Abstract
Inflammatory responses represent a hallmark of numerous pathologies including sepsis, bacterial infection, insulin resistance, and malign obesity. Here we describe an unexpected coactivator function for the nuclear receptor interacting protein 140 (RIP140) for nuclear factor κB (NFκB), a master transcriptional regulator of inflammation in multiple tissues. Previous work has shown that RIP140 suppresses the expression of metabolic gene networks, but we have found that genetic as well as acute deficiency of RIP140 leads to the inhibition of the proinflammatory program in macrophages. The ability of RIP140 to function as a coactivator for cytokine gene promoter activity relies on direct protein-protein interactions with the NFκB subunit RelA and histone acetylase cAMP-responsive element binding protein (CREB)-binding protein (CBP). RIP140-dependent control of proinflammatory gene expression via RelA/CBP may, therefore, represent a molecular rational for the cellular integration of metabolic and inflammatory pathways.
Introduction
Metabolic diseases, such as insulin resistance, obesity, and atherosclerosis, have recently been recognized as low-grade, subacute inflammatory conditions, contributing to the development of type II diabetes and cardiovascular failure.1 Similar to acute inflammation, all of these conditions are characterized by elevated levels of proinflammatory cytokines such as interleukin-1β (IL-1β) and IL-6, and tumor necrosis factor α (TNFα).2,3 Toward this end, levels of IL-6, IL-1β, and TNFα are elevated in obese patients and mouse models of insulin resistance and obesity.2-4 In this respect, ablation of the TNFα gene or of its receptor renders mice resistant to the development of insulin resistance and associated metabolic disorders.5,6 A common polymorphism has been identified in the IL-6 receptor gene that is associated with energy intake and obesity in humans,7 underlining the critical impact of cytokine signaling for metabolic diseases.
The inflammatory response emerging in the presence of insulin resistance and obesity seems to reside predominantly in adipose tissue.1,8 Indeed, transgenic overexpression of monocyte chemotactic protein 1 (MCP1) in adipose tissue results in enhanced macrophage infiltration, inflammation, and insulin resistance.9,10 On the other hand, impairment of macrophage migration into adipose tissue by genetic knockout of the MCP1 receptor chemotactic cytokine receptor 2 (CCR2) has been found to substantially improve tissue inflammation and insulin sensitivity.10,11 Importantly, macrophages have recently been shown to accumulate under obese conditions in adipose tissue of both mice and humans.12,13 Resident macrophages in adipose tissue, therefore, seem to be in large part responsible for the cytokine release of this tissue and the systemic inflammation associated with obesity.12,14 These studies highlighted the importance of adipose tissue as a key site for the interaction of metabolic cells with effectors of the immune system, specifically macrophages, to control systemic energy homeostasis and to trigger metabolic dysfunction under pathophysiologic conditions.8 Consequently, not only adipose tissue quantity but also quality, as exemplified by its macrophage content and inflammatory status, seems to represent a critical determinant for the onset of insulin resistance and other components of the metabolic syndrome.8,15
In this respect, the cytokine release from macrophages in response to external signals is largely determined by transcriptional mechanisms. A number of transcriptional regulators have been identified as critical checkpoints for the proinflammatory response of macrophages, including activator protein 1 (AP1), E26-transformation–specific (Ets) factors, and nuclear factor κB (NFκB), acting through the signal-dependent assembly of cofactor complexes on respective cytokine target gene promoters.1,16,17
Receptor-interacting protein 140 (RIP140) has been characterized as a nuclear receptor cofactor, interacting with a number of nuclear receptor family members, such as peroxisome proliferator-activated receptors (PPARs), liver X receptor (LXR), estrogen receptor-related receptor (ERR), and estrogen receptor (ER).18 RIP140 has been defined as a critical factor for female fertility and oxidative metabolism in skeletal muscle and adipose tissue.19-21 Whole-body RIP140 knockout mice are lean and protected against diet-induced obesity resulting from an increase in mitochondrial biogenesis, respiratory activity, and thermogenesis.22 In this respect, RIP140 has been shown to be recruited to the uncoupling protein 1 (UCP-1) gene promoter in these cells, thereby efficiently controlling cellular energy expenditure.23
The delicate functional organization and structural proximity between adipocytes and macrophages in adipose tissue, therefore, prompted us to explore the function of RIP140 in macrophages, given that RIP140 has been described as an important checkpoint for adipose tissue biology. Our studies discovered an unforeseen coactivator function of RIP140 for NFκB, the transcriptional master regulator of inflammation in multiple cellular contexts.
Methods
All procedures were performed in accordance with the guidelines for animal care and use of the United Kingdom Home Office.
Plasmids, cell culture, and transfections
Promoter luciferase constructs containing the murine TNFα 5′-flanking region, 3xCRE-Luc, pκB-Luc, pκBmut-Luc, and the expression plasmids HA-CBP, Gal4-CBP, pCMV-HA-p300, p50, RelA (p65), RelB, c-Rel, mRIP140, Gal4-DBD-RelA, GST-RelA wild-type, GST-RelA(1-305) (RHD), GST-RelA(441-551) (TA), GST-CBP fragments, and pBK-HA-RIP140 were kind gifts from I. S. Singh, M. D. Conkright, M. Mayo, P. L. Puri, B. Baumann, L. N. Wei, M. Hottiger, M. Tini, J. Nyborg, and E. Treuter. pCI-Gal4-RIP140 full, pCI-Gal4-RIP140 (aa's 78-333; RD1), pCI-Gal4-RIP140 (aa's 410-700; RD2), pCI-Gal4-RIP140 (aa's 735-885; RD3), and pCI-Gal4-RIP140 (aa's 1118-1158; RD4) have been described previously.24 GST-CBP (722-1100), Flag-RIP140, and VP16-RIP140 were generated by standard polymerase chain reaction (PCR) procedures. The −121/−88-bp mouse TNFα promoter region was cloned into pGL3 using the following oligonucleotides: wt, 5′-CCGCTTCCTCCACATGAGATCATGGTTTTCTCCACA-3′; muEts, 5′-CCGCTGTTGTCACATGAGATCATGGTTTTCTCCACA-3′; muAP1, 5′-CCGCTTCCTCCACATGTCTACATGG-TTTTCTCCACA-3; and muκB, 5′-CCGCTTCCTCCACATGAGATC-ATGGATATCTCCACA-3′.
Murine RAW264.7 macrophages, murine 3T3-L1, and human embryonic kidney (HEK293T) cells were maintained in DMEM supplemented with 10% FBS and transfected using SuperFect reagent (RAW264.7; Promega, Mannheim, Germany), Lipofectamine (3T3-L1) (Invitrogen, Karlsruhe, Germany), or the calcium phosphate precipitation method (HEK293T) as described.25 Cells were left untreated or treated with LPS (1 μg/mL) or forskolin (10 μM; Sigma, Munich, Germany) for 24 hours and were harvested for luciferase assays 48 hours after transfection. The luciferase activity was normalized to beta galactosidase activity.
RNA interference
Oligonucleotides targeting mouse RIP140 (RNAi 1: 5′-GCTGCAGAAACTAAAGCTTCT-3′; RNAi 2: 5′-GCCACCTTTCAGAGTCCAATG-3′) were annealed and cloned into pENTRY RNAi vector (BLOCK-iT U6 RNAi Entry Vector Kit; Invitrogen). Nonspecific oligonucleotides (5′-GATCTGATCGACACTGTAATG-3′) with no significant homology to any mammalian gene sequence were used as nonsilencing controls in all experiments.
Recombinant adenoviruses
Adenoviruses expressing RIP140-specific or nonspecific control small hairpin RNAs (shRNAs) were produced using the BLOCK-iT Adenoviral RNAi expression system (Invitrogen) according to the manufacturer's instructions and purified by CsCl gradients as described.25 RAW264.7 macrophages were exposed to virus at a multiplicity of infection of 100 for 48 hours, treated with LPS (100 ng/mL), polyI:C (10 μg/mL), or Pam3CSK4 (100 ng/mL) for 8 hours, or left untreated, and supernatants and cells were harvested for enzyme-linked immunosorbent assay (ELISA) or RNA isolation, respectively.
Quantitative PCR
Total RNA from primary or RAW264.7 macrophages was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) and used for cDNA synthesis. cDNAs were analyzed by quantitative PCR using assays-on-demand specific for TNFα, TNF ligand superfamily 4 (TNFSF4), IL-6, IL-1β, interferon B1 (IFN-B1), acetyl-CoA acyltransferase 2 (ACAA2), carnitine palmitoyltransferase 1B (CPT1B), medium-chain acyl-CoA dehydrogenase (MCAD), long-chain acyl-CoA dehydrogenase (LCAD), acetyl-CoA carboxylase β (ACACβ), isocitrate dehydrogenase 3α (IDH3α), lipoprotein lipase (LPL), scavenger receptor CD36, runt-related transcription factor 1 (RUNX1), lysozyme (LYZS), colony-stimulating factor 1 receptor (CSF1R), epidermal growth factor module-containing mucinlike receptor 1 (EMR1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), RIP140, or TATA-box binding protein (TBP; Applied Biosystems, Darmstadt, Germany).
Cytokine release
Cellular supernatants of RAW264.7 macrophages infected with control or RIP140-specific shRNA adenoviruses were assayed for IL-1β, IL-6, and TNFα levels by ELISA according to manufacturer's instructions (R&D Systems, Minneapolis, MN).
Animal experiments
Bone marrow–derived macrophages were isolated from wild-type or RIP140 knockout mice19 and cultured for 5 to 7 days before the experiment. Adherent macrophages were treated with LPS (10 ng/mL) or polyI:C (10 μg/mL) for 2 hours or 6 hours, respectively, or left untreated, and were used for RNA isolation followed by Affymetrix (Santa Clara, CA) or quantitative PCR analysis.
Protein analysis
Protein was extracted from RAW264.7 cells, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and blotted onto nitrocellulose membrane. Western blot assays were performed as previously described25 using antibodies specific for CBP, RelA, RelB, c-Rel, p50 (Santa Cruz, Heidelberg, Germany), CREB (Upstate, Lake Placid, NY), FLAG M2, β-actin (Sigma), or RIP140.
Chromatin immunoprecipitation assay
RAW264.7 cells treated with 100 ng/mL LPS for 2 hours were cross-linked with 1% formaldehyde. Cells were lysed, sonicated, and subjected to immunoprecipitation using anti-RIP140 (mouse monoclonal antibody generated against full-length protein) or anti-HA (mouse monoclonal; Santa Cruz) antibody. Precipitated DNA fragments were analyzed by quantitative PCR using 2 distinct primer pairs directed against the IL-1β and IL-6 promoters. Primers for GAPDH were used as additional negative controls. Primer sequences are available upon request.
GST-pulldown assay
GST fusion proteins were produced in BL21 cells and affinity purified using glutathione sepharose (Amersham Biosciences, Darmstadt, Germany). In vitro transcription/translation was performed using the TNT T7/T3 quick coupled transcription/translation system (Promega) according to the manufacturer's instructions. GST and in vitro translated proteins were incubated at 4°C overnight. After extensive washing, GST-precipitated proteins were separated by SDS-PAGE and detected by autoradiography.
Immunoprecipitation
HEK293T cells were either cotransfected with a NFκB family member expression vector plus Flag-RIP140 or an empty Flag vector, or HEK293T cells were transfected with Flag-RIP140 or an empty Flag vector and treated with TNFα (1 μg/mL; Alexis Biochemicals, Grünberg, Germany) for 1 hour before harvesting the cells. Subsequently, cells were lysed and centrifuged, and the supernatant was incubated with anti-FLAG M2 Agarose (Sigma) for 2 hours. The immunoprecipitates were subsequently analyzed by Western blot as described.
Gene expression profiling
Gene expression profiling was done for macrophages from RIP140 knockout or wild-type mice. RNA isolation, cDNA and cRNA synthesis, and hybridization to arrays of type Mouse Genome 430 2.0 from Affymetrix were performed according to the manufacturer's recommendations. Three arrays per genotype were hybridized. Microarray data were analyzed based on ANOVA using a commercial software package (Micro Array Solution, version 1.0; SAS Institute, Cary, NC). Standard settings were used, except for the following specifications: log-linear mixed models26 were fitted for values of perfect matches, with genotype considered to be constant and the array identification, random. Custom CDF27 with Unigene-based gene/transcript definitions was used to annotate the arrays. Affected biologic pathways (taken from Subramanian et al28 [including KEGG]) reflected by the differential gene expression were determined by ORA29 based on Fisher exact test. The raw and normalized data were deposited in the Gene Expression Omnibus database as accession no. GSE10386.30
Statistical analyses
Statistical analyses were performed using Student t test or, for 2 paired samples, a 2-way analysis of variance (ANOVA). In the ANOVA, Bonferroni-adjusted comparisons between individual experimental groups were performed as indicated. The significance level was P equals .05.
Results
RIP140 controls proinflammatory gene expression and cytokine release of macrophages
To investigate a role for RIP140 in macrophages, we initially isolated and cultured primary, bone marrow–derived macrophages from RIP140 knockout and wild-type mice19 and analyzed their gene expression profiles using Affymetrix microarrays. Intriguingly, analysis of differentially regulated genomic pathways revealed that RIP140 deficiency significantly affected the expression of 4 distinct gene clusters (Figure 1A), most prominently the autoimmune/inflammatory pathway including cytokines (Figure 1A; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Differential expression of representative cytokine genes between RIP140 knockout and wild-type macrophages was subsequently confirmed by quantitative PCR analysis (Figure 1B). RIP140 deficiency led to the consistent inhibition of these genes (Figure 1B). To rule out the possibility that the constitutive RIP140 deficiency impairs macrophage differentiation, thereby indirectly influencing cytokine gene expression, we analyzed specific differentiation markers31 by quantitative PCR analysis. Importantly, RIP140 deficiency did not affect RUNX1, lysozyme, CSF1R, or EMR1 gene expression, demonstrating unaltered differentiation capacity of RIP140 knockout macrophages compared with wild-type cells (Figure S1). In contrast to cytokine genes, no major change in expression levels was observed for a number of key regulatory genes in metabolic pathways that had previously been identified as RIP140 targets in skeletal muscle and adipocytes20,32 (Figure 1C). Notably, only the fatty acid transporter CD36 was found to be elevated upon RIP140 knockout (Figure 1C).
Proinflammatory gene expression is impaired in primary RIP140 knockout macrophages. (A) Pathway analysis of differential gene expression in wild-type and RIP140 knockout primary macrophages. Significantly changed pathways are shown. Percentage of genes associated with the corresponding pathway among all genes within the 4 pathways indicated. (B) Real-time PCR analysis of mRNA levels for IL-1β, IL-6, TNFα, and TNFSF4, in bone marrow–derived macrophages from wild-type (wt) or RIP140 knockout (ko) littermates. (C) Real-time PCR analysis of mRNA levels for ACAA2, CPT1B, MCAD, LCAD, ACACB, IDH3A, LPL, CD36, and GAPDH in same animals as in panel B. (D,E) Real-time PCR analysis of mRNA levels for IL-6, IL-1β, TNFα, TNFSF4, and IFNB1 in bone marrow–derived macrophages from wild-type (wt) or RIP140 knockout (ko) littermates. Cells were treated with polyI:C (10 μg/mL) for 6 hours (D) or LPS (10 ng/mL) for 2 hours (E) or left untreated as indicated. Data are means plus or minus SEM (n ≥ 3, each done in duplicate). *P < .05.
Proinflammatory gene expression is impaired in primary RIP140 knockout macrophages. (A) Pathway analysis of differential gene expression in wild-type and RIP140 knockout primary macrophages. Significantly changed pathways are shown. Percentage of genes associated with the corresponding pathway among all genes within the 4 pathways indicated. (B) Real-time PCR analysis of mRNA levels for IL-1β, IL-6, TNFα, and TNFSF4, in bone marrow–derived macrophages from wild-type (wt) or RIP140 knockout (ko) littermates. (C) Real-time PCR analysis of mRNA levels for ACAA2, CPT1B, MCAD, LCAD, ACACB, IDH3A, LPL, CD36, and GAPDH in same animals as in panel B. (D,E) Real-time PCR analysis of mRNA levels for IL-6, IL-1β, TNFα, TNFSF4, and IFNB1 in bone marrow–derived macrophages from wild-type (wt) or RIP140 knockout (ko) littermates. Cells were treated with polyI:C (10 μg/mL) for 6 hours (D) or LPS (10 ng/mL) for 2 hours (E) or left untreated as indicated. Data are means plus or minus SEM (n ≥ 3, each done in duplicate). *P < .05.
In resting macrophages, cytokines are expressed at only very low levels.16 To investigate the impact of RIP140 on cytokine gene expression under activated conditions, we exposed primary wild-type and RIP140 knockout macrophages to 2 different inflammatory stimuli, triggering distinct subtypes of Toll-like receptor (TLR) signaling pathways.33 As shown in Figure 1D, TLR3 activation through its agonist polyI:C strongly stimulated IL-6, IL-1β, TNFα, TNFSF4, and IFN-B1 mRNA expression in wild-type macrophages (Figure 1D). Consistent with the inhibitory effect of RIP140 deficiency on basal expression (Figure 1B), activation of these proinflammatory genes was significantly impaired in primary RIP140 knockout macrophages (Figure 1D), showing that RIP140 was indeed required for the full activation of these genes. Identical results were obtained in cells exposed to the TLR4 ligand lipopolysaccharide (LPS; Figure 1E).
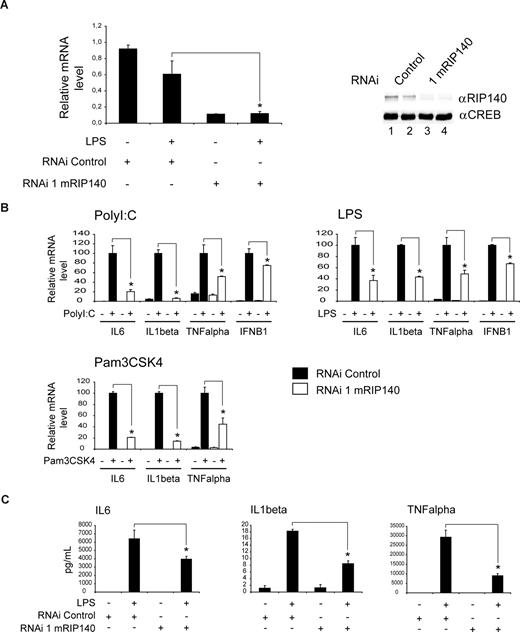
To investigate the effects of RIP140 on cytokine gene expression in an independent model, we acutely disrupted the activity of RIP140 in cultured RAW264.7 macrophages by delivering an adenovirus expressing a RIP140-specific or control shRNA construct. shRNA treatment efficiently down-regulated RIP140 mRNA (Figure 2A left panel) as well as protein levels compared with control shRNA-infected cells (Figure 2A right panel, compare lanes 1,2 to lanes 3,4). Consistent with findings in primary macrophages, acute RIP140 gene knockdown specifically diminished mRNA expression of IL-6, IL-1β, TNFα, and IFN-B1 compared with controls in polyI:C- and LPS-treated cells (Figure 2B). Whereas polyI:C and LPS signaling via TLR3/TLR4 is conferred through TRIF and MyD88/TRIF adaptor protein complexes, respectively, TLR2 uses MyD88 exclusively.33 To extend our studies to this distinct TLR pathway, we subsequently performed quantitative PCR analysis of representative RIP140 target genes also in the presence of the TLR2 agonist Pam3CSK4. As shown for TLR3/TLR4 ligand treatment, RIP140 deficiency impaired Pam3CSK4-stimulated cytokine gene expression significantly (Figure 2B). The expression of a number of unrelated genes including GAPDH remained unchanged upon acute RIP140 depletion in TLR-activated macrophages (data not shown). Importantly, identical results were obtained using a second, independent RIP140-specific shRNA construct in parallel studies (Figures S2,S3), supporting the specificity of the shRNA effects. Thus, we conclude that RIP140 is a key regulatory factor in the proinflammatory genetic program controlled by TLR2, TLR3, or TLR4.
RIP140 deficiency impairs TLR-mediated cytokine gene expression. (A) shRNA-mediated knockdown of RIP140 expression in macrophages. Real-time PCR analysis (left) and Western blot analysis (right) of RAW264.7 macrophages infected with an adenovirus expressing a RIP140-specific or control shRNA construct. Sixty hours after infection, cells were treated with LPS (100 ng/mL) for 8 hours as indicated. Proteins were detected by Western blot using RIP140- or CREB-specific antibody. Lane 1, shRNA control; lane 2, shRNA control plus LPS; lane 3, shRNA mRIP140; lane 4, shRNA mRIP140 plus LPS. (B) Real-time PCR analysis of mRNA levels for IL-6, IL-1β, TNFα, and IFN-B1 in RAW264.7 macrophages infected with an adenovirus expressing a RIP140-specific or control shRNA construct. Sixty hours after infection, RAW264.7 cells were treated with polyI:C (10 μg/mL), LPS (100 ng/mL), or Pam3CSK4 (100 ng/mL) for 8 hours as indicated. (C) Release of IL-6, IL-1β, and TNFα from RAW264.7 cells infected and treated with LPS as in panel B. Cytokine content in the cell supernatant was determined by ELISA. Data are means plus or minus SEM (n = 9). *P < .05.
RIP140 deficiency impairs TLR-mediated cytokine gene expression. (A) shRNA-mediated knockdown of RIP140 expression in macrophages. Real-time PCR analysis (left) and Western blot analysis (right) of RAW264.7 macrophages infected with an adenovirus expressing a RIP140-specific or control shRNA construct. Sixty hours after infection, cells were treated with LPS (100 ng/mL) for 8 hours as indicated. Proteins were detected by Western blot using RIP140- or CREB-specific antibody. Lane 1, shRNA control; lane 2, shRNA control plus LPS; lane 3, shRNA mRIP140; lane 4, shRNA mRIP140 plus LPS. (B) Real-time PCR analysis of mRNA levels for IL-6, IL-1β, TNFα, and IFN-B1 in RAW264.7 macrophages infected with an adenovirus expressing a RIP140-specific or control shRNA construct. Sixty hours after infection, RAW264.7 cells were treated with polyI:C (10 μg/mL), LPS (100 ng/mL), or Pam3CSK4 (100 ng/mL) for 8 hours as indicated. (C) Release of IL-6, IL-1β, and TNFα from RAW264.7 cells infected and treated with LPS as in panel B. Cytokine content in the cell supernatant was determined by ELISA. Data are means plus or minus SEM (n = 9). *P < .05.
To test whether the RIP140-dependent changes in proinflammatory gene activity are translated into altered cytokine release, we measured IL-1β, IL-6, and TNFα production of macrophages infected with RIP140-specific or control shRNA adenovirus by ELISA. As shown in Figure 2C, RIP140 knockdown reduced the release of these cytokines from LPS-activated cells, demonstrating that the regulation of gene expression via RIP140 is directly translated into alterations of biologically active, extracellular cytokine levels.
RIP140 impacts proinflammatory gene expression via NFκB response elements
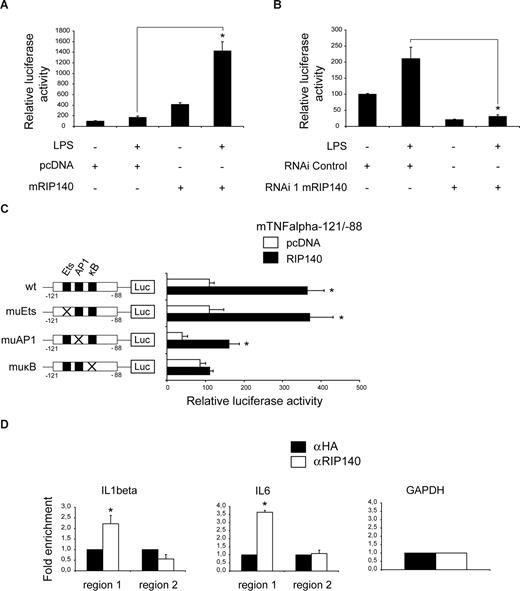
To investigate the significance of RIP140 for cytokine promoter control, we transfected cultured macrophages with a reporter gene construct carrying 1080 bp of the TNFα promoter in the presence or absence of RIP140. RIP140 stimulated TNFα promoter activity 4-fold under basal conditions (Figure 3A). LPS alone only weakly triggered TNFα promoter transcription, consistent with previous reports,34 but RIP140 expression potentiated LPS-induced TNFα reporter activity by 7-fold (Figure 3A), again indicating that RIP140 is functionally involved in the induction of TNFα gene expression. In contrast, shRNA-mediated RIP140 gene ablation (Figure 2A) inhibited LPS-induced as well as basal TNFα promoter activity (Figure 3B). Along with endogenous mRNA expression results from primary and cultured macrophages, these results substantiate the hypothesis that RIP140 plays a critical role in proinflammatory gene expression by promoter activation.
RIP140 regulates proinflammatory gene expression via direct promoter association. (A,B) Transient transfection assay of RAW264.7 cells cotransfected with a luciferase reporter containing − 1080 bp of the TNFα 5′-flanking region and an expression vector for RIP140 or empty pcDNA vector (A) or with plasmids carrying RIP140-specific or nonspecific control shRNA (B). Twenty-four hours after transfection, cells were either left untreated or treated with LPS (1 μg/mL) for 24 hours as indicated. Data are means plus or minus SEM (n = 9). *P < .05. (C) Transient transfection assay of RAW264.7 macrophages cotransfected with TNFα luciferase reporter carrying wild-type (wt) sequence from −121 to −88 bp or mutations in binding sites for Ets (muEts), AP1 (muAP1), or NFκB (muκB), together with an expression vector for RIP140 or empty pcDNA vector. Twenty-four hours after transfection, cells were treated with LPS (1 μg/mL) for 24 hours as indicated. Data are means plus or minus SEM (n = 9). *P < .05. Schematic representations of wild-type and mutated promoter constructs are shown. (D) Chromatin immunoprecipitation (ChIP) assay of RAW264.7 macrophages using antibodies specific for RIP140 (αRIP140) or nonspecific IgG (αHA). Precipitated fragments were analyzed by real-time PCR using IL-1β, IL-6, and GAPDH promoter primers. Data show fold enrichment relative to control IgG. Region 1, including NFκB site; region 2, without NFκB site. Data are means plus or minus SEM (n = 2). *P < .05.
RIP140 regulates proinflammatory gene expression via direct promoter association. (A,B) Transient transfection assay of RAW264.7 cells cotransfected with a luciferase reporter containing − 1080 bp of the TNFα 5′-flanking region and an expression vector for RIP140 or empty pcDNA vector (A) or with plasmids carrying RIP140-specific or nonspecific control shRNA (B). Twenty-four hours after transfection, cells were either left untreated or treated with LPS (1 μg/mL) for 24 hours as indicated. Data are means plus or minus SEM (n = 9). *P < .05. (C) Transient transfection assay of RAW264.7 macrophages cotransfected with TNFα luciferase reporter carrying wild-type (wt) sequence from −121 to −88 bp or mutations in binding sites for Ets (muEts), AP1 (muAP1), or NFκB (muκB), together with an expression vector for RIP140 or empty pcDNA vector. Twenty-four hours after transfection, cells were treated with LPS (1 μg/mL) for 24 hours as indicated. Data are means plus or minus SEM (n = 9). *P < .05. Schematic representations of wild-type and mutated promoter constructs are shown. (D) Chromatin immunoprecipitation (ChIP) assay of RAW264.7 macrophages using antibodies specific for RIP140 (αRIP140) or nonspecific IgG (αHA). Precipitated fragments were analyzed by real-time PCR using IL-1β, IL-6, and GAPDH promoter primers. Data show fold enrichment relative to control IgG. Region 1, including NFκB site; region 2, without NFκB site. Data are means plus or minus SEM (n = 2). *P < .05.
Remarkably, the majority of genes associated with the proinflammatory pathway down-regulated by RIP140 deficiency as identified by expression profiling represents prototypical targets of the NFκB transcription factor complex,35 a key regulator of the proinflammatory program in various tissues.16 This prompted us to test whether RIP140 controls proinflammatory gene expression in concert with this transcriptional regulator. To this end, we used a −88 to −121 bp TNFα promoter fragment harboring a conserved cluster of binding sites for Ets, AP1, and NFκB transcriptional regulators, which has been shown to be critical for LPS-dependent TNFα promoter activation.17 Promoter constructs comprising this NFκB-responsive region were cotransfected with RIP140 or control expression vectors into LPS-stimulated RAW264.7 macrophages. RIP140 overexpression activated the reporter approximately 3- to 4-fold, and this was maintained following mutation of the Ets- or AP1-binding sites (Figure 3C). In contrast, mutation of the NFκB site completely abolished this effect (Figure 3C muκB), demonstrating the functional importance of the NFκB recognition sequence for the ability of RIP140 to induce proinflammatory gene expression.
To explore whether RIP140 confers its activating effect on proinflammatory gene expression via direct promoter association, we performed ChIP studies in LPS-treated RAW264.7 macrophages. IL-1β and IL-6 promoter fragments containing NFκB recognition sequences (region 1) were readily recovered from precipitates using RIP140-specific (αRIP140) but not nonspecific control (HA) antibody (Figure 3D). In contrast, IL-1β, IL-6, and GAPDH promoter fragments lacking NFκB recognition sequences (region 2) were not detected in RIP140 precipitates (Figure 3D), indicating that RIP140 is directly recruited to NFκB-dependent proinflammatory gene promoters in vivo.
RIP140 coactivates NFκB through direct interaction with RelA
We next sought to explore whether RIP140 can serve as a direct NFκB coactivator. As homodimers and heterodimers of the NFκB subunit RelA have been shown to be largely responsible for proinflammatory cytokine gene expression,36 we overexpressed RIP140 alone or in combination with RelA along with the TNFα promoter in RAW264.7 macrophages. To initially verify the experimental system, we performed titration experiments showing that increasing amounts of RelA expression plasmid resulted in an up to 20-fold induction of TNFα promoter activity (data not shown). Because coregulators seem to exhibit more pronounced effects at low levels of coexpressed DNA-binding partners,37 we subsequently used amounts of RelA expression vector that caused only a 2-fold promoter induction compared with basal activity (Figure 4A). In this setting, coexpression of RIP140 synergistically induced RelA-driven reporter activity to 5-fold (Figure 4A). Furthermore, the 6- to 7-fold TNFα promoter induction found in the presence of higher amounts of RelA was blunted to 2-fold upon cotransfection of RIP140 shRNA construct (Figure 4B). Importantly, the RIP140 shRNA did not influence the expression of RelA in RAW264.7 macrophages (Figure S4). Together, these results suggest that endogenous RIP140 is required for the full activity of the NFκB/RelA transcriptional complex and can serve as a RelA coactivator.
RIP140 acts as a coactivator for NFκB. (A) Transient transfection assay of RAW264.7 macrophages cotransfected with a luciferase reporter containing −1080 bp of the TNFα promoter and expression vectors for RIP140 and RelA as indicated. (B) Same as in panel A using plasmids carrying RIP140-specific or nonspecific control shRNA constructs as indicated. (C) Transient transfection assay of RAW264.7 macrophages cotransfected with pκB-Luc (containing wild-type NFκB-binding sites) or pκB-mut-Luc (containing mutated NFκB-binding sites) together with RelA and RIP140-specific or nonspecific control shRNA constructs as indicated. Data are means plus or minus SEM (n = 9). *P < .05.
RIP140 acts as a coactivator for NFκB. (A) Transient transfection assay of RAW264.7 macrophages cotransfected with a luciferase reporter containing −1080 bp of the TNFα promoter and expression vectors for RIP140 and RelA as indicated. (B) Same as in panel A using plasmids carrying RIP140-specific or nonspecific control shRNA constructs as indicated. (C) Transient transfection assay of RAW264.7 macrophages cotransfected with pκB-Luc (containing wild-type NFκB-binding sites) or pκB-mut-Luc (containing mutated NFκB-binding sites) together with RelA and RIP140-specific or nonspecific control shRNA constructs as indicated. Data are means plus or minus SEM (n = 9). *P < .05.
To test this assumption in an isolated system, we cotransfected a luciferase reporter vector carrying 3 isolated NFκB-binding sites along with plasmids encoding RelA or a RIP140-specific shRNA construct in cultured macrophages. RelA alone induced activity of a wild-type reporter gene by 20-fold (Figure 4C, pκB-Luc). Coexpression of RIP140 shRNA plasmid inhibited RelA-driven transcription to approximately 5-fold (Figure 4C), further demonstrating a direct coactivating role of RIP140 for RelA and the requirement of endogenous RIP140 for full RelA transcriptional activity. Neither RelA nor RIP140 shRNA plasmid significantly affected the activity of a reporter construct comprising mutated RelA elements (Figure 4C pκB-mut-Luc), underlining the specificity of the observed effects. Identical results were obtained using a second, independent RIP140-specific shRNA construct along with the pκB-Luc promoter (Figure S5), thereby verifying the functional specificity of the experimental system.
To address the mechanism of the observed functional cooperation between RelA and RIP140, we tested their interaction in human embryonic kidney (HEK) cells expressing Flag-tagged RIP140 protein (Figure 5A lane 1). Coimmunoprecipitation studies demonstrated that endogenous RelA was recovered from precipitates of Flag-RIP140–transfected cells using Flag-specific antibody (Figure 5A lane 1, anti-RelA) but not control cells (Figure 5A lane 2), indicating that RelA and RIP140 form a complex in vivo. Consistent with this notion, a VP16-RIP140 fusion protein increased the activity of GAL4-RelA in mammalian-2-hybrid assays in both HEK and RAW264.7 cells (Figure 5B left and right panels, respectively), independently confirming the in vivo interaction of both proteins.
RIP140 physically interacts with RelA. (A) Coimmunoprecipitation of HEK293T cells transfected with FlagRIP140 or an empty vector using anti-FLAG M2 antibody. Bound proteins were resolved by SDS-PAGE and subsequently detected by Western blot using RelA and FLAG M2 antibodies. Cells were treated with TNFα (1 μg/mL) for 1 hour before harvesting. (B) Mammalian-2-hybrid assay in HEK293T (left) or RAW264.7 (right) cells using a GAL4-Luc reporter cotransfected with Gal4-DNA–binding domain (DBD)-RelA or the empty Gal4-DBD and VP16-RIP140 or VP16 alone as indicated. Data are means plus or minus SEM (n = 9). *P < .05. (C,D) Pulldown assays performed with full-length RelA, the transactivation domain (TA), or the Rel-homology domain (RHD) of RelA fused to GST and GST alone as a control. GST fusion proteins were incubated with in vitro translated full-length RIP140 (C) or in vitro translated repression domains 1 to 4 (RD1-4) of RIP140 (D). Bound proteins were resolved by SDS-PAGE and visualized by autoradiography. Input lanes represent 10% of the input. Schematic representations of RIP140-RelA interactions shown.
RIP140 physically interacts with RelA. (A) Coimmunoprecipitation of HEK293T cells transfected with FlagRIP140 or an empty vector using anti-FLAG M2 antibody. Bound proteins were resolved by SDS-PAGE and subsequently detected by Western blot using RelA and FLAG M2 antibodies. Cells were treated with TNFα (1 μg/mL) for 1 hour before harvesting. (B) Mammalian-2-hybrid assay in HEK293T (left) or RAW264.7 (right) cells using a GAL4-Luc reporter cotransfected with Gal4-DNA–binding domain (DBD)-RelA or the empty Gal4-DBD and VP16-RIP140 or VP16 alone as indicated. Data are means plus or minus SEM (n = 9). *P < .05. (C,D) Pulldown assays performed with full-length RelA, the transactivation domain (TA), or the Rel-homology domain (RHD) of RelA fused to GST and GST alone as a control. GST fusion proteins were incubated with in vitro translated full-length RIP140 (C) or in vitro translated repression domains 1 to 4 (RD1-4) of RIP140 (D). Bound proteins were resolved by SDS-PAGE and visualized by autoradiography. Input lanes represent 10% of the input. Schematic representations of RIP140-RelA interactions shown.
We then sought to determine whether the observed RelA/RIP140 interaction requires the presence of additional factors in general. To this end, wild-type RelA or deletion mutants thereof were purified as GST fusion proteins (Figure S6) and used in GST-pulldown assays using in vitro transcribed/translated RIP140. As shown in Figure 5C, wild-type RIP140 interacted with wild-type RelA as well as with the rel homology domain (RHD) of RelA, but not with the transactivation domain (TA; Figure 5C, compare lanes 3 and 5 with lane 4), showing that RIP140 contacts RelA through direct protein-protein interaction with the RHD domain. To further define this interaction, we subsequently used various RIP140 deletion mutants, covering previously identified repression domains 1 to 4 (RD1-4)24 along with the GST-RelA RHD fusion protein (Figure S7) in GST-pulldown experiments. In contrast to the lack of any interaction between RelA RHD and repression domains 2 to 4 (Figure 5D), pulldown of GST-RelA RHD recovered RIP140 RD1 (Figure 5D lane 3). Together, these data demonstrate that RIP140 serves as a noncanonical coactivator for RelA, and we propose that the RelA RHD recruits RIP140 via its RD1 domain.
Finally, to test whether the observed interaction was specific for RelA within the NFκB protein family, we performed additional coimmunoprecipitation studies in human embryonic kidney (HEK) cells, overexpressing a Flag-tagged RIP140 protein and different NFκB transcriptions factors (Figure S8 lane 1). In addition to RelA, we found that RelB, and to a minor extent c-Rel, were recovered from precipitates of Flag-RIP140–transfected cells using Flag-specific antibody (Figure S8 lane 1) but not control cells (Figure S8 lane 2). In contrast, no interaction could be observed between RIP140 and p50, an NFκB family member lacking a classical transactivation domain.36 These results indicate that RIP140 can potentially cooperate with multiple components of the NFκB transcriptional complex in addition to RelA.
RIP140 cooperates with acetyl-transferase CBP
In the majority of studies, RIP140 has been characterized as a transcriptional corepressor of metabolic target genes,18 which is in striking contrast to the observed coactivating function on RelA in the control of proinflammatory genes. Transfection studies using GAL4-RIP140 fusion proteins and GAL4 luciferase reporter vectors demonstrated that RIP140 by default lacks an intrinsic activation function in various cell culture systems, including HEK and RAW264.7 cells (data not shown). These results suggest that the coactivator potential of RIP140 is not determined by the specific cell context, but may be conferred by the RelA-associated transcriptional coactivator complex.
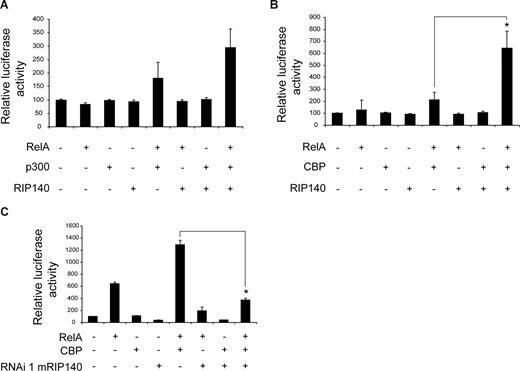
Therefore, we tested the hypothesis that RelA coactivation by RIP140 is based on the interaction of RIP140 with other RelA cofactors rather than on an intrinsic activation function. In particular, the histone acetyltransferases p300/CBP have been characterized as coactivators of RelA on proinflammatory gene promoters such as TNFα.38 Based on initial titration experiments (data not shown), we used low doses of expression vectors that alone only mildly affected reporter gene activity. Under these conditions, RIP140 overexpression only slightly influenced p300/RelA-mediated transcription at the TNFα promoter in macrophages (Figure 6A). However, RIP140 clearly enhanced CBP/RelA-driven TNFα promoter activity in these cells (Figure 6B), suggesting that RIP140 preferentially cooperates with the CBP coactivator complex on RelA-regulated gene promoters. We then sought to verify that endogenous RIP140 was required for maximum TNFα promoter activity by depleting cells of the protein using RIP140-specific shRNA. As shown in Figure 6C, we found that the ability of CBP to enhance RelA-dependent TNFα promoter activity by 2-fold was completely abolished (Figure 6C), demonstrating that endogenous RIP140 is required for full CBP/RelA transcriptional activity in this context. We next sought to determine whether RIP140 acts as a CBP coactivator also in other contexts. The cAMP-responsive DNA element (CRE) has been established as a prototypical response sequence for CREB/CBP-driven transcription.39 Interestingly, RIP140 shRNA did not block the cAMP-stimulated activity of an isolated CRE in transient transfection assays (Figure S9), indicating that the coactivating action of RIP140 is, at least to a certain degree, specific for the CBP/RelA context.
RIP140 functionally cooperates with CBP. (A,B) Transient transfection assay of RAW264.7 cells cotransfected with a luciferase reporter containing −1080 bp of the TNFα 5′-flanking region and expression vectors for RIP140, RelA, and p300 (A) or RIP140, RelA, and CBP (B) as indicated. (C) Transient transfection assays of RAW264.7 cells cotransfected with a luciferase reporter containing −1080 bp of the TNFα promoter and expression vectors for RelA and CBP along with RIP140-specific or control shRNA vectors as indicated. Data are means plus or minus SEM (n = 9). *P < .05.
RIP140 functionally cooperates with CBP. (A,B) Transient transfection assay of RAW264.7 cells cotransfected with a luciferase reporter containing −1080 bp of the TNFα 5′-flanking region and expression vectors for RIP140, RelA, and p300 (A) or RIP140, RelA, and CBP (B) as indicated. (C) Transient transfection assays of RAW264.7 cells cotransfected with a luciferase reporter containing −1080 bp of the TNFα promoter and expression vectors for RelA and CBP along with RIP140-specific or control shRNA vectors as indicated. Data are means plus or minus SEM (n = 9). *P < .05.
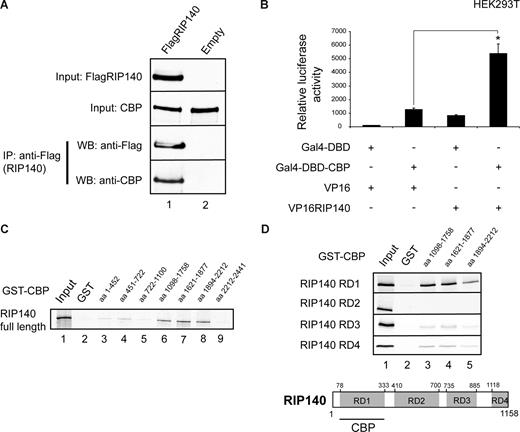
To test whether the cooperation between RIP140 and CBP relies on direct complex formation, we performed coimmunoprecipitation studies. Indeed, in HEK cells endogenous CBP was recovered from precipitates of Flag-RIP140–transfected cells using Flag-specific antibody (Figure 7A lane 1, anti-CBP) but not from control cells (Figure 7A lane 2), demonstrating that CBP and RIP140 interact in vivo. Moreover, a VP16-RIP140 fusion protein strongly increased the activity of GAL4-CBP in mammalian-2-hybrid assays (Figure 7B), which again is in line with RIP140-CBP complex formation in vivo.
RIP140 associates with CBP in vivo. (A) Coimmunoprecipitation of HEK293T cells transfected with FlagRIP140 or an empty Flag vector using anti-FLAG M2 antibody. Bound proteins were resolved by SDS-PAGE and subsequently detected by Western blot using CBP and FLAG M2 antibodies. Cells were treated with TNFα (1 μg/mL) for 1 hour before harvesting. (B) Mammalian-2-hybrid assay in HEK293T cells using a GAL4-Luc reporter cotransfected with Gal4-DBD-CBP or the empty Gal4-DBD and VP16-RIP140 or the empty VP16 alone as indicated. Data are means plus or minus SEM (n = 9). *P < .05. (C,D) Pulldown assays performed with different CBP deletion mutants (as indicated) fused to GST and GST alone used as a control. GST deletion constructs were incubated with in vitro translated full-length RIP140 (C) or in vitro translated repression domains 1 to 4 (RD1-4) of RIP140 (D). Bound proteins were resolved by SDS-PAGE and visualized by autoradiography. Input lanes represent 10% of the input. Schematic representation of RIP140/CBP interactions shown.
RIP140 associates with CBP in vivo. (A) Coimmunoprecipitation of HEK293T cells transfected with FlagRIP140 or an empty Flag vector using anti-FLAG M2 antibody. Bound proteins were resolved by SDS-PAGE and subsequently detected by Western blot using CBP and FLAG M2 antibodies. Cells were treated with TNFα (1 μg/mL) for 1 hour before harvesting. (B) Mammalian-2-hybrid assay in HEK293T cells using a GAL4-Luc reporter cotransfected with Gal4-DBD-CBP or the empty Gal4-DBD and VP16-RIP140 or the empty VP16 alone as indicated. Data are means plus or minus SEM (n = 9). *P < .05. (C,D) Pulldown assays performed with different CBP deletion mutants (as indicated) fused to GST and GST alone used as a control. GST deletion constructs were incubated with in vitro translated full-length RIP140 (C) or in vitro translated repression domains 1 to 4 (RD1-4) of RIP140 (D). Bound proteins were resolved by SDS-PAGE and visualized by autoradiography. Input lanes represent 10% of the input. Schematic representation of RIP140/CBP interactions shown.
To investigate the RIP140-CBP interaction in more detail, we performed GST-pulldown experiments using various GST-CBP fragments (Figure S10), spanning the entire CBP full-length protein sequence along with in vitro transcribed/translated RIP140 protein. These studies demonstrated that wild-type RIP140 interacts with fragments comprising the C-terminal CH2, CH3, and Q-rich domain of CBP40 (Figure 7C lanes 6-8). In turn, these GST-CBP fusion proteins (Figure S11) efficiently recovered the RIP140 RD1 domain in subsequent GST interaction assays (Figure 7D RD1, lanes 3-5) but not RDs 2 to 4 (Figure 7D RD2-4). Together, these data indicated that the CBP-RIP140 interaction specifically relies on the CBP C-terminus and the RIP140 RD1 domain, and that the ability of RIP140 to function as a coactivator for RelA can be explained, at least in part, by the interaction with acetyl-transferase CBP.
Discussion
Inflammatory reactions are critical hallmarks of the body's response to injuries or the invasion of exogenous pathogens and the activation of the innate immune system.41 More recently, inflammation has been recognized as an important factor in the development of metabolic diseases, such as atherosclerosis, obesity, and insulin resistance. Aberrant production and secretion of a variety of proinflammatory cytokines from various cell types in this context seem to be largely determined at the gene promoter level through transcriptional control mechanisms.1,16 In this report, we identify an unexpected coactivating function for the nuclear receptor corepressor RIP140 in the control of NFκB-dependent proinflammatory gene expression.
Global gene expression profiling in macrophages showed that RIP140 deficiency specifically impairs the execution of the proinflammatory program (Figures 1,2). Whole-body RIP140 knockout animals are lean, display resistance against diet-induced obesity, and maintain insulin sensitivity, suggesting that RIP140 globally promotes fat accumulation and insulin resistance.22 Our results are consistent with the assumption/hypothesis that proinflammatory RIP140 action in macrophages and other cell types contributes to insulin resistance susceptibility by triggering cellular cytokine production and release under insulin-resistant or obese conditions, when adipose tissue is characterized by a relative increase in invading macrophages.12,14 Interestingly, our data suggest that RIP140 is not specifically associated with a distinct TLR pathway in these cells, as RIP140 deficiency equally impaired cytokine gene activation by TLR2, TLR3, and TLR4 signaling (Figures 1,2). Indeed, this lack of TLR specificity of RIP140 action is consistent with the role of the NFκB transcriptional com-plex as a common point of convergence for multiple TLR signaling cascades.33
RIP140 was initially identified as a cofactor for the estrogen receptor and other members of the nuclear receptor family.42 Although individual studies have reported an activating function for RIP140 in diverging contexts,43-45 RIP140 has been considered mainly as an atypical transcriptional repressor. Thus, the expression of multiple genes involved in mitochondrial biogenesis, oxidative phosphorylation, and thermogenesis were found to be negatively regulated by RIP140 action.20,22,23 The repressive capacity of RIP140 in this context seems to rely on the interaction with the C-terminal binding protein (CtBP) corepressor46 and the recruitment of histone deacetylases (HDACs) via distinct repressor domains within the RIP140 protein.47 More recently, RIP140 has been found to potentiate the transcription of a number of genes required for triglyceride synthesis,44 but the mechanism by which this is achieved was not elucidated.
Unexpectedly, our study has identified a role for RIP140 in the activation of proinflammatory gene expression. The stimulatory function of RIP140 in the inflammatory pathway seems to depend on its interaction with RelA and the CBP coactivator (Figures 5 and 7). Given the ubiquitous expression of RelA and CBP, RIP140 might be of general importance for the induction of transcriptional programs regulated by RelA/CBP also in other tissues and cell types. Given the association of RIP140 with the NFκB/RelA transcription factor complex and the dependence of its activity on NFκB DNA regulatory elements, RIP140 may represent a novel component of the transcriptional response unit contributing to the maximum induction of cytokine gene transcription by RelA. Indeed, previous reports have shown that RIP140 alleviates repression of TNFα by the ER,48 and RIP140 can revert inhibition of RelA by glucocorticoids on artificial reporter systems.49 The impact of RIP140 might also be extended to RelB and c-Rel target genes, and it is conceivable that RIP140 may play a role in the activation of specific RelB dimer-dependent subsets of target genes, potentially distinct classes of chemokines.36
As transcription factor interactions provide an attractive target for small molecule–guided therapeutic approaches, the identification of the RelA/CBP/RIP140 transcriptional unit may pave the way to a novel molecular interference strategy for low-grade inflammation and its long-term complications by targeting cellular cytokine production during obesity, atherosclerosis, or insulin resistance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank B. Baumann (Ulm University, Ulm, Germany), M. Conkright (Scripps Research Institute, Jupiter, FL), M. O. Hottiger (University of Zurich, Zurich, Switzerland), M. Mayo (University of Virginia, Charlottesville), J. Nyborg (Colorado State University, Fort Collins), P. L. Puri (Dulbecco Telethon Institute, Rome, Italy), I. S. Singh (University of Maryland, Baltimore), M. Tini (University of Western Ontario, London, ON), L. N. Wei (University of Minnesota, Minneapolis) for reagents and members of our laboratories for critical discussions.
This work was supported by the Novartis Foundation for Therapeutic Research (Nürnberg, Germany), the Emmy-Noether Program (Deutsche Forschungsgemeinschaft), and a Marie Curie Excellence Grant (European Union, to S.H.).
Authorship
Contribution: I.Z., U.H., A.K.-H., A.V., M.B.D., J.M., D.S., and R.Z. performed the research; X.Y. and N.G. analyzed the results; T.G.H., M.C., R.W., and M.G.P. provided new material; S.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephan Herzig, German Cancer Research Center Heidelberg, Molecular Metabolic Control A170, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; e-mail: s.herzig@dkfz.de.