Abstract
Procoagulant factor VIII (FVIII) is either produced endogenously under physiologic conditions, or administered exogenously as a therapeutic hemostatic drug in patients with hemophilia A. In the circulation, FVIII interacts with a multitude of glycoproteins, and may be used for coagulation at the sites of bleeding, eliminated by scavenger cells, or processed by the immune system, either as a self-constituent or as a foreign antigen. The fate of FVIII is dictated by the immune status of the individual, the location of FVIII in the body at a given time point, and the inflammatory microenvironment. It also depends on the local concentration of FVIII and of each interacting partner, and on the affinity of the respective interactions. FVIII, by virtue of its promiscuity, thus constitutes the core of a dynamic network that links the coagulation cascade, cells of the immune system, and, presumably, the inflammatory compartment. We describe the different interactions that FVIII is prone to establish during its life cycle, with a special focus on players of the innate and adaptive immune response. Lessons can be learned from understanding the dynamics of FVIII interactions—lessons that should pave the way to the conception of long-lasting hemostatic drugs devoid of iatrogenic immunogenicity.
Promiscuity of the FVIII molecule under physiologic conditions
The life cycle of FVIII
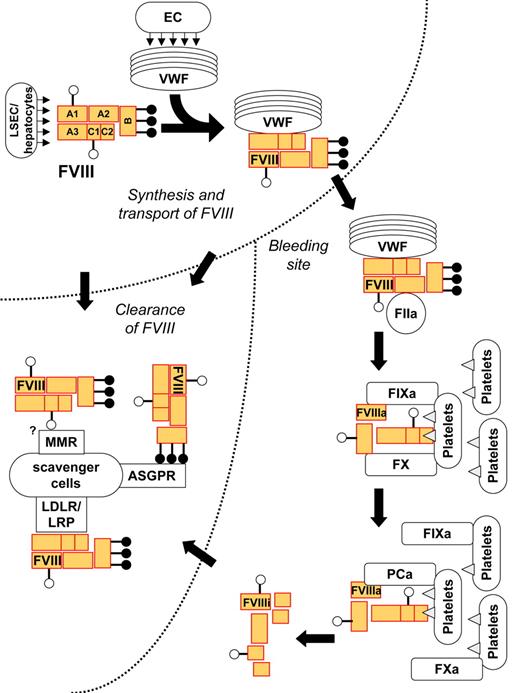
The life cycle of FVIII has been described in great detail previously.1 Here we recapitulate the key steps of the FVIII life cycle, and highlight the different molecules that FVIII may interact with at different stages of its life. In the circulation, FVIII binds to at least 10 different identified proteins, some of them behaving as physiologic chaperones, some that participate in the coagulation cascade, and others being involved in removal of biologic wastes (Figure 1). While binding to different proteins may involve the same structures on the FVIII molecule, the sequence of FVIII interactions with its physiologic partners is ordered temporally and spatially, depending on the quiescent, activated, or inactivated state of the molecule. Three phases in the life cycle of FVIII may thus be distinguished: transport of FVIII from the site of secretion to the bleeding site; participation of FVIII in the coagulation cascade; and clearance of FVIII from the circulation. Because it is not pertinent for the present discussions, we leave aside the intramolecular interactions of FVIII within the cells that produce it.2
Life cycle of FVIII under physiologic conditions. Following secretion by liver sinusoidal endothelial cells (LSECs) or hepatocytes, the heterodimeric FVIII binds to circulating von Willebrand factor (VWF). VWF transports FVIII to the bleeding site, where FVIII is cleaved and activated by thrombin (FIIa). Activated FVIII binds to phospholipids (shown as triangles) on the membrane of activated cells and platelets and forms a complex with activated factor IX (FIXa) and factor X (FX), which results in activation of FX. The complex disassembles and FVIII is inactivated by spontaneous dissociation of its subunits, or cleavage by activated protein C (APC). Native, activated, or inactivated FVIII may be eliminated by binding to catabolic receptors on scavenger cells: the asialoglycoprotein receptors (ASGPRs) binds to galactose-ending glycans on the B domain of the FVIII, and receptors of the LDL receptor (LDLR) family bind to protein moieties on the heavy and/or light chain of the molecule. A role for the macrophage mannose receptors (MMRs) in FVIII catabolism is being investigated. VWF prevents the early elimination of FVIII by blocking its binding to the scavenger receptors.
Life cycle of FVIII under physiologic conditions. Following secretion by liver sinusoidal endothelial cells (LSECs) or hepatocytes, the heterodimeric FVIII binds to circulating von Willebrand factor (VWF). VWF transports FVIII to the bleeding site, where FVIII is cleaved and activated by thrombin (FIIa). Activated FVIII binds to phospholipids (shown as triangles) on the membrane of activated cells and platelets and forms a complex with activated factor IX (FIXa) and factor X (FX), which results in activation of FX. The complex disassembles and FVIII is inactivated by spontaneous dissociation of its subunits, or cleavage by activated protein C (APC). Native, activated, or inactivated FVIII may be eliminated by binding to catabolic receptors on scavenger cells: the asialoglycoprotein receptors (ASGPRs) binds to galactose-ending glycans on the B domain of the FVIII, and receptors of the LDL receptor (LDLR) family bind to protein moieties on the heavy and/or light chain of the molecule. A role for the macrophage mannose receptors (MMRs) in FVIII catabolism is being investigated. VWF prevents the early elimination of FVIII by blocking its binding to the scavenger receptors.
Transport of FVIII from the site of secretion to the bleeding site.
FVIII is synthesized mainly in the liver by sinusoidal endothelial cells3,4 and possibly by hepatocytes.5,6 Production of FVIII by human microvascular lung endothelial cells has also been reported.7 Indeed, transplantation experiments in dogs have indicated that extrahepatic FVIII synthesis suffices for hemostasis.8-10 The mature FVIII is released in the circulation as a heterodimeric glycoprotein that consists of 2332 amino acids and encompasses a heavy chain (HC; domains A1-a1-A2-a2-B, residues 1-1648) and a light chain (LC; domains a3-A3-C1-C2, residues 1649-2332).1 The molecule contains 25 consensus sequences (Asn-Xxx-Thr/Ser) that allow N-linked glycosylation, of which 19 have been shown to be glycosylated. The overall sugar content of recombinant human FVIII is of high mannose type and biantennary, triantennary, and tetra-antennary complex-type sugar chains.11,12
Upon its release in the circulation, FVIII binds in a noncovalent manner to circulating endogenous von Willebrand factor (VWF).13 The binding between the 2 molecules involves the a3 and the C2 domains of the light chain of FVIII, and the D′D3 region of VWF, with an affinity of 0.2 to 0.4 nM.13 This means that, under physiologic conditions and at the concentrations of FVIII and VWF that circulate in blood (1 and 50 nM, respectively), approximately 94% of the FVIII molecules are bound to VWF, while the remaining 6% circulate in a free form.
The binding of VWF to FVIII is critical at different stages of the life cycle of FVIII. It protects FVIII from binding to the surface of activated platelets and endothelial cells14 and from proteolytic attack by a variety of lipid-bound serine proteases, including activated protein C and activated factor X (FXa).15,16 Importantly, by virtue of its binding to the exposed collagen, VWF directs FVIII to the site of bleeding.
Participation of FVIII in the coagulation cascade.
At the site of bleeding, VWF displays FVIII for being cleaved and activated by thrombin.17 Thrombin-mediated proteolytic cleavage of FVIII occurs within the light chain at Arg1689 and within the heavy chain at Arg372 and Arg740. FVIII may also be cleaved and activated by FXa.18 Cleavage of FVIII at Arg1689 results in conformational changes in its light chain that alter the binding affinity of activated FVIII (FVIIIa) for VWF, thus allowing its release and integration into the tenase complex.
In the tenase complex, FVIIIa acts as a tridirectional magnet. First, by binding to FX through its A1 domain (residues 337-372)19 and through residues 558 to 565, 698 to 710, and 1811 to 1818 to activated FIX (FIXa; reviewed in Lenting et al1 ), it brings the 2 molecules together, thus increasing the catalytic efficiency of FIXa in generating FXa by many-fold.20 In this respect, FVIIIa acts merely as a passive intermediary, as evidenced by the fact that it can be replaced by an engineered monoclonal antibody with bispecificity for both FX and FIXa.21
In contrast to the latter monoclonal antibody, however, activated FVIII anchors the tenase complex to the phosphatidyl serines on the membrane of activated platelets or endothelial cells,14 thus restricting the amplification loop of thrombin activation at the place of vascular injury. Upon dissociation of the tenase complex, FVIIIa either simply disassembles into subunits or is hydrolyzed by activated protein C, both of which result in its inactivation.1 Proteolytic inactivation of FVIIIa also involves cleavages by FIXa and FXa at positions 336 and 562.
Clearance of FVIII from the circulation.
The half-life of FVIII in the circulation ranges from 12 to 16 hours.22,23 Degradation of FVIII has been proposed to occur in the liver, and probably also occurs in the spleen (A.-M.N., S. Delignat, and S.L.-D., manuscript in preparation). FVIII is removed from the circulation by pinocytosis or phagocytosis or through specific interactions with catabolic receptors expressed by scavenger cells, such as macrophages. Catabolic receptors for FVIII include asialoglycoprotein receptors (ASGPRs), heparan sulfate proteoglycans (HSPGs), or members of the low-density lipoprotein receptor (LDLR) family.24-27 Disruption of the expression of hepatic low-density lipoprotein receptor-related protein (LRP/CD91) in conditional knockout mice, or saturation of receptors of the LDLR family using the receptor-associated protein (RAP), results in increased FVIII residence time in the blood.24,27-29 Similarly, saturation of the liver-expressed ASGPR using the ASGPR antagonist asialo-orosomucoid leads to prolonged half-life of circulating FVIII.30
By preventing the interaction of FVIII with LRP, VWF plays an important role in reducing FVIII catabolism.25 We have recently demonstrated that VWF prevents the binding of FVIII to the macrophage mannose receptor (MMR/CD206) and its subsequent endocytosis in vitro.31 We are currently investigating whether MMR also participates in FVIII catabolism in vivo. Interestingly, some mutations in VWF are associated (1) with a loss or a drastic reduction in the affinity of VWF for FVIII, which results in a pathology referred to as type 2N von Willebrand disease (VWD),32,33 or (2) with a complete lack of endogenous VWF, as seen in patients with type 3 VWD.34 Circulating levels of FVIII in patients with type 2N or type 3 VWD are between 10% to 30% or less than 5%, respectively, of the levels found in the plasma of healthy individuals, and the half-life of FVIII is reduced to 2 to 3 hours.35 Conversely, the infusion of VWF into VWF-deficient patients elevates circulating FVIII to above-normal levels.36
FVIII as seen by the immune system under physiologic conditions
Tolerance toward self-antigens.
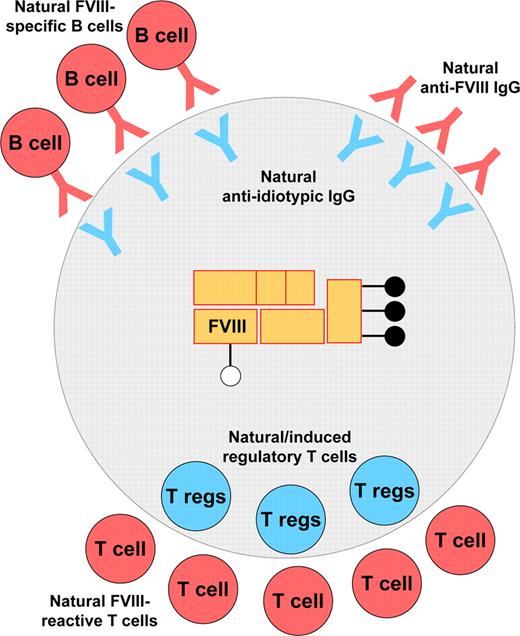
While performing its protective role against infection, the immune system discriminates foreign organisms and detrimental molecules from self-constituents. To achieve efficient discrimination between self and nonself, the immune system has evolved delicate and complex mechanisms that include physical or functional elimination of autoreactive T and B lymphocytes. Thus, strongly autoreactive T cells are eliminated during ontogeny in the thymus.37 Similarly, most self-reactive B lymphocytes are eliminated or negatively regulated by one of several mechanisms including clonal deletion and receptor editing. Nevertheless, central tolerance is not foolproof: self-reactive T and B cells, and autoreactive antibodies are found at the periphery. The pathologic manifestation of self-reactive T and B cells is tightly controlled by different regulatory mechanisms under physiologic conditions: the presentation of self-antigens by dendritic cells in steady state ensures the anergy or deletion of autoreactive clones38 ; regulatory T cells monitor the state of activation of self-reactive T cells, B lymphocytes, and antigen-presenting cells (APCs)39-41 ; anti-idiotypic antibodies neutralize potentially harmful circulating self-reactive antibodies and possibly down-regulate the functions of autoreactive B cells.42 In agreement with this, and as explained in “Recognition of FVIII by CD4+ T lymphocytes in healthy individuals,” FVIII is not invisible to the immune system of healthy individuals. Tolerance to FVIII instead relies on a delicate equilibrium between recognition by immune effectors and regulation of these effectors (Figure 2), thus complying with the gnothi seauton (ie, “know yourself”) theory once postulated by Stratis Avrameas.43
Steady-state interaction of FVIII with the immune system under physiologic conditions. At the humoral level, tolerance to FVIII under physiologic conditions relies on an equilibrium between the recognition of FVIII by naturally occurring potentially inhibitory anti-FVIII antibodies and their control by neutralizing anti-idiotypic antibodies. Neutralizing anti-idiotypic antibodies may also regulate the B-cell clones that secrete the FVIII-specific autoantibodies. At the T-cell level, natural FVIII-reactive T cells may be down-regulated by natural regulatory T cells (Tregs; ie, CD4+CD25+FoxP3+ Tregs) and/or by induced transforming growth factor β (TGF-β)–secreting Tregs.
Steady-state interaction of FVIII with the immune system under physiologic conditions. At the humoral level, tolerance to FVIII under physiologic conditions relies on an equilibrium between the recognition of FVIII by naturally occurring potentially inhibitory anti-FVIII antibodies and their control by neutralizing anti-idiotypic antibodies. Neutralizing anti-idiotypic antibodies may also regulate the B-cell clones that secrete the FVIII-specific autoantibodies. At the T-cell level, natural FVIII-reactive T cells may be down-regulated by natural regulatory T cells (Tregs; ie, CD4+CD25+FoxP3+ Tregs) and/or by induced transforming growth factor β (TGF-β)–secreting Tregs.
Recognition of FVIII by CD4+ T lymphocytes in healthy individuals.
The proliferation of circulating CD4+ T cells from healthy individuals upon exposure to FVIII was described more than 10 years ago.44 It has since been confirmed using, as antigens, both intact FVIII and pools of synthetic peptides that span different domains of the FVIII molecule. Depending on the reports, circulating CD4+ T cells from 52% to 78% of the donors proliferated when incubated with FVIII in vitro.45,46 Conti-Fine and coworkers demonstrated transient proliferative responses toward pools of peptides spanning the C2 domain (Reding et al47 ), the A3 domain (Reding et al48 ), and the A2 domain (Hu et al49 ) of FVIII. The detection of FVIII-reactive CD4+ T cells in these experi-ments may have been underestimated, however; indeed, naturally occurring self-reactive T lymphocytes may be controlled by regulatory T cells.
Regulatory CD4+ T cells (Tregs) either exist as naturally occurring CD4+CD25+FoxP3+ Tregs, which are generated in the thymus,50 or can be induced at the periphery as Tr1 cells, which secrete interleukin-10, or as Th3 cells, which secrete transforming growth factor β (TGF-β). Both natural and induced Tregs have been implicated in the regulation of the natural anti-FVIII T-cell immune response. Kamate et al demonstrated that the depletion of CD4+CD25high Tregs may enhance, or unveil, the in vitro FVIII-induced proliferation of CD4+ T cells from healthy individuals.51 A possible implication of induced TGF-β–producing Th3 cells in tolerance to FVIII has been evoked as a mechanism to prevent FVIII-reactive T-cell proliferation.45 These observations suggest that both natural and induced Tregs may be associated with the control of the anti-FVIII immune response under physiologic conditions.
FVIII, natural antibodies to FVIII, and neutralizing antiidiotypic antibodies: a humoral triad.
The first evidence for the presence of natural anti-FVIII antibodies came in 1992 with the demonstration that heat-treated plasma of 17% of unselected healthy blood donors with otherwise normal levels of circulating FVIII contained antibodies that are able to inhibit the procoagulant activity of FVIII in a functional coagulation assay.52 Titers of antibodies with inhibitory activity (FVIII inhibitors) in plasma ranged between 0.4 and 2.0 Bethesda units (BU). The prevalence of inhibitory activity was equally distributed between males and females. The inhibitory activity copurified with the IgG fraction of normal plasma and was found in the F(ab′)2 fragments prepared from the IgG.52 There was no direct relationship between the inhibitor titer and the FVIII-binding activity, as assessed by enzyme-linked immunosorbent assay (ELISA), suggesting that normal IgGs contain both antibodies with inhibitory activity to FVIII and antibodies that bind to FVIII but are devoid of functional inhibitory ability. Natural anti-FVIII IgGs belong to the 4 IgG subclasses.52,53 The existence of natural anti-FVIII IgG was later confirmed by independent studies wherein anti-FVIII antibodies were isolated by affinity-purification on FVIII either from the serum of healthy subjects with normal levels of FVIII,53 or from therapeutic preparations of pooled normal IgG, intravenous immunoglobulins (IVIgs). Anti-FVIII IgG isolated from IVIgs preferentially bound to the C2 domain of FVIII and to a lesser extent to its A2 domain. The inhibitory titer found in the affinity-purified anti-FVIII fraction of IVIgs was in the order of 16 BU per milligram of IgG.54
Several lines of evidence indicate that natural anti-FVIII IgGs with inhibitory activity against FVIII are subjected to idiotypic regulation by antiidiotypic antibodies in normal plasma. IgGs purified from the plasma of healthy donors were found to bind to murine monoclonal anti-FVIII IgG.53 Further, these IgGs inhibited the binding of both mouse monoclonal and human polyclonal anti-FVIII antibodies to FVIII, thus suggesting that natural antiidiotypic antibodies are produced spontaneously in healthy individuals.52,55
Breakdown of tolerance to FVIII
In rare instances (ie, one individual in a million56 ), there is a rupture of tolerance to FVIII. An autoimmune disorder then develops that is referred to as acquired hemophilia and is characterized by the detection of pathologic levels of inhibitory antibodies directed toward endogenous FVIII.57 Anti-FVIII autoantibodies thus develop spontaneously in individuals without previous abnormalities of hemostasis (ie, idiopathic cases), during or after pregnancy, or in patients with a variety of underlying conditions including autoimmune disorders, malignancies, or allergic reactions to medication.58 Acquired hemophilia is more frequent in elderly individuals than in young adults. Anti-FVIII autoantibodies are more often of the IgG isotype, and preferentially target the A2, A3, and C2 domains of FVIII. Monoclonal IgA or IgM antibodies have been reported in acquired hemophilia patients with hematologic malignancies.59 The disease is fatal in 6% to 40% of the cases.58,60 Interestingly, a survey of the literature reveals that, possibly apart from the nature of the underlying pathology, none of the parameters scored at the time of diagnosis of acquired hemophilia, including the residual levels of circulating FVIII, the inhibitory titer, or the activated partial thromboplastin time, is predictive of survival 1 year after diagnosis. This underscores the complexity of the etiology of acquired hemophilia and questions the relevance of perturbed immune homeostasis, and in particular of FVIII recognition by immune effectors, to the mortality of the patients in the long run.
Whether the sudden breakdown of tolerance toward endogenous FVIII may be attributed to a specific yet uncharacterized inflammatory state of the patient, whether it is associated with perturbations in the idiotypic network, thus favoring the emergence of FVIII-specific pathogenic B-cell clones, or whether it is consecutive to a dysregulation in populations of regulatory T cells remains unexplained. Seminal work by Kazatchkine and Sultan has shown, however, that spontaneous remission from acquired hemophilia is associated with the generation of neutralizing anti-idiotypic IgG (Sultan et al61 ). In addition, treatment of patients with IVIg has demonstrated beneficial effects in some instances.62 IVIg, which contains natural anti-FVIII IgG with inhibitory potential toward FVIII,54 was also shown to contain naturally occurring anti-idiotypic antibodies capable of neutralizing disease-associated anti-FVIII antibodies.63 IVIg has been proposed to impact on the populations of FVIII-specific pathogenic B-cell clones.62 To our knowledge, a role for Tregs in remission from acquired hemophilia has not yet been documented.
FVIII as seen by the immune system of patients with hemophilia A
Genetic and phenotypic heterogeneity of hemophilia A
Hemophilia A is characterized by the absence of functional endogenous FVIII. The inherent absence of FVIII hampers the positive feedback loop of thrombin generation. The result is the absence of consolidation of the fibrin clot, hence incomplete coagulation and failure to repair the breached vascular endothelium. Hemophilia A is a clinically heterogeneous disorder, resulting from a variety of molecular defects in the F8 gene. Different types of molecular defects (eg, intron 22 inversion, large deletions, stop mutations or small insertions) result in 3 different categories of hemophilia A: severe hemophilia A when the level of FVIII in the plasma of the patient is less than 2% of the FVIII found in normal plasma, moderate hemophilia A (2%-5%), or mild hemophilia A (6%-40%).64 Importantly, patients with severe hemophilia A may be further distinguished according to the presence (“cross-reactive material–positive” patients) or absence (“cross-reactive material–negative” patients) of endogenous nonfunctional FVIII. In other words, some patients who completely lack functional FVIII may present with traces, or even normal levels, of circulating nonfunctional FVIII antigen.
Alloimmunization to therapeutic FVIII
The classic treatment of bleeding episodes in patients with hemophilia A is the intravenous administration of exogenous FVIII so as to replace the missing coagulation factor. Treatment of hemophilia A patients with therapeutic FVIII results, in 15% to 30% of the cases, in the emergence of anti-FVIII antibodies (inhibitors) that neutralize the procoagulant activity of the therapeutically administered FVIII.65 The occurrence of FVIII inhibitors is considered to reflect an allogeneic immune response to the repeated administration of an exogenous FVIII protein. The development of FVIII inhibitors represents both a major medical hurdle and a critical societal concern; patients with FVIII inhibitors become resistant to conventional replacement therapy and hence their quality of life is dramatically affected. The occurrence of an inhibitor to therapeutic FVIII in a patient increases by more than 3-fold the cost of the treatment.66 In addition, the development of FVIII inhibitors poses a challenge to immunologists. Indeed, the immunogenicity of FVIII is intriguing for a molecule that is administered at low doses, intravenously, and in the absence of adjuvant.
Different risk factors have been proposed to be associated with inhibitor development. These include risk factors associated with the type of preparation of therapeutic FVIII (ie, either the plasmatic or recombinant origin of FVIII), with the inflammatory state or the HLA haplotype of the patient, or with polymorphisms in immune genes such as genes encoding tumor-necrosis factor, interleukin-10, or CTLA-4.67-69 The only proven risk factor, however, is the type of mutation in the F8 gene that causes hemophilia A, and more specifically the presence or absence of traces of endogenous FVIII antigen in the circulation of the patient. Thus, the incidence of inhibitor development in the 3 categories of patients reflects the severity of the molecular defect: FVIII inhibitors develop in 20% to 35% of patients with severe hemophilia A70,71 and in 3% to 13% of mild/moderate patients.72 Indeed, the various mutations that result in the absence or severe truncation of the FVIII protein are associated with the highest risk for inhibitor formation. This indicates that a complete lack of endogenous circulating FVIII protein prevents the patient's immune system from mounting physiologic tolerance mechanisms toward FVIII. The immune system of the patient then “treats” the incoming therapeutic FVIII molecule as a “non-self”-antigen. Conversely, in cross-reactive material–positive patients, FVIII, or part of the molecule, is “known” to the immune system. Hence, tolerance to FVIII may have been established during ontogeny, and risk for inhibitor development is reduced or absent.
Dynamics of the development of the anti-FVIII immune response
After intravenous administration to the patients, therapeutic FVIII meets with several possible fates: as shown in mice,29,30 FVIII is eliminated in the liver upon binding to the catabolic receptors CD91 and ASGPR expressed by scavenger cells; by virtue of its association with VWF, a nonnegligible fraction of the administered FVIII is transported to, and presumably accumulates at, the sites of bleeding; interestingly, the fact that 10% to 30% of patients with hemophilia A develop an alloimmune response to therapeutic FVIII indicates that FVIII is not solely attracted to the sites of bleeding or to catabolic organs, but also has a tropism toward lymphoid organs and professional APCs.
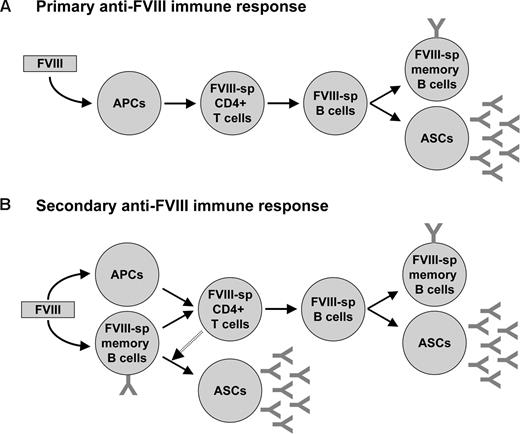
The response of the immune system to therapeutic exogenous FVIII is believed to develop as a classical immune response to an external antigen (Figure 3). According to this view, the administered antigen is recognized, internalized, and processed by patrolling APCs, then presented to antigen-specific T cells in secondary lymphoid organs. Activated T helper cells provide activation signals to antigen-specific B lymphocytes that in turn differentiate into antibody-secreting plasma cells or memory B cells. What is unclear, however, is the location where therapeutic FVIII “encounters” the immune system for the first time, the type of APCs that are involved in the process, and the site where the anti-FVIII immune response develops. Answering these questions is crucial for conceiving strategies aimed at selectively preventing the onset of the deleterious anti-FVIII immune response.
Immune response to FVIII in patients with hemophilia A. (A) The primary immune response is initiated by the internalization of the therapeutically administered FVIII by professional antigen presenting cells (APCs; eg, dendritic cells) and its subsequent presentation to naive FVIII-specific (FVIII-sp) CD4+ T cells. Activated CD4+ T cells in turn activate FVIII-specific naive B cells, which proliferate and differentiate either into plasmocytes (or antibody-secreting cells, ASCs) or into FVIII-specific memory B cells. (B) During the secondary immune response, FVIII-specific memory B cells generated during the primary immune response act as APCs and activate FVIII-specific CD4+ T cells. With the help of CD4+ T cells, FVIII-specific memory B cells further differentiate into ASCs. In parallel, uptake of FVIII by professional APCs results in activation of T cells that in turn activate new FVIII-specific B cells and thus generate additional ASCs and memory B cells.
Immune response to FVIII in patients with hemophilia A. (A) The primary immune response is initiated by the internalization of the therapeutically administered FVIII by professional antigen presenting cells (APCs; eg, dendritic cells) and its subsequent presentation to naive FVIII-specific (FVIII-sp) CD4+ T cells. Activated CD4+ T cells in turn activate FVIII-specific naive B cells, which proliferate and differentiate either into plasmocytes (or antibody-secreting cells, ASCs) or into FVIII-specific memory B cells. (B) During the secondary immune response, FVIII-specific memory B cells generated during the primary immune response act as APCs and activate FVIII-specific CD4+ T cells. With the help of CD4+ T cells, FVIII-specific memory B cells further differentiate into ASCs. In parallel, uptake of FVIII by professional APCs results in activation of T cells that in turn activate new FVIII-specific B cells and thus generate additional ASCs and memory B cells.
Two nonexclusive hypotheses may be proposed to describe the “first encounter” of FVIII with immune effectors. In a first scenario, the spleen plays a major role both as a meeting point and as a site of initiation of the immune response. Indeed, the spleen filters blood-borne antigens and provides the immunologic environment for the initiation of adaptive immune responses. This view is supported by our unpublished work wherein we demonstrate that FVIII administered to FVIII-deficient mice accumulates in the spleen, and removal of the spleen before repeated FVIII administration reduces the extent of the anti-FVIII immune response (A.-M.N., S. Delignat, and S.L.-D., manuscript in preparation). In a second scenario, therapeutic FVIII may be captured by APCs at the site of bleeding and then transported to secondary lymphoid organs (ie, the draining lymph nodes and/or the spleen) for presentation to CD4+ T cells. For instance, repeated bleeding in the joints creates a chronic inflammatory microenvironment, as indicated by the development of arthropathy in patients with hemophilia A.73 The inflammatory atmosphere in turn promotes the local recruitment of APCs and immune effectors, and may provide the appropriate signals for the activation of the professional APCs that have endocytosed FVIII. FVIII-educated APCs may then initiate the anti-FVIII immune response on site and/or in secondary lymphoid organs upon migration.
Actors of the anti-FVIII immune response
Antigen-presenting cells and endocytosis of FVIII.
Different types of APCs may be implicated in the uptake of therapeutic FVIII in the patients, among which dendritic cells (DCs), macrophages, and B lymphocytes are the most potent. The type of APCs involved in FVIII uptake and processing may, however, differ depending on the “experience” the immune system of the patient has, of exogenous FVIII.
In previously untreated patients who have never been exposed to FVIII, FVIII-specific B lymphocytes have not been triggered and are not likely to be present at a frequency high enough to serve as APCs. In this context, and in view of their capacity to stimulate naive T cells, DCs may be the major cell type that activates FVIII-specific CD4+ T lymphocytes, although a role for macrophages in priming immune responses cannot be excluded. Using human monocyte-derived DCs, we have demonstrated recently that FVIII is endocytosed by the macrophage mannose receptor (MMR/CD206) that recognizes mannose-ending glycans on both the heavy and light chains of FVIII.31 Importantly, MMR-mediated internalization of FVIII is followed by presentation of FVIII-derived peptides to CD4+ T cells. In our experiments, LRP/CD91, which was found to be expressed and to be functional on approximately 20% of DCs, failed to mediate FVIII endocytosis.74 Although controversial, VWF has been proposed to reduce the immunogenicity of FVIII in patients with hemophilia A.75,76 VWF prevented the binding of FVIII to MMR,31 and blocked in a dose-dependent manner the endocytosis of FVIII by monocyte-derived DCs.77
In patients who have already developed anti-FVIII IgG, presentation of FVIII to T cells may proceed after endocytosis of FVIII (1) in the form of immune complexes with circulating anti-FVIII IgG through Fc receptors expressed at the surface of B cells, DCs, or macrophages, (2) by B-cell receptors (BcRs) present at the surface of FVIII-specific memory B lymphocytes, and (3) by endocytic receptors expressed by DCs and/or macrophages such as MMR.
Importance and nature of FVIII-reactive T lymphocytes.
The requirement for T lymphocytes in the development of the anti-FVIII immune response is indicated by observations in hemophilia A patients and by experimental studies in murine models of severe hemophilia A.
The accumulation of somatic mutations in the V genes encoding human anti-FVIII IgG demonstrates that FVIII-specific B cells undergo clonal selection and affinity maturation,78-83 thus suggesting help from helper CD4+ T cells upon encounter with FVIII. Indeed, the presence of circulating CD4+ T lymphocytes that proliferate when stimulated in vitro with FVIII or FVIII-derived peptides has been documented in inhibitor-positive patients with hemophilia A.47,84-86 FVIII-reactive T cells were found to preferentially use Vβ genes of the BV2, BV5, and BV9 families.87 Interestingly, patients with a history of high inhibitor titers more than 5 BU were found to lose FVIII inhibitors after infection by HIV.88 This was associated with a drop in CD4+ T-cell counts, and the lack of an anamnestic response upon subsequent intravenous administration of therapeutic FVIII. Furthermore, analyses in FVIII-deficient mice have demonstrated that abrogation of T-cell help using antibodies to CD40-ligand (CD154), CD80, or CD86, or using CTLA4-Ig constructs, which impairs the cross-talk between APCs and T cells, prevented the anti-FVIII immune response as well.89-93
Interestingly, some patients who completely lack the endogenous FVIII molecule, and presumably do not develop natural tolerance to FVIII, never develop FVIII inhibitors, while others do. This suggests that the emergence of FVIII inhibitors in severe hemophilia A patients is not merely due to a lack of natural tolerance to FVIII. A recent preliminary study suggests that the inhibitor-positive or negative status of the patients may reside in the inability or ability of the patients' immune system to induce regulatory TGF-β–producing Th3 cells in the periphery.45
Anti-FVIII antibodies.
The anti-FVIII alloantibodies that may develop in hemophilia A patients after repeated administration of therapeutic FVIII are of the IgG isotype and use both lambda and kappa light chains. The isotypic distribution of polyclonal anti-FVIII IgG follows the physiologic profile of IgG subclasses, with a preference, however, for the IgG1 and IgG4 isotypes.94-96 Noticeably, the 2 published human monoclonal FVIII inhibitors, the anti-C2 BO2C11 and anti-C1 LE2E9, are both of the IgG4κ isotype.82,83 Anti-FVIII IgGs are polyclonal in patients and encompass antibodies that are endowed with inhibitory properties toward FVIII procoagulant activity, and antibodies that bind FVIII without affecting its functional activity as measured using the Bethesda assay.97 Several mechanisms have been described or proposed by which anti-FVIII IgG may lower the efficacy of therapeutically administered FVIII:
(a) Anti-FVIII IgGs that inhibit FVIII in the Bethesda assay are directed to functional epitopes of the molecule. By steric hindrance, they prevent the interaction of FVIII with different molecules participating in the coagulation cascade98 (eg, FIXa, FX, thrombin, or phospholipids; Figure 1). Accordingly, 3 major clusters of B-cell epitopes have been identified in the A2, A3, and C2 domains of FVIII.
(b) Both inhibitory and noninhibitory anti-FVIII IgGs may form immune complexes with the administered FVIII.99 Although never formally demonstrated, the formation of immune complexes between FVIII and anti-FVIII IgG has been proposed to accelerate the clearance of therapeutic FVIII from the circulation and decrease its half-life.
Prevention or neutralization of adverse immune reactions to FVIII in hemophilia A patients
Today, in most developed countries where the risks of contamination of FVIII products by infectious agents are minimized, the treatment of patients with hemophilia A is confronted with 2 major issues. The first one, as explained in “Alloimmunization to therapeutic FVIII,” is related to the immunogenicity of FVIII and the development of an anti-FVIII immune response after administration of exogenous FVIII. The second one relates to the cumbersomeness of the treatment that requires life-long and frequent injections of FVIII, especially in patients undergoing prophylaxis. Deciphering the promiscuity of FVIII unravels the different knots to be untied to achieve tolerance to the molecule (Figure 4), both at the level of the patient and at that of the FVIII molecule.
Prevention and blocking of adverse immune reactions to FVIII in hemophilia A patients. Elimination of FVIII inhibitors in hemophilia A patients who have developed an alloimmunization to FVIII may be achieved by FVIII-specific targeting of immune effectors, for instance upon manipulation of the idiotypic network. Alternatively, immune reactions to therapeutic FVIII may be avoided in previously untreated patients by using structurally modified FVIII, of which the dominant T- and B-cell epitopes and/or structures of FVIII that mediate its internalization by APCs have been altered. Simultaneously, assessment of the inflammatory state of the patient may help clinicians to reduce risks of alloimmunization.
Prevention and blocking of adverse immune reactions to FVIII in hemophilia A patients. Elimination of FVIII inhibitors in hemophilia A patients who have developed an alloimmunization to FVIII may be achieved by FVIII-specific targeting of immune effectors, for instance upon manipulation of the idiotypic network. Alternatively, immune reactions to therapeutic FVIII may be avoided in previously untreated patients by using structurally modified FVIII, of which the dominant T- and B-cell epitopes and/or structures of FVIII that mediate its internalization by APCs have been altered. Simultaneously, assessment of the inflammatory state of the patient may help clinicians to reduce risks of alloimmunization.
Immunointervention in patients with FVIII inhibitors
Available therapies for the eradication of FVIII inhibitors include the administration of high-dose FVIII to induce specific tolerance (referred to as immune tolerance induction, or ITI), or the depletion of B cells by off-label use of a monoclonal anti-CD20 antibody. ITI is purely empiric and the underlying mechanisms remain unclear, even though recent evidence in mice suggests that it might be associated with the deletion of FVIII-specific memory B cells.103 Its application is restricted by its prohibitive cost, and by the length and the complexity of the treatment. The anti-CD20 monoclonal therapy is hampered by its lack of specificity for FVIII-reactive B cells, uncertain efficacy, and possible adverse effects in the long run.104 Alternative approaches toward the control of FVIII-specific immune effectors are being investigated.
Abrogation of the cross-talk between APCs and T cells, using for instance an anti-CD40L monoclonal antibody or CTLA4-Ig constructs, has shown promising results in FVIII-deficient mice,89,90 but may also suffer from a lack of specificity toward FVIII-specific immune effectors and by adverse secondary effects in the human.105 In contrast, induction of specific tolerance to FVIII has been achieved in hemophilic mice by gene-transferred FVIII-IgG fusion proteins expressed in B cells or through preventive mucosal exposure of the animals to FVIII domains.106,107 Whether such strategies are applicable to human patients in a therapeutic approach is subject to further investigation.
As summarized in “FVIII as seen by the immune system under physiologic conditions,” the humoral immune response to FVIII is tightly regulated under physiologic conditions by anti-idiotypic antibodies that neutralize naturally occurring FVIII inhibitors. Recovery from acquired hemophilia and successful eradication of FVIII inhibitors by ITI in congenital hemophilia A are both associated with the spontaneous generation of neutralizing anti-idiotypic antibodies. Hemophilia thus appears as one of the few human diseases in which manipulation of the idiotypic network may prove efficient and realistic. These observations provide the platform for the development of therapeutic strategies based on the use of monoclonal anti-idiotypic antibodies aimed at neutralizing FVIII inhibitors in vivo.108
Interestingly, the bleeding phenotype may be corrected in the presence of FVIII inhibitors, as shown in FVIII-deficient mice by ectopic targeting of the expression of FVIII to platelets.109
Modification of the FVIII molecule so as reduce its immunogenicity
The prediction of epitopes on FVIII that bind to MHC II, T-cell receptors, or BcR may be achieved via in vitro assays110 or in silico modeling. FVIII contains 2 mannose-terminating glycans that allow specific endocytosis by DCs through mannose-sensitive receptors.31 Removing glycosylation sites or immunodominant epitopes by site-directed mutagenesis may lead to the development of structurally modified FVIII molecules that are “invisible” to immune sentinels and devoid of iatrogenic immunogenicity. The structure of FVIII should, however, be altered within limits that are compatible with its functional integrity and avoid the generation of novel linear or conformational epitopes with unpredicted immunogenicity. Interestingly, modification of moieties implicated in the binding of FVIII to endocytic receptors may also have a favorable impact on the half-life of therapeutic FVIII in the patients.111
Assessment and modulation of the inflammatory status of the patients
One of the proposed risk factors that may favor the development of FVIII inhibitors is related to the inflammatory state of the patient at the time of FVIII administration. The inflammatory state of each patient may be monitored by analyzing the levels of circulating cytokines and the polymorphism of immune genes. Introduction of FVIII in patients at high risk of developing neutralizing antibody responses could be delayed or avoided, and alternative drugs, such as activated factor VII, administered. Alternatively, the inflammatory environment of the patients could be neutralized before or at the time of administration of FVIII. This may be achieved using therapeutic monoclonal antibodies to inflammatory cytokines,112 or immunosuppressive agents (eg, steroids). Importantly, such approaches would not only reduce the activation state and endocytic capacity of APCs and impart anergy to T-cell effectors, but also restore normal functions of Tregs.113
Conclusion: immunogenicity of FVIII–lessons from promiscuity
For an immune response to develop against FVIII, there are several prerequisites: (1) The antigen-specific effector T and B cells should not have been eliminated during ontogeny, and natural tolerance to the antigen should not have been established. This is the case especially in cross-reactive material–negative patients with hemophilia A. In these patients, FVIII has not been presented to T cells during their development in the thymus, nor to T and B cells at the periphery, hence the immune system of the patients has not been educated vis-à-vis FVIII during its ontogeny and FVIII-reactive cells persist in the circulation. (2) FVIII needs to be recognized by APCs, internalized, processed, and presented to antigen-specific T cells. As explained earlier, FVIII is endocytosed by APCs, at least in part through lectin receptors such as the macrophage mannose receptor, processed, and presented to T cells in an MHC II–dependent manner. (3) According to the “danger” theory postulated by Matzinger, presentation of the antigen by APCs to T cells has to occur in the context of danger for the functional activation of T cells.114 The nature of the danger signals that are provided to APCs in the case of FVIII remains unknown. Recent results suggest that FVIII does not provide direct maturation signals to DCs,115 although this has not been confirmed in the case of macrophages or B lymphocytes. The immunogenicity of FVIII may also indirectly relate to the absence of its procoagulant function, that is, chronic hemorrhages create local proinflammatory environments prone to the attraction and maturation of APCs and effector T and B cells.
Based on the observation of the extreme promiscuity of the FVIII molecule, we would like to propose here an alternative hypothesis. FVIII is at the center of an interaction network that links molecules of coagulation, innate immunity (endocytic receptors, natural antibodies), catabolic machinery (catabolic receptors), and possibly inflammation. We thus propose that the homeostasis of the organism relies in part on the maintenance of a dynamic equilibrium between the different actors of this network. In this context, it is not surprising that deprivation of FVIII, as seen in hemophilia A patients, introduces a disequilibrium prone to triggering the immune and/or inflammatory cascades. Similarly, out-of-range variations in circulating levels of FVIII that may be associated with different disorders such as neoplastic or autoimmune diseases may initiate anti-FVIII autoimmune manifestations as seen in acquired hemophilia.
Acknowledgment
The authors thank Aditi Varthaman for proofreading and constructive discussion.
This work was supported by Inserm, by Centre National de la Recherche Scientifique (CNRS), and by grants from Agence Nationale de la Recherche (ANR-05-MRAR-030-01, ANR-07-JCJC-0100-01, ANR-07-RIB-002-02, ANR-07-MRAR-028-01). S.D. and A.-M.N. are recipients of fellowships from Fédération de la Recherche Médicale (FRM) and from Région Ile-de-France (Paris, France), respectively.
Authorship
Contribution: All authors contributed to the writing and proofreading of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sébastien Lacroix-Desmazes, Inserm UMR S 872 Equipe 16, Centre de Recherche des Cordeliers, 15 rue de l'école de médecine, 75006 Paris, France; e-mail: sebastien.lacroix-desmazes@crc.jussieu.fr.