ACh; 10−7 to 10−4 mol/L) was recorded in the absence (■,▴) and presence (□,▵) of the NO antagonist l-NAME (100 μM). Data are presented as mean (± SEM) for n = 9 vessels from n = 7 control mice, and n = 8 vessels from n = 6 sph/sph mice. Normal mice were all older than 12 weeks of age; n = 3 sph/sph mice were 7 to 10 weeks of age, and n = 3 were 13 to 18 weeks of age. No differences in vessel dilation were observed in younger versus older sph/sph mice. *P < .005, control vessel dilation without l-NAME compared with sph/sph vessel dilation without l-NAME.
Impaired dilation of the facialis artery in sph/sph mice in response to stimulation with acetylcholine. Facialis arteries (180-250 μm) were isolated from control (■,□) and sph/sph (▴,▵) mice as described under “Methods.” Pressurized arteries were preconstricted with the thromboxane A2 agonist U46619 (10−9-10−8 mol/L; 15-hydroxy-11α,9α-(epoxymethano)prosta-5,13-dienoic acid). Vessel dilation in response to stimulation with acetylcholine (ACh; 10−7 to 10−4 mol/L) was recorded in the absence (■,▴) and presence (□,▵) of the NO antagonist l-NAME (100 μM). Data are presented as mean (± SEM) for n = 9 vessels from n = 7 control mice, and n = 8 vessels from n = 6 sph/sph mice. Normal mice were all older than 12 weeks of age; n = 3 sph/sph mice were 7 to 10 weeks of age, and n = 3 were 13 to 18 weeks of age. No differences in vessel dilation were observed in younger versus older sph/sph mice. *P < .005, control vessel dilation without l-NAME compared with sph/sph vessel dilation without l-NAME.
Increased markers of endothelial damage in sph/sph mice
The presence of increased levels of soluble VCAM-1 (sVCAM-1), E-selectin (sE-sel), and P-selectin (sP-sel) in plasma is indicative of increased vascular injury.26,27,42 As shown in Figure 3A, levels of sVCAM-1 in the plasma of sph/sph mice were approximately 3.5-fold higher than those in the plasma of normal (+/+, sph/+) mice (P < .0001). Similarly elevated plasma levels of sP-sel were found in sph/sph compared with normal (+/+, sph/+) mice (Figure 3C; P < .0001). Analysis of plasma levels of sE-sel also revealed significant increases in sph/sph mice compared with normal (+/+, sph/+) mice (Figure 3E; P < .005). To evaluate whether unique characteristics of hemolytic plasma, such as elevated bilirubin or LDH, might be generating a “false positive” signal in the above assays, separate sets of plasma from both +/+and sph/sph mice were tested by ELISA without the addition of the secondary antibody. No detectable spectrophotometric signal above background was detected in any of these samples, indicating that the ELISA results are not the result of nonspecific binding of factors in either +/+ or sph/sph plasma.
Increased workers of endothelial damage in sph/sph mice. Plasma levels of sVCAM-1 (A), sP-sel (C), and sE-sel (E) are increased in sph/sph mice compared with control mice. Levels of soluble murine vascular cell adhesion molecule-1 (sVCAM-1, A), soluble P-selectin (sP-sel, C), and soluble E-selectin (sE-sel, E) were determined by ELISA of plasma samples from control wild-type (+/+, □), control heterozygote (sph/+,  ), and sph/sph (■) mice. Data are presented as mean (± SEM) for the following numbers: (A) n = 7 +/+ [all more than 8 weeks of age], n = 7 sph/+[all more than 8 weeks of age], n = 6 sph/sph [all more than 8 weeks of age]; (C,E) n = 13 +/+ [n = 6, 6-8 weeks of age; n = 7, more than 8 weeks of age], n = 12 sph/+[n = 6, 6-8 weeks of age; n = 6, more than 8 weeks of age], n = 17 sph/sph [n = 9, 6-8 weeks of age; n = 8, more than 8 weeks of age]. No significant difference between age groups was noted for sP-sel and sE-sel values. *Significant difference between sph/sph and control (+/+, sph/+) values: P < .0001 for sVCAM-1 (A) and sP-sel (C); P < .005 for sE-sel (E). Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in the plasma of sph/sph and control mice are variably reduced by ultracentrifugation. Plasma from +/+ (□ and gray-striped bars) mice more than 6 weeks of age and sph/sph mice (black and black-striped bars) more than 8 weeks of age was subjected to ultracentrifugation to remove microparticles as described under “Methods.” Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in plasma were compared before (“−UCS,” open and black bars) and after (“+UCS,” gray-striped and black-striped bars) ultracentrifugation. The difference between sVCAM-1 levels in A and B and sP-sel levels in C and D reflects the fact that plasma samples from separate sets of +/+ and sph/sph mice were used for the assays. Data are presented as mean (± SEM) for the following numbers: (B) n = 4 +/+ and n = 3 sph/sph; (D) n = 3 +/+ and n = 3 sph/sph; (F) n = 3 +/+ and n = 4 sph/sph. *Significant difference between “−UCS” and “+UCS” values, P < .03.
), and sph/sph (■) mice. Data are presented as mean (± SEM) for the following numbers: (A) n = 7 +/+ [all more than 8 weeks of age], n = 7 sph/+[all more than 8 weeks of age], n = 6 sph/sph [all more than 8 weeks of age]; (C,E) n = 13 +/+ [n = 6, 6-8 weeks of age; n = 7, more than 8 weeks of age], n = 12 sph/+[n = 6, 6-8 weeks of age; n = 6, more than 8 weeks of age], n = 17 sph/sph [n = 9, 6-8 weeks of age; n = 8, more than 8 weeks of age]. No significant difference between age groups was noted for sP-sel and sE-sel values. *Significant difference between sph/sph and control (+/+, sph/+) values: P < .0001 for sVCAM-1 (A) and sP-sel (C); P < .005 for sE-sel (E). Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in the plasma of sph/sph and control mice are variably reduced by ultracentrifugation. Plasma from +/+ (□ and gray-striped bars) mice more than 6 weeks of age and sph/sph mice (black and black-striped bars) more than 8 weeks of age was subjected to ultracentrifugation to remove microparticles as described under “Methods.” Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in plasma were compared before (“−UCS,” open and black bars) and after (“+UCS,” gray-striped and black-striped bars) ultracentrifugation. The difference between sVCAM-1 levels in A and B and sP-sel levels in C and D reflects the fact that plasma samples from separate sets of +/+ and sph/sph mice were used for the assays. Data are presented as mean (± SEM) for the following numbers: (B) n = 4 +/+ and n = 3 sph/sph; (D) n = 3 +/+ and n = 3 sph/sph; (F) n = 3 +/+ and n = 4 sph/sph. *Significant difference between “−UCS” and “+UCS” values, P < .03.
Increased workers of endothelial damage in sph/sph mice. Plasma levels of sVCAM-1 (A), sP-sel (C), and sE-sel (E) are increased in sph/sph mice compared with control mice. Levels of soluble murine vascular cell adhesion molecule-1 (sVCAM-1, A), soluble P-selectin (sP-sel, C), and soluble E-selectin (sE-sel, E) were determined by ELISA of plasma samples from control wild-type (+/+, □), control heterozygote (sph/+,  ), and sph/sph (■) mice. Data are presented as mean (± SEM) for the following numbers: (A) n = 7 +/+ [all more than 8 weeks of age], n = 7 sph/+[all more than 8 weeks of age], n = 6 sph/sph [all more than 8 weeks of age]; (C,E) n = 13 +/+ [n = 6, 6-8 weeks of age; n = 7, more than 8 weeks of age], n = 12 sph/+[n = 6, 6-8 weeks of age; n = 6, more than 8 weeks of age], n = 17 sph/sph [n = 9, 6-8 weeks of age; n = 8, more than 8 weeks of age]. No significant difference between age groups was noted for sP-sel and sE-sel values. *Significant difference between sph/sph and control (+/+, sph/+) values: P < .0001 for sVCAM-1 (A) and sP-sel (C); P < .005 for sE-sel (E). Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in the plasma of sph/sph and control mice are variably reduced by ultracentrifugation. Plasma from +/+ (□ and gray-striped bars) mice more than 6 weeks of age and sph/sph mice (black and black-striped bars) more than 8 weeks of age was subjected to ultracentrifugation to remove microparticles as described under “Methods.” Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in plasma were compared before (“−UCS,” open and black bars) and after (“+UCS,” gray-striped and black-striped bars) ultracentrifugation. The difference between sVCAM-1 levels in A and B and sP-sel levels in C and D reflects the fact that plasma samples from separate sets of +/+ and sph/sph mice were used for the assays. Data are presented as mean (± SEM) for the following numbers: (B) n = 4 +/+ and n = 3 sph/sph; (D) n = 3 +/+ and n = 3 sph/sph; (F) n = 3 +/+ and n = 4 sph/sph. *Significant difference between “−UCS” and “+UCS” values, P < .03.
), and sph/sph (■) mice. Data are presented as mean (± SEM) for the following numbers: (A) n = 7 +/+ [all more than 8 weeks of age], n = 7 sph/+[all more than 8 weeks of age], n = 6 sph/sph [all more than 8 weeks of age]; (C,E) n = 13 +/+ [n = 6, 6-8 weeks of age; n = 7, more than 8 weeks of age], n = 12 sph/+[n = 6, 6-8 weeks of age; n = 6, more than 8 weeks of age], n = 17 sph/sph [n = 9, 6-8 weeks of age; n = 8, more than 8 weeks of age]. No significant difference between age groups was noted for sP-sel and sE-sel values. *Significant difference between sph/sph and control (+/+, sph/+) values: P < .0001 for sVCAM-1 (A) and sP-sel (C); P < .005 for sE-sel (E). Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in the plasma of sph/sph and control mice are variably reduced by ultracentrifugation. Plasma from +/+ (□ and gray-striped bars) mice more than 6 weeks of age and sph/sph mice (black and black-striped bars) more than 8 weeks of age was subjected to ultracentrifugation to remove microparticles as described under “Methods.” Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in plasma were compared before (“−UCS,” open and black bars) and after (“+UCS,” gray-striped and black-striped bars) ultracentrifugation. The difference between sVCAM-1 levels in A and B and sP-sel levels in C and D reflects the fact that plasma samples from separate sets of +/+ and sph/sph mice were used for the assays. Data are presented as mean (± SEM) for the following numbers: (B) n = 4 +/+ and n = 3 sph/sph; (D) n = 3 +/+ and n = 3 sph/sph; (F) n = 3 +/+ and n = 4 sph/sph. *Significant difference between “−UCS” and “+UCS” values, P < .03.
A potential source of “soluble” VCAM-1, P-sel, and E-sel in plasma is the presence of microparticles in the plasma. Plasma from +/+ and sph/sph mice (separate sets from those used in Figure 3A,C,E) was assessed both before and after ultracentrifugation (200 000g for 2 hours, 4°C) of plasma to remove microparticles. Ultracentrifugation eliminated 88% of the sVCAM-1 signal in plasma from normal mice (Figure 3B; P < .03) but only slightly reduced the sVCAM-1 signal in plasma from sph/sph mice (Figure 3B). Likewise, ultracentrifugation reduced sP-sel signal by 95% in plasma from normal mice (Figure 3D; P < .03) but reduced sP-sel signal by only 40% in plasma from sph/sph mice (Figure 3D; P < .03). Finally, analysis of sE-sel levels showed that although ultracentrifugation eliminated 90% of the sE-sel signal in plasma from +/+ mice, it eliminated only 50% of the sE-sel signal in plasma from sph/sph mice (Figure 3F; P < .03). Taken together, these results suggest that endothelial damage is occurring in sph/sph mice. In addition, the presence of microparticles in plasma from sph/sph mice may only partially explain the increase in these markers of endothelial damage in sph/sph mice.
Increased plasma hemoglobin and NO scavenging capacity in sph/sph mice
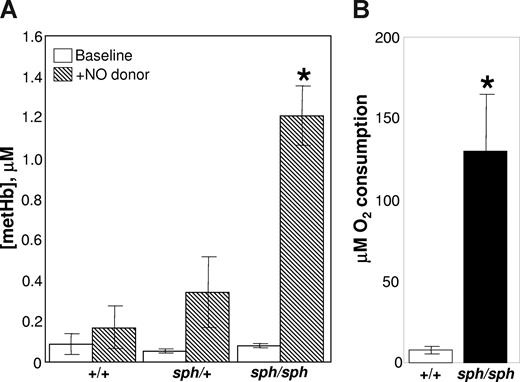
One potential source of endothelial dysfunction is increased plasma hemoglobin released by the intravascular lysis of sph/sph RBCs.9,10,20-25 EPR studies, at liquid helium temperatures, show that there is not an increase in basal methemoglobin in plasma from sph/sph mice (Figure 4A open bars). These results may reflect the relatively short half-life of metHb in plasma. However, when the exogenous NO donor PROLI/NO is added to convert all plasma free oxyHb to metHb, a significantly higher level of metHb is generated in the plasma from sph/sph mice compared with plasma from normal (+/+, sph/+) animals (Figure 4A hatched bars; P < .01). These results indicate that plasma free hemoglobin is significantly increased in sph/sph mice, providing a potential source of both endothelial oxidative damage and endothelial dysfunction via NO scavenging. In addition, these data also suggest that a significant portion of the hemolysis in sph/sph mice occurs in the intravascular rather than extravascular space.
Nitric oxide scavenging and oxidizing potential of plasma from sph/sph mice is greater than that of plasma from normal (+/+, sph/+) mice. (A) Helium EPR measurements of plasma metHb levels before (□) and after ( ) the addition of the NO donor proline/NO. Data are presented as mean (± SEM) for n = 3 +/+, n = 5 sph/+, and n = 4 sph/sph mice. All mice were between 6 and 9 weeks of age. *P < .02 compared with +/+ or sph/+plasma with proline/NO added. (B) Oxygen uptake assay of oxidizing potential of plasma from normal (+/+, □) and sph/sph (■) mice. Data presented as mean (± SEM) for n = 4 mice per group. Mice in both groups were between 7 and 13 weeks of age; there were no differences between data from younger versus older mice. *P < .013 compared with +/+ plasma.
) the addition of the NO donor proline/NO. Data are presented as mean (± SEM) for n = 3 +/+, n = 5 sph/+, and n = 4 sph/sph mice. All mice were between 6 and 9 weeks of age. *P < .02 compared with +/+ or sph/+plasma with proline/NO added. (B) Oxygen uptake assay of oxidizing potential of plasma from normal (+/+, □) and sph/sph (■) mice. Data presented as mean (± SEM) for n = 4 mice per group. Mice in both groups were between 7 and 13 weeks of age; there were no differences between data from younger versus older mice. *P < .013 compared with +/+ plasma.
Nitric oxide scavenging and oxidizing potential of plasma from sph/sph mice is greater than that of plasma from normal (+/+, sph/+) mice. (A) Helium EPR measurements of plasma metHb levels before (□) and after ( ) the addition of the NO donor proline/NO. Data are presented as mean (± SEM) for n = 3 +/+, n = 5 sph/+, and n = 4 sph/sph mice. All mice were between 6 and 9 weeks of age. *P < .02 compared with +/+ or sph/+plasma with proline/NO added. (B) Oxygen uptake assay of oxidizing potential of plasma from normal (+/+, □) and sph/sph (■) mice. Data presented as mean (± SEM) for n = 4 mice per group. Mice in both groups were between 7 and 13 weeks of age; there were no differences between data from younger versus older mice. *P < .013 compared with +/+ plasma.
) the addition of the NO donor proline/NO. Data are presented as mean (± SEM) for n = 3 +/+, n = 5 sph/+, and n = 4 sph/sph mice. All mice were between 6 and 9 weeks of age. *P < .02 compared with +/+ or sph/+plasma with proline/NO added. (B) Oxygen uptake assay of oxidizing potential of plasma from normal (+/+, □) and sph/sph (■) mice. Data presented as mean (± SEM) for n = 4 mice per group. Mice in both groups were between 7 and 13 weeks of age; there were no differences between data from younger versus older mice. *P < .013 compared with +/+ plasma.
Increased oxidizing potential of plasma from sph/sph mice
The oxidizing potential of WBB6F1-+/+ and sph/sph plasma was analyzed by an oxygen uptake assay using linoleic acid as the target for oxidative agents present in the plasma. As shown in Figure 4B, plasma from +/+ mice (open bar) generated little oxidation of linoleic acid, as evidenced by minimal oxygen consumption. This is to be expected, as oxidative reactions in plasma are normally kept under tight control.9,25,30,43-45 In contrast, when plasma from sph/sph mice was tested, a dramatically higher amount of oxygen was consumed as a result of the oxidation of linoleic acid (Figure 4B filled bar; P < .013). This is most likely reflective of the increased levels of plasma free hemoglobin in sph/sph compared with +/+ mice, and indicates that the plasma environment in sph/sph mice has a high oxidative potential.
Immunohistochemistry for XD/XO in normal and sph/sph mice
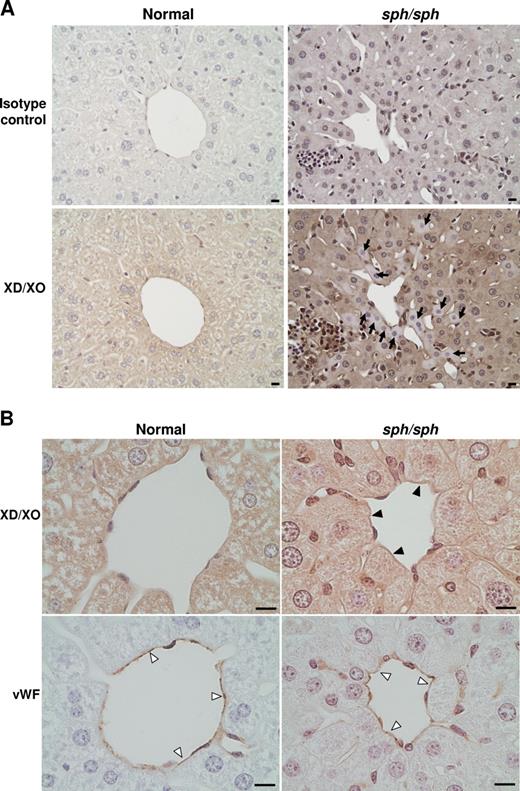
In response to oxidative stress, the enzyme XD, normally stored in the hepatocytes of the liver, is rapidly released into the plasma and converted to XO, a potent generator of superoxide.34,44 Immunohistochemistry (IHC) using a rabbit polyclonal antibody that detects both XD and XO showed diffuse staining in the hepatocytes of the liver of normal mice (Figure 5A). In contrast, hepatocyte staining for XD/XO in some areas of sph/sph liver sections was patchy; some hepatocytes showed little or no XD/XO staining (arrows, Figure 5A). This suggests that liver XD has been released into the circulation of sph/sph mice. In addition, staining with the same antibody showed the presence of XO on liver blood vessels in sph/sph but not normal mice (filled arrowheads, Figure 5B). These results indicate that tissue injury is likely occurring in sph/sph mice, and that XO, a potent generator of superoxide and thus further potential oxidative damage, is abnormally present along the endothelial lining of at least one vascular bed in the mutant mice.
IHC reveals release of XO from liver and presence of XO on liver vascular endothelium of sph/sph mice. (A) DAB-based IHC of liver sections from normal (+/+) and sph/sph mice with an antibody that detects both XD(the form stored in liver hepatocytes) and XO (generated by cleavage of XD by plasma proteases). Pictures shown are representative of results obtained from 3 different mice of each genotype (+/+, sph/sph; all mice more than 12 weeks old). Diffuse staining is seen in hepatocytes from normal mice (botom left panel). Sections from sph/sph mice (bottom right panel) show the presence of hepatocytes with little or no staining for XD/XO ( ). Bar, 10 μm. (B) High magnification of liver sections from normal and sph/sph mice. Top panels, staining for XD/XO, with presence of XO staining on endothelium of blood vessel in sph/sph (▶) but not +/+ mice. Bottom panels: adjacent sections stained (▷) with an antibody to VWF, an endothelial-specific marker. Bar, 10 μm. Pictures shown are representative of results obtained from 3 different mice of each genotype as described for Figure 5A.
). Bar, 10 μm. (B) High magnification of liver sections from normal and sph/sph mice. Top panels, staining for XD/XO, with presence of XO staining on endothelium of blood vessel in sph/sph (▶) but not +/+ mice. Bottom panels: adjacent sections stained (▷) with an antibody to VWF, an endothelial-specific marker. Bar, 10 μm. Pictures shown are representative of results obtained from 3 different mice of each genotype as described for Figure 5A.
IHC reveals release of XO from liver and presence of XO on liver vascular endothelium of sph/sph mice. (A) DAB-based IHC of liver sections from normal (+/+) and sph/sph mice with an antibody that detects both XD(the form stored in liver hepatocytes) and XO (generated by cleavage of XD by plasma proteases). Pictures shown are representative of results obtained from 3 different mice of each genotype (+/+, sph/sph; all mice more than 12 weeks old). Diffuse staining is seen in hepatocytes from normal mice (botom left panel). Sections from sph/sph mice (bottom right panel) show the presence of hepatocytes with little or no staining for XD/XO ( ). Bar, 10 μm. (B) High magnification of liver sections from normal and sph/sph mice. Top panels, staining for XD/XO, with presence of XO staining on endothelium of blood vessel in sph/sph (▶) but not +/+ mice. Bottom panels: adjacent sections stained (▷) with an antibody to VWF, an endothelial-specific marker. Bar, 10 μm. Pictures shown are representative of results obtained from 3 different mice of each genotype as described for Figure 5A.
). Bar, 10 μm. (B) High magnification of liver sections from normal and sph/sph mice. Top panels, staining for XD/XO, with presence of XO staining on endothelium of blood vessel in sph/sph (▶) but not +/+ mice. Bottom panels: adjacent sections stained (▷) with an antibody to VWF, an endothelial-specific marker. Bar, 10 μm. Pictures shown are representative of results obtained from 3 different mice of each genotype as described for Figure 5A.
Discussion
Five major conclusions can be drawn from the data presented in this article: (1) pulmonary hypertension is evident in sph/sph mice; (2) impaired systemic arterial vasodilation is present in sph/sph mice; (3) there is evidence of endothelial activation and injury in sph/sph mice; (4) plasma from sph/sph mice has increased plasma free hemoglobin, nitric oxide scavenging capacity, and oxidative potential; and (5) oxidative injury and stress occurs in sph/sph mice, by a mechanism that likely involves both cell-free Hb and the production of superoxide by xanthine oxidase.
RVSP, a surrogate measure for pulmonary artery pressure (PAP), was increased by 26% in sph/sph with severe HS compared with control mice (Figure 1A). It is important to note that previous studies6-8 (N.J.W., unpublished data) have not documented pulmonary thrombi/emboli or other lung pathology in sph/sph mice. Therefore, the pulmonary hypertension seen in sph/sph mice is not secondary to existing lung pathology. Hsu et al46 recently demonstrated the presence of pulmonary hypertension in Berkeley sickle mice by a 2-fold increase in PAP compared with hemizygous control mice; there was a similar lack of lung pathology in these mice. Interestingly, the increase in PAP/RVSP, relative to controls, was greater for sickle mice than for sph/sph mice with severe HS, despite the fact that sph/sph mice have higher levels of hemolysis (30%-50% reticulocytosis in Berkeley sickle mice vs 95%-100% reticulocytosis in sph/sph mice4,5,46,47 ). In contrast, right ventricular hypertrophy was increased 33% in Berkeley sickle mice relative to control,46 versus a 78% increase in sph/sph mice relative to control (Figure 1B), reflective of the greater anemia in sph/sph versus Berkeley sickle mice (hematocrit 0.22 in sph/sph versus 0.28 in Berkeley sickle mice4,5,46,47 ). These contrasting data suggest that, in addition to hemolysis, sickle hemoglobin may have a unique role in generating pulmonary hypertension; this hypothesis is echoed by the findings of Hsu and colleagues.46
Impaired endothelial-dependent vasodilation of the facialis artery in response to acetylcholine stimulation in sph/sph mice (Figure 2) indicates that vasoregulation is abnormal in the systemic as well as pulmonary vasculature in the mutant mice. The degree of inhibition of vasodilation in the facialis artery of sph/sph mice is similar to what has been documented in the same arterial bed from Berkeley sickle mice.48 In addition, impaired vasoregulation of the systemic circulation in Berkeley sickle mice has been documented in other vascular beds by several laboratories.46,49-51 Global impairment of vasoregulation in sph/sph mice may be reflective of a vascular response to long-term nitric oxide scavenging by plasma free hemoglobin released by the severe hemolysis of sph/sph red blood cells (Figure 4A).
In the current report, the plasma of sph/sph mice with severe HS was found to have increased levels of sVCAM-1, sP-sel, and sE-sel (Figure 3A,C,E). This is consistent with recent studies in patients with sickle cell disease,27-29,52-54 thalassemia,26,55 and hemolytic uremic syndrome,56,57 as well as mice with sickle cell disease.42,58,59 The increased plasma levels of soluble endothelial adhesion molecules (VCAM-1, P-sel, and E-sel) are indicative of increased exposure of these molecules on the endothelial cell surface, suggesting that the vascular endothelium in sph/sph mice is both activated and damaged. Interestingly, the majority of the soluble VCAM-1, P-sel, and E-sel signal in plasma from normal mice was removed by ultracentrifugation, suggesting that these molecules in normal mice are circulating on microparticles (Figure 3B,D,F). In contrast, ultracentrifugation had a much more limited effect on soluble VCAM-1, P-sel, and E-sel signal in plasma from sph/sph mice (Figure 3B,D,F), suggesting that the increased levels of these markers of endothelial damage is at best only partially explained by the presence of microparticles in sph/sph plasma.
The increased nitric oxide scavenging capacity by plasma free hemoglobin (Figure 4A) and the increased oxidizing potential (Figure 4B) of plasma from sph/sph compared with control mice suggests that one potential route by which the endothelium might be damaged and activated is through oxidative injury. The importance of oxidative damage in the vascular disruption in sph/sph mice is further supported by the release of xanthine dehydrogenase from the liver of sph/sph mice (Figure 5A), and the appearance of xanthine oxidase on the vascular endothelium of the liver of sph/sph mice (Figure 5B). These results imply that oxidative injury and stress is occurring in sph/sph mice by a mechanism that likely includes the production of superoxide by xanthine oxidase. Similar results have been seen in mice with sickle cell disease,34,48,50 suggesting that oxidative damage initiated by plasma free hemoglobin is a common factor contributing to endothelial dysfunction in hemolytic anemia.
In conclusion, our studies show that sph/sph mice with severe hereditary spherocytosis have evidence of endothelial activation, injury, and oxidative stress. These mice also have pulmonary and systemic vascular dysfunction, similar to mice with sickle cell disease. The vascular dysfunction, oxidative stress, and endothelial activation/injury documented in these studies likely influences the development of thrombosis and stroke6-8 in sph/sph mice. However, future studies comparing temporal variations in these parameters with the temporal development of thrombosis/stroke8 in sph/sph mice are necessary to further define the interrelationship(s) between these pathologic phenomena. Importantly, although sph/sph mice have higher levels of hemolysis than mice with sickle cell disease, they do not appear to have a more pronounced vascular dysfunction. These data suggest that, whereas red blood cell hemolysis is a major contributor to the development of pulmonary hypertension in hemolytic anemia, additional unique mechanisms may be provoked in sickle cell disease. Thus, sph/sph mice with severe HS serve as an excellent model to specifically study the role of hemolysis in the evolution of endothelial injury and overt vascular dysfunction. Further studies will permit the discrimination between pathologic mechanisms that are solely related to hemolysis and those that are further influenced by other contributing factors, such as sickle hemoglobin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We greatly appreciate the technical assistance of Noah Leigh and thank Drs Cheryl Hillery, J. Paul Scott, and Paula North for critical review of the manuscript and helpful scientific discussions.
This work was supported by American Heart Association grants 0265250Z (N.J.W.) and 0530073N (N.J.W.); National Institutes of Health grants HL071214 (K.A.P.), HL081139 (K.A.P.), EB001980 (National Biomedical EPR Center at Medical College of Wisconsin), GM55792 (N.H.), and HL066328 (K.A.F.); and support to N.J.W. from the Midwest Athletes Against Childhood Cancer (MACC) Fund.
National Institutes of Health
Authorship
Contribution: A.C.F. performed research; performed statistical analysis; and collected, analyzed, and interpreted data. Y.G. performed research and collected, analyzed, and interpreted data. D.W.J. and K.A.P. analyzed and interpreted data. K.A.F. designed and performed research; collected, analyzed, and interpreted data; contributed vital new analytical tools, and performed statistical analysis. N.H. designed and performed research; collected, analyzed, and interpreted data; contributed vital new analytical tools; and performed statistical analysis. N.J.W. designed research, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nancy J. Wandersee, Department of Pediatrics, Division of Hematology/Oncology, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: nancy.wandersee@bcw.edu.

![Figure 3. Increased workers of endothelial damage in sph/sph mice. Plasma levels of sVCAM-1 (A), sP-sel (C), and sE-sel (E) are increased in sph/sph mice compared with control mice. Levels of soluble murine vascular cell adhesion molecule-1 (sVCAM-1, A), soluble P-selectin (sP-sel, C), and soluble E-selectin (sE-sel, E) were determined by ELISA of plasma samples from control wild-type (+/+, □), control heterozygote (sph/+, ), and sph/sph (■) mice. Data are presented as mean (± SEM) for the following numbers: (A) n = 7 +/+ [all more than 8 weeks of age], n = 7 sph/+[all more than 8 weeks of age], n = 6 sph/sph [all more than 8 weeks of age]; (C,E) n = 13 +/+ [n = 6, 6-8 weeks of age; n = 7, more than 8 weeks of age], n = 12 sph/+[n = 6, 6-8 weeks of age; n = 6, more than 8 weeks of age], n = 17 sph/sph [n = 9, 6-8 weeks of age; n = 8, more than 8 weeks of age]. No significant difference between age groups was noted for sP-sel and sE-sel values. *Significant difference between sph/sph and control (+/+, sph/+) values: P < .0001 for sVCAM-1 (A) and sP-sel (C); P < .005 for sE-sel (E). Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in the plasma of sph/sph and control mice are variably reduced by ultracentrifugation. Plasma from +/+ (□ and gray-striped bars) mice more than 6 weeks of age and sph/sph mice (black and black-striped bars) more than 8 weeks of age was subjected to ultracentrifugation to remove microparticles as described under “Methods.” Levels of sVCAM-1 (B), sP-sel (D), and sE-sel (F) in plasma were compared before (“−UCS,” open and black bars) and after (“+UCS,” gray-striped and black-striped bars) ultracentrifugation. The difference between sVCAM-1 levels in A and B and sP-sel levels in C and D reflects the fact that plasma samples from separate sets of +/+ and sph/sph mice were used for the assays. Data are presented as mean (± SEM) for the following numbers: (B) n = 4 +/+ and n = 3 sph/sph; (D) n = 3 +/+ and n = 3 sph/sph; (F) n = 3 +/+ and n = 4 sph/sph. *Significant difference between “−UCS” and “+UCS” values, P < .03.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/2/10.1182_blood-2007-12-126714/4/m_zh80150821300003.jpeg?Expires=1768018009&Signature=QvfWDUK9ZpTyFBwaOePzsuB-gJ8~naYmKAiR~lUtF3wZr~nZDIlCtqX4K0xHOne1dUQhQ5Hn61QJgYqZ~iZ5CJtZ~N3O~rYLNlpLxv5IBCJuzmajBb9vhWmWy-1j6y2-YXo~7DdzVzdRfYP14b50Q7mqFIELNOfQPu4JOR1rXWbVDYDEpIH~hSabFc2oqImrBheTz6I7PJNL0flfRFlSmKWuNNq~d6mK0-Od1q0NaBEzx6UbO26sAxuYueW-WxzNsh6HhvvXcu4uraBYavNTkkOQ5l~etBl8k66XktyFjXNjSn74S3bjtpfKJDKhmNc0J4kJ3olcXRN4hs3~454img__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal