Abstract
Aberrations of the Wnt canonical pathway (WCP) are known to contribute to the pathogenesis of various types of cancer. We hypothesize that these defects may exist in mantle cell lymphoma (MCL). Both the upstream and downstream aspects of WCP were examined in MCL cell lines and tumors. Using WCP-specific oligonucleotide arrays, we found that MCL highly and consistently expressed Wnt3 and Wnt10. β-catenin, a transcriptional factor that is a downstream target of WCP, is localized to the nucleus and transcriptionally active in all 3 MCL cell lines examined. By immunohistochemistry, 33 (52%) of 64 MCL tumors showed nuclear localization of β-catenin, which significantly correlated with the expression of the phosphorylated/inactive form of GSK3β (p-GSK3β; P = .011, Fisher). GSK3β inactivation is directly linked to WCP stimulation, since addition of recombinant sFRP proteins (a naturally occurring decoy for the Wnt receptors) resulted in a significant decrease in p-GSK3β. Down-regulation of DvL-2 (an upstream signaling protein in WCP) by siRNA or selective inhibition of β-catenin using quercetin significantly decreased cell growth in MCL cell lines. To conclude, WCP is constitutively activated in a subset of MCL and it appears to promote tumorigenesis in MCL.
Introduction
Mantle cell lymphoma (MCL) is a specific type of non-Hodgkin B-cell lymphoma recognized by the World Health Organization (WHO) Classification Scheme.1 The genetic hallmark of this disease is the recurrent chromosomal abnormality, the t(11;14)(q13;q32), which brings the cyclin D1 gene under the influence of the enhancer of the immunoglobulin heavy chain (IgH) gene, leading to cyclin D1 overexpression.2 Although cyclin D1 overexpression is likely to be pathogenetically important in MCL, evidence suggests that additional biochemical defects are necessary for lymphomagenesis. For instance, using Eμ-cyclin D1 transgenic mice, 2 research groups have previously shown that enforced cyclin D1 expression in B cells is not sufficient to induce tumor formation.3,4 Furthermore, large-scale cDNA microarray studies using frozen MCL tumors have revealed a relatively large number of biochemical abnormalities in MCL, with these defects frequently implicated in the regulation of apoptosis, cell cycle progression, and DNA repair.5-10 Examination of specific cellular signaling pathways also provided additional insights into the biology of these tumors. For instance, a relatively recent study reported constitutive activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway in a subset of MCL tumors, particularly those with a blastoid morphology.11
The Wnt canonical pathway (WCP) is important for normal cell growth and development.12,13 Defects of this pathway have been shown to play roles in the pathogenesis of a variety of human cancers, particularly those of epithelial type.14 Normally, WCP is activated via ligation of the Wnt proteins, which are secreted glycoproteins, to their respective dimeric cell surface receptors composed of the frizzled proteins and the low-density lipoprotein-receptor–related proteins (LRPs).15 The binding of the Wnt proteins to their receptors is negatively regulated by the Wnt inhibitory factor and the sFRP proteins (sFRP1-5). Upon ligation to their receptors, the disheveled proteins (DvLs), a family of the upstream WCP signaling proteins, are phosphorylated.16,17 In WCP, Wnt stimulation results in inactivation/phosphorylation of GSK3β, as well as the dissociation of the “destruction complex.” The normal function of the destruction complex, composed of several proteins including axin, adenomatous polyposis coli, and GSK3β, has been recognized as a crucial regulator of a wide range of cellular functions including apoptosis and cell proliferation. One of the known biologic functions of this complex is to inhibit β-catenin, by sequestration and promotion of its proteasome degradation.12 With GSK3β inactivation and dissociation of the destruction complex, β-catenin is released and allowed to accumulate in the cell and translocate to the nucleus, where it up-regulates the transcription of multiple genes including cyclin D1. WCP is particularly interesting in the context of MCL, in view of its regulatory role on cyclin D1 expression.
The status of WCP has not been comprehensively studied in MCL. Specifically, the expression profile of various Wnt proteins and their receptors, as well as the activation/phosphorylation status of GSK3β and the DvL protein, are not known. In light of the biologic significance of WCP in normal and cancer cells, we attempted to determine whether defects in WCP may exist in MCL, and whether these defects may contribute to the pathogenesis of these tumors.
Methods
MCL cell lines and primary tumors
The characteristics of the 3 MCL cell lines, Jeko-1, Mino, and SP53, have been previously described.18 Briefly, these 3 cell lines have the mature B-cell immunophenotype, carry the t(11;14)(q13;q32), and express cyclin D1. All 3 cell lines are negative for the Epstein-Barr virus (EBV) nuclear antigen. All MCL primary tumors were diagnosed at the Cross Cancer Institute and the diagnostic criteria were based on those described in the WHO Classification Scheme.1 Five of the 64 MCL tumors used in this study were of the blastoid variant. All cases were confirmed to express cyclin D1 by immunohistochemistry. The use of these tissues has been approved by University of Alberta Cancer Board Institutional Ethics Committee.
Reagents
Quercetin, a β-catenin inhibitor, was purchased from Sigma-Aldrich (St Louis, MO). Stock solutions were prepared by dissolving in dimethyl sulfoxide (DMSO; Sigma-Aldrich) following the manufacturer's instruction. Addition of DMSO without quercetin served as the negative controls.
Subcellular protein fractionation, Western blots, and antibodies
For subcellular protein fractionation, we used a kit purchased from Active Motif (Carlsbad, CA) and followed the manufacturer's instructions.
Preparation of cell lysates for Western blots is described as follows: cells were washed with phosphate-buffered saline (PBS), and cellular proteins were precipitated with ice-cold 5% trichloroacetic acid (TCA) overnight at 4°C and centrifuged twice for 15 minutes at 15 000g at 4°C. Subsequently, the TCA-precipitated protein pellets were dissolved in the modified Laemmli buffer containing 90 mM Tris-HCl (pH 7.9), 2% SDS, 10% glycerol, 5 mM EDTA, 125 mg/mL urea, 0.1 M dithiothreitol, 0.02% bromophenol blue, which was supplemented with 40.0 μg/mL leupeptin, 1 μM pepstatin, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, and 0.1 mM phenylmethylsulfonyl-fluoride. The protein concentration of the samples was determined by the modified Lowry method. Cell lysates were then electrophoresed on 8% or 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad, Richmond, CA). After the membranes were blocked with 5% milk in TBS buffer (20 mM Tris-HCL, pH 7.6, 150 mM NaCl), primary antibodies were added to the membranes. After washings with PBS, secondary antibody conjugated with the horseradish peroxidase (Jackson Immunoresearch Laboratories, West Grove, PA) was added to the membrane. After washings with PBS, proteins were detected using the enhanced chemiluminescence detection kit (Amersham Life Sciences, Arlington Heights, IL). Antibodies used in this study included anti–cyclin D1, anti–Bcl-2, anti–Bcl-XL, anti–α-tubulin (all of which were purchased from Santa Cruz Biotechnology, Santa Cruz, CA), anti–p-GSK3β, anti-pAktser473, anti-pAktthr308, anti-PARP, anti–caspase-3, anti–caspase-7, anti–caspase-8, anti–caspase-9, anti–cleaved caspase-3, anti–cleaved caspase-7, anti–cleaved caspase-8, anti–cleaved caspase-9, anti–DvL-3 (all of which were purchased from Cell Signaling, Danvers, MA), anti–DvL-1, anti–DvL-2 (both of which were purchased from BIOMOL, Plymouth Meeting, PA), anti–β-catenin, and anti–β-actin (both of which were from Sigma-Aldrich). A dilution of 1:1000 was used for all antibodies with the exception of anti–β-actin, for which a 1:2000 dilution was used. Western blot results were analyzed using the Scion Image software (Scion, Frederick, MD).
Immunofluorescence and confocal microscopy
Cells were grown on coverslip previously treated with poly-l-lysine (Sigma-Aldrich) in a 6-well plate and fixed with 3% paraformaldehyde in PBS (pH 7.4). Cell were rinsed 3 times with PBS, permeabilized with Triton, washed again with PBS, and incubated with 200 μL anti–β-catenin (1:50; Sigma-Aldrich) overnight at room temperature in a humidified chamber, followed by rinsing 3 times with PBS. After incubation with 200 μL Alexa Fluor 488 secondary antibody (1:250; Invitrogen, Burlington, CA) for 1 hour at room temperature, cells were rinsed with PBS and the procedure was completed with a mounting media (Dako, Mississauga, ON) added to the slides. Cells were visualized with a Zeiss LSM510 confocal microscope (40×/1.3 NA oil; Carl Zeiss, Heidelberg, Germany) at the Core Cell Imaging Facility, Cross Cancer Institute.
β-catenin transcriptional activity assessed by luciferase reporter assay
To assess the transcriptional activity of β-catenin in MCL, we used the TOP/FOP system. This method has been previously described in detail.19 MCL cells were transiently transfected with β-catenin responsive firefly luciferase reporter plasmids TopFlash (Millipore, Billerica, MA) and the negative control FopFlash (Millipore) using the Amaxa nucleofector system (Amaxa, Cologne, Germany). After 48 hours, cells were harvested and cell extracts were prepared using a lysis buffer containing 100 mM Tris (pH 7.8), 0.5% NP-40, and freshly added 1 mM DTT. Cells extracts were collected and stored at −80°C until further use. Luciferase activity was assessed using 10 μL cell lysate and 100 μL luciferase assay reagent containing 20 mM Tricine, 1.07 mM MgCO3, 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM DTT, 270 μM coenzyme A, 530 μM ATP, and 470 μM luciferin. The luciferase activity measured was normalized against the β-galactosidase activity, which was measured by incubating 20 μL cell lysates in a covered microtiter plate with 100 μL o-nitrophenyl-β-d-galactopyranoside solution (0.8 mg/mL) measured at 420 nm at 37°C. Data are reported as means plus or minus standard deviations of 3 separate experiments, each of which was performed in triplicates.
Gene expression array analysis of the Wnt pathway
We used the reverse transcription (RT2) Profiler polymerase chain reaction (PCR) Array Human Wnt Signaling Pathway array purchased from SuperArray Bioscience (Frederick, MD). The complete gene list is available on http://www.superarray.com. Total RNA from MCL cell lines and frozen tumors was isolated using the TRIZOL Reagent (Invitrogen) and measured using the DU1640 Beckman spectrophotometer (Beckman Coulter, Mississauga, ON). First-strand cDNA synthesis reaction was performed as follows: 2 μg of extracted RNA was mixed with 10 μL of the SuperArray RT cocktail mix. The products were then incubated at 37°C for 1 hour and heated at 95°C for 5 minutes. Real-time–based SYBR green PCR was performed using an ABI 7900HT instrument (Applied Biosystems, Streetsville, ON) and the following thermal cycling condition was used: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Data analysis and the cycle threshold (CT) values, which were defined as the fractional cycle number at which the fluorescence passes an arbitrarily set threshold, were analyzed using the SDS (version 2.2.2) program (Applied Biosystems). The CT value of each gene was normalized to that of GAPDH, which is included in this commercially available kit.
MTS assay and cell viability
MCL cells lines in RPMI 1640 were seeded at 20 000 cells/well in a 96-well plate and the assay was conducted following the manufacturer's instructions (Promega, Nepean, ON). The measurements were obtained at a wavelength of 450 nM using a Bio-Rad Microplate Reader. The absorbance values were normalized to the untreated cells using the microplate Manager 5.2.1 software (Bio-Rad). All experiments were performed in triplicates. Cell viability was determined by the trypan blue exclusion test.
Immunohistochemistry
Immunohistochemistry was performed using standard techniques as previously described.20 Briefly, formalin-fixed, paraffin-embedded tissue sections of 4-μM thickness were deparaffinized and hydrated. Heat-induced epitope retrieval was performed using citrate buffer (pH = 6) and a microwave histoprocessor (RHS; Milestone, Bergamo, Italy). Tissue sections were then incubated with anti–p-GSK3β (1:500) or anti–β-catenin (1:1200) overnight in a humidified chamber at 4°C. All of these primary antibodies were the same as those used for Western blots. After 3 washes with PBS, tissue sections were incubated with anti–rabbit IgG (EnVision; Dako) for 30 minutes at room temperature. Tissue sections were incubated with 3,3′-diaminobenzidine/H2O2 (Dako) for color development and counterstained with hematoxylin. For p-GSK3β staining, tumors showing staining in 30% or more of the cells were categorized as positive cases. For β-catenin staining, tumors with more than 50% of the cells showing definitive nuclear staining were regarded as positive cases. These arbitrary cutoffs were used to achieve the highest statistical significance. Pictures were taken on a Zeiss Axioscope 2 microscope (Carl Zeiss) with Axiovision software version 0.63 on a 40×/1.3 NA oil objective.
Short interfering RNA
Short interfering RNA (siRNA) reagents were purchased from Invitrogen. The sequences of DvL-2 siRNA were as follows: 5′-GGUUCCUCCUCCAUGAGCACCAUUA-3′ and 5′-UAAUGGUGCUCAUGGAGGAGGAACC-3′. Transient transfections of Jeko-1 and SP53 cells were performed using the Amaxa Nucleofector system according to the manufacturer's protocol. Cells were harvested at 24 hours after transfection. The DvL-2 protein level detected by Western blot analysis was used to assess the efficiency of DvL-2 inhibition.
Recombinant sFRP
Human sFRP1 and sFRP4 were purchased from R&D Systems (Minneapolis, MN). Human recombinant sFRP2 and sFRP3 are not commercially available. Jeko-1 and SP53 cells were treated with or without sFRPs proteins in a serum-free medium. After 3 hours of treatment, the cells were lysed and analyzed by Western blot.
Assessment of cyclin D1 expression using quantitative RT-PCR
The expression of cyclin D1 in MCL cells treated with quercetin was assessed using an quantitative RT-PCR (qRT-PCR) assay adapted from a previously published method.21 The assay was performed using the Applied Biosystems 7900 HT, and the EXPRESS SYBR GreenER qPCR SuperMixes and a 2-step qRT-PCR kit from Invitrogen were used. Triplicate experiments were performed and the statistical significance of the differences was assessed using the Student t test.
Cell-cycle analysis by flow cytometry
Cells were synchronized by serum starvation for 24 hours. Subsequently, cells at a concentration of 106 cells/mL were resuspended in DMEM with 10% FBS. To prepare cells for cell-cycle analysis, cells were washed twice with PBS and fixed with 70% cold ethanol for 2 hours. After washes with PBS, cells were treated with RNase (1 mg/mL) at 37°C for 30 minutes. Cells were then washed 3 times with PBS and staining was achieved by incubating cells with 100 μg/mL propidium iodide containing 0.1% Triton X-100. Cells were analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA).
Statistical analysis
Data are expressed as mean plus or minus standard derivation. Statistical significance was tested using the Student t test and was achieved when the P value was less than .05.
Results
Nuclear localization and transcriptional activity of β-catenin in MCL cells
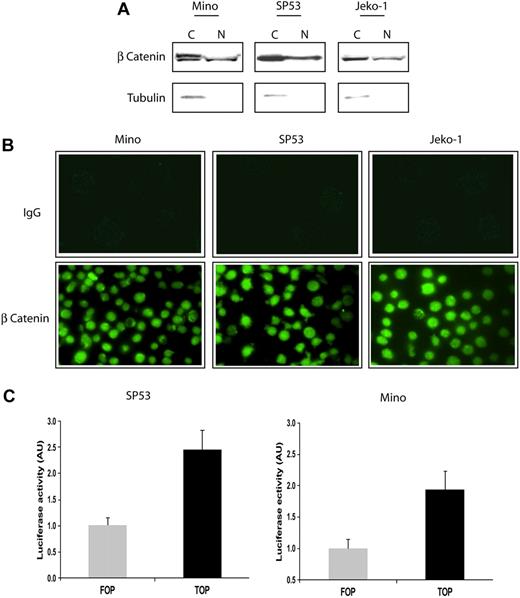
We first assessed the activity of β-catenin, the downstream effectors of WCP, in 3 EBV-negative MCL cell lines. Since β-catenin is a transcriptional factor that migrates to the nucleus in response to WCP activation, we assessed its nuclear localization. Using subcellular protein fractionation and Western blots, β-catenin was found in both the cytoplasmic and nuclear fractions in all 3 MCL cell lines (Figure 1A). α-Tubulin, a protein that is normally confined to the cytoplasm, was used as a control for the efficiency of the subcellular protein fractionation. Of note, the cytoplasmic portion of β-catenin in Mino cells, and to a lesser extent in the other 2 MCL cell lines, contained a slower-migrating β-catenin band that was present only in the cytoplasmic portion. This band has been described to represent the phosphorylated form of β-catenin that is destined for proteosome degradation.22 This finding suggests that the negative regulatory mechanism for β-catenin, which is known to promote its proteosome degradation, is at least partially functional in these cells.
Localization and in vitro activity of β-catenin in MCL cell lines. (A) Subcellular fractionation using the cell lysates of 3 MCL cell lines revealed that β-catenin was localized to the nucleus (N). The expression of α-tubulin in the cytoplasm (C) served as a control for the efficiency of subcellular fractionation. (B) Confocal microscopy revealed the nuclear accentuation of the β-catenin staining in MCL cells (bottom panel). The use of secondary antibody served only as negative controls (top panel). (C) The use of the TOP/FOP system confirmed that β-catenin is transcriptionally active in SP53 and Mino cells. Luciferase activity is expressed as arbitrary units (AU). Error bars indicate SD. Experiments were performed in triplicates and the differences are statistically significant (P < .05, Student t test).
Localization and in vitro activity of β-catenin in MCL cell lines. (A) Subcellular fractionation using the cell lysates of 3 MCL cell lines revealed that β-catenin was localized to the nucleus (N). The expression of α-tubulin in the cytoplasm (C) served as a control for the efficiency of subcellular fractionation. (B) Confocal microscopy revealed the nuclear accentuation of the β-catenin staining in MCL cells (bottom panel). The use of secondary antibody served only as negative controls (top panel). (C) The use of the TOP/FOP system confirmed that β-catenin is transcriptionally active in SP53 and Mino cells. Luciferase activity is expressed as arbitrary units (AU). Error bars indicate SD. Experiments were performed in triplicates and the differences are statistically significant (P < .05, Student t test).
The nuclear translocation of β-catenin was further demonstrated by immunofluorescence staining and confocal microscopy. As shown in Figure 1B, staining with the Alexa Fluor 488–conjugated secondary antibody showed no detectable signals in all 3 MCL cell lines (top panel). In contrast, inclusion of the anti–β-catenin primary antibody showed nuclear accentuation of the staining in the majority of the cells. To verify that β-catenin is indeed transcriptionally active in these cells, we used the TOP/FOP system, as described in “β-catenin transcriptional activity assessed by luciferase reporter assay.” As illustrated in Figure 1C, SP53 and Mino cells transfected with the TOP + gal vectors had higher luciferase activities compared with cells transfected with FOP + gal. This result demonstrates that β-catenin is transcriptionally active in MCL cells.
Expression of nuclear β-catenin and p-GSK3β in MCL tumors
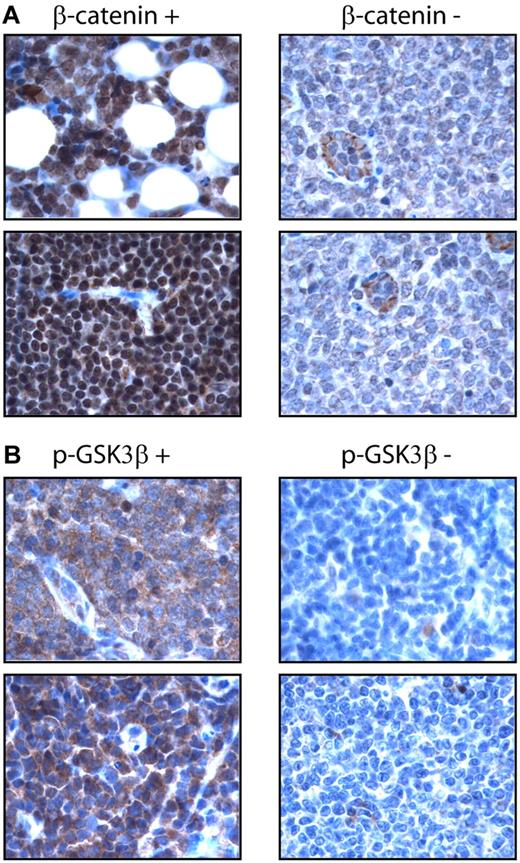
Using an anti–β-catenin antibody and immunohistochemistry, we surveyed the expression of nuclear β-catenin in a cohort of formalin-fixed, paraffin-embedded MCL tumors (n = 64). Nuclear β-catenin was detected in 33 (52%) cases. Similarly, we surveyed the expression of p-GSK3β using a monoclonal antibody and immunohistochemistry. p-GSK3β was detectable in 36 (56%) cases. Importantly, the expression of these 2 markers significantly correlated with one another (P = .011, Fisher exact test). The staining results are illustrated in Figure 2. In the positive cases, β-catenin staining was mostly nuclear, although a weak cytoplasmic staining was often detectable. In β-catenin–negative cases, we were able to consistently identify relatively weak cytoplasmic staining. The staining for pGSK-3β was cytoplasmic in the positive cases. Tonsils were included as the negative controls; both nuclear β-catenin and p-GSK3β were not detectable using our immunohistochemical methods.
Expression of β-catenin in MCL tumors. (A) Immunohistochemistry using paraffin-embedded MCL tumors showed nuclear staining of β-catenin in a positive case (left panel). Only weak cytoplasmic staining was found in the tumor cells in a negative case, although endothelial cells showed intense cytoplasmic staining (right panel). (B) Immunohistochemistry using paraffin-embedded MCL tumors showed intense cytoplasmic staining of p-GSK3β in a positive case (left panel), but no appreciable staining in a negative case (right panel).
Expression of β-catenin in MCL tumors. (A) Immunohistochemistry using paraffin-embedded MCL tumors showed nuclear staining of β-catenin in a positive case (left panel). Only weak cytoplasmic staining was found in the tumor cells in a negative case, although endothelial cells showed intense cytoplasmic staining (right panel). (B) Immunohistochemistry using paraffin-embedded MCL tumors showed intense cytoplasmic staining of p-GSK3β in a positive case (left panel), but no appreciable staining in a negative case (right panel).
Status of the upstream WCP in MCL cells
We comprehensively surveyed the expression of various Wnt-related genes using Wnt pathway–specific oligonucleotide arrays. All of the results were normalized to the housekeeping genes included in this assay kit. As summarized in Table 1, of all the Wnt members detected in the 3 MCL cell lines and 3 frozen primary MCL tumors, Wnt3 and Wnt10A were consistently and most highly expressed Wnt members in these samples. The median CT values of these 2 Wnt members (ie, 23.26 and 24.15) were close to that of cyclin D1 (ie, 21.40), which is known to be highly expressed in MCL. In contrast, the CT values of Wnt1 and Wnt6 (ie, 34.01 and 34.07) were substantially lower. We also detected relatively high expression levels of several frizzled receptor members as well as LRP5 in these MCL cell lines and tumors, indicating that functional receptors for Wnt are expressed on MCL cells.
Oligonucleotide microarray studies to examine the expression of various genes involved in the Wnt canonical pathway in 3 MCL cell lines and 3 primary tumors
| Gene name . | Mino . | Jeko-1 . | SP53 . | MCL-tumor 1 . | MCL-tumor 2 . | MCL-tumor 3 . | Median CT . |
|---|---|---|---|---|---|---|---|
| WNT3 | 23.92 | 23.73 | 22.78 | 23.73 | 20.92 | 20.95 | 23.26 |
| WNT10A | 21.61 | 23.84 | 24.87 | 25.42 | 20.62 | 24.43 | 24.15 |
| WNT16 | 25.28 | 24.86 | 28.00 | 24.21 | 24.97 | 27.87 | 25.14 |
| WNT5B | 25.31 | 27.79 | 27.70 | 29.65 | 23.61 | 28.59 | 27.75 |
| WNT8A | ND | 29.31 | 29.58 | 29.26 | 28.32 | 29.24 | 29.29 |
| WNT3A | 30.44 | 28.44 | 30.15 | 28.49 | 28.98 | 30.20 | 29.57 |
| WNT9A | ND | 27.82 | 37.36 | 29.79 | 27.22 | 29.74 | 29.77 |
| WNT5A | 31.67 | 26.34 | 34.82 | 31.30 | 29.67 | 28.37 | 30.49 |
| WNT2 | 30.57 | 30.56 | 33.00 | 33.36 | 28.32 | 31.52 | 31.05 |
| WNT7B | 33.62 | 30.65 | 29.77 | 31.43 | 29.22 | 31.54 | 31.04 |
| WNT11 | 36.95 | 28.85 | 31.16 | 29.92 | 32.27 | 31.98 | 31.57 |
| WNT4 | 33.92 | 29.84 | 31.35 | 32.80 | ND | 32.31 | 32.56 |
| WNT1 | 36.23 | 32.38 | 33.56 | 34.38 | 33.64 | 36.44 | 34.01 |
| WNT6 | 31.56 | 32.63 | 35.50 | 37.10 | 32.33 | 36.32 | 34.07 |
| WNT2B | ND | ND | 33.80 | 29.70 | 29.38 | 28.90 | 31.75 |
| WNT7A | ND | ND | ND | 32.39 | ND | 31.65 | ND |
| FZD3 | 20.86 | 22.58 | 22.85 | 27.21 | 22.23 | 23.92 | 22.72 |
| FZD2 | 21.95 | 24.61 | 23.44 | 27.23 | 26.20 | 24.96 | 24.79 |
| FZD6 | 21.54 | 27.21 | 24.71 | 25.63 | 22.92 | 25.54 | 25.13 |
| FZD7 | 25.22 | 24.14 | 26.25 | 25.43 | 25.00 | 25.44 | 25.33 |
| FZD8 | 29.00 | 28.77 | 27.53 | 27.27 | 27.57 | 27.32 | 27.55 |
| FZD4 | 32.21 | 31.23 | 29.70 | 26.46 | 24.39 | 27.14 | 28.42 |
| FZD1 | 33.27 | 34.77 | 35.34 | 34.95 | 37.10 | 34.51 | 34.86 |
| LRP5 | 21.16 | 22.49 | 23.39 | 24.68 | 26.28 | 24.53 | 23.96 |
| LEF1 | 19.92 | 21.81 | 24.59 | 24.78 | 19.85 | 24.3 | 25.54 |
| TCF7 | 26.38 | 27.46 | 28.31 | 28.28 | 26.54 | 27.69 | 27.44 |
| CCND1 | 18.86 | 20.61 | 18.93 | 22.54 | 25.25 | 22.18 | 21.40 |
| Gene name . | Mino . | Jeko-1 . | SP53 . | MCL-tumor 1 . | MCL-tumor 2 . | MCL-tumor 3 . | Median CT . |
|---|---|---|---|---|---|---|---|
| WNT3 | 23.92 | 23.73 | 22.78 | 23.73 | 20.92 | 20.95 | 23.26 |
| WNT10A | 21.61 | 23.84 | 24.87 | 25.42 | 20.62 | 24.43 | 24.15 |
| WNT16 | 25.28 | 24.86 | 28.00 | 24.21 | 24.97 | 27.87 | 25.14 |
| WNT5B | 25.31 | 27.79 | 27.70 | 29.65 | 23.61 | 28.59 | 27.75 |
| WNT8A | ND | 29.31 | 29.58 | 29.26 | 28.32 | 29.24 | 29.29 |
| WNT3A | 30.44 | 28.44 | 30.15 | 28.49 | 28.98 | 30.20 | 29.57 |
| WNT9A | ND | 27.82 | 37.36 | 29.79 | 27.22 | 29.74 | 29.77 |
| WNT5A | 31.67 | 26.34 | 34.82 | 31.30 | 29.67 | 28.37 | 30.49 |
| WNT2 | 30.57 | 30.56 | 33.00 | 33.36 | 28.32 | 31.52 | 31.05 |
| WNT7B | 33.62 | 30.65 | 29.77 | 31.43 | 29.22 | 31.54 | 31.04 |
| WNT11 | 36.95 | 28.85 | 31.16 | 29.92 | 32.27 | 31.98 | 31.57 |
| WNT4 | 33.92 | 29.84 | 31.35 | 32.80 | ND | 32.31 | 32.56 |
| WNT1 | 36.23 | 32.38 | 33.56 | 34.38 | 33.64 | 36.44 | 34.01 |
| WNT6 | 31.56 | 32.63 | 35.50 | 37.10 | 32.33 | 36.32 | 34.07 |
| WNT2B | ND | ND | 33.80 | 29.70 | 29.38 | 28.90 | 31.75 |
| WNT7A | ND | ND | ND | 32.39 | ND | 31.65 | ND |
| FZD3 | 20.86 | 22.58 | 22.85 | 27.21 | 22.23 | 23.92 | 22.72 |
| FZD2 | 21.95 | 24.61 | 23.44 | 27.23 | 26.20 | 24.96 | 24.79 |
| FZD6 | 21.54 | 27.21 | 24.71 | 25.63 | 22.92 | 25.54 | 25.13 |
| FZD7 | 25.22 | 24.14 | 26.25 | 25.43 | 25.00 | 25.44 | 25.33 |
| FZD8 | 29.00 | 28.77 | 27.53 | 27.27 | 27.57 | 27.32 | 27.55 |
| FZD4 | 32.21 | 31.23 | 29.70 | 26.46 | 24.39 | 27.14 | 28.42 |
| FZD1 | 33.27 | 34.77 | 35.34 | 34.95 | 37.10 | 34.51 | 34.86 |
| LRP5 | 21.16 | 22.49 | 23.39 | 24.68 | 26.28 | 24.53 | 23.96 |
| LEF1 | 19.92 | 21.81 | 24.59 | 24.78 | 19.85 | 24.3 | 25.54 |
| TCF7 | 26.38 | 27.46 | 28.31 | 28.28 | 26.54 | 27.69 | 27.44 |
| CCND1 | 18.86 | 20.61 | 18.93 | 22.54 | 25.25 | 22.18 | 21.40 |
Data are expressed in CT values normalized against GAPDH.
ND indicates not detectable.
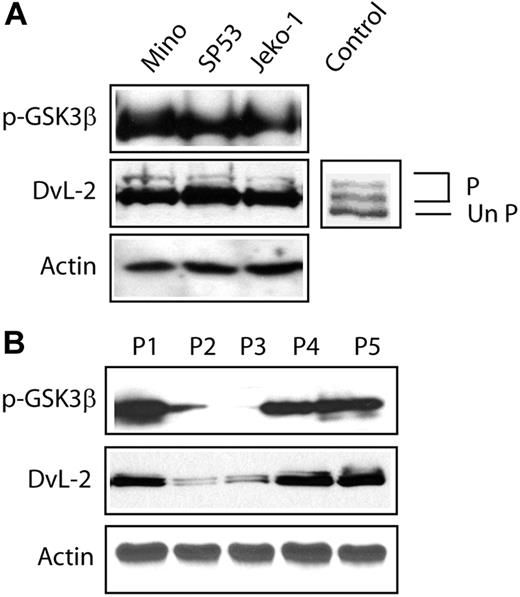
To further evaluate whether Wnt stimulation indeed occurs in MCL, we assessed the phosphorylation status of the DvL proteins as a surrogate marker. In the 3 MCL cell lines and 5 frozen MCL tumors used to assess the expression of p-GSK3β in this study, we clearly identified the presence of the slow migrating/phosphorylated species of DvL-2 (Figure 3). Mouse stem cell lysates, which are known to contain the phosphorylated forms of DvL-2, were used as a positive control.17,23 Interestingly, the expression level of DvL-2 correlated with that of p-GSK3β expression, as tumor P2 and P3 (with no or weak expression of p-GSK3β) had relatively low levels of DvL-2 (Figure 3B). Results for DvL-3 followed the same pattern as those for DvL-2. DvL-1 was not detectable in all MCL cell lines and tumors examined.
Activation status of the WNT pathway in MCL. (A) Western blot studies revealed the strong expression of p-GSK3β in all 3 MCL cell lines. In addition, DvL-2 was highly expressed. Importantly, the slowly migrating/phosphorylated forms of DvL-2 were detected in all 3 cell lines. Mouse stem cell lysates were used as positive controls for the phosphorylated forms of DvL proteins. P indicates the phosphorylated form of DvL-2 and UnP indicates the unphosphorylated form of DvL-2. (B) Western blot studies revealed the expression pattern of p-GSK3β in 5 MCL primary tumors. P1, P3, and P5 had a relatively high level of p-GSK3β, which was associated with relatively a high level of DvL-2 and the presence of its slowly migrating forms. In contrast, P2 and P3 had weak or undetectable p-GSK3β, which correlated with a weak DvL-2 expression.
Activation status of the WNT pathway in MCL. (A) Western blot studies revealed the strong expression of p-GSK3β in all 3 MCL cell lines. In addition, DvL-2 was highly expressed. Importantly, the slowly migrating/phosphorylated forms of DvL-2 were detected in all 3 cell lines. Mouse stem cell lysates were used as positive controls for the phosphorylated forms of DvL proteins. P indicates the phosphorylated form of DvL-2 and UnP indicates the unphosphorylated form of DvL-2. (B) Western blot studies revealed the expression pattern of p-GSK3β in 5 MCL primary tumors. P1, P3, and P5 had a relatively high level of p-GSK3β, which was associated with relatively a high level of DvL-2 and the presence of its slowly migrating forms. In contrast, P2 and P3 had weak or undetectable p-GSK3β, which correlated with a weak DvL-2 expression.
Blockade of the upstream WCP led to decreased p-GSK3β
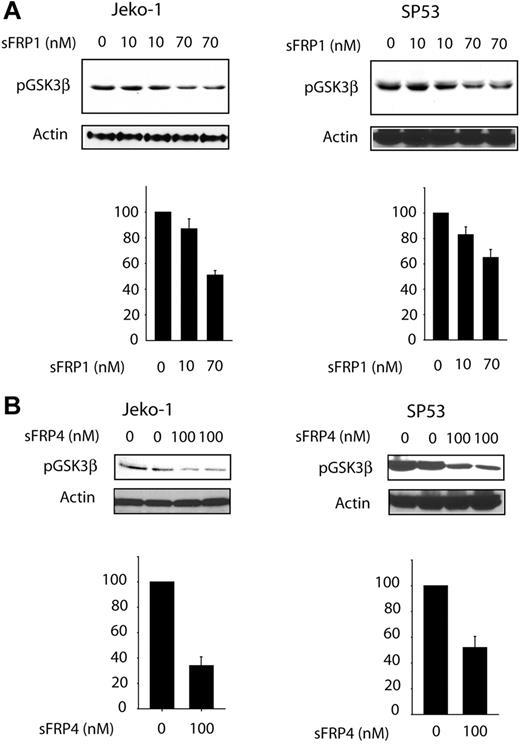
To determine whether GSK3β phosphorylation/inactivation is directly linked to Wnt activation, we added soluble recombinant sFRP1 or sFRP4 (naturally occurring decoys of the Wnt receptors)24 to the 3 MCL cell lines. Using quantitative RT-PCR, we have previously determined that these 2 factors were not produced or were produced at a low level in these 3 MCL cell lines (P.G., unpublished data, September 2006). As shown in Figure 4A, sFRP1 induced a statistically significant and dose-dependent decrease in the p-GSK3β levels for both Jeko-1 and SP53 cells. The decrease in p-GSK3β in both cell lines was approximately 50%. Significant differences were also observed when sFRP4 was used, with approximately 60% reduction in the level of pGSK-3β in both cell lines. These findings support the concept that phosphorylation and inactivation of GSK3β can be directly attributed to WCP.
Effect of WNT pathway inhibition on GSF3β phosphorylation status. SP53 and Jeko-1 cells were treated with different concentrations of natural Wnt inhibitor: sFRP1 (A) or sFRP4 (B). Treatments with either of these 2 sFRP proteins led to a significant decrease in p-GSK3β. The bottom graph on each figure represents densitometry measurement of the p-GSK3β expression levels. Error bars indicate SD.
Effect of WNT pathway inhibition on GSF3β phosphorylation status. SP53 and Jeko-1 cells were treated with different concentrations of natural Wnt inhibitor: sFRP1 (A) or sFRP4 (B). Treatments with either of these 2 sFRP proteins led to a significant decrease in p-GSK3β. The bottom graph on each figure represents densitometry measurement of the p-GSK3β expression levels. Error bars indicate SD.
Blockade of WCP resulted in a significant reduction in cell growth
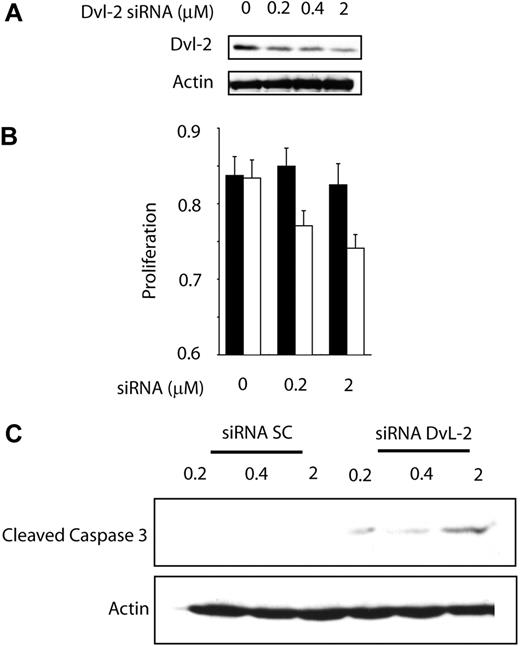
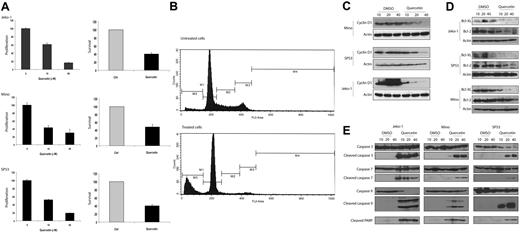
To test whether activation of WCP is biologically significant in MCL, we assessed whether cell growth is altered after the WCP is blocked either by siRNA knockdown of DvL-2 or using quercetin, a pharmacologic agent reported to be relatively specific for β-catenin.25,26 The choice of DvL-2 (instead of DvL-3) is based on our finding that the phosphorylated form of DvL-2 is more strongly expressed than that of DvL-3. As shown in Figure 5A, transfection of siRNA results in a concentration-dependent reduction in the expression of DvL-2 detectable by Western blots. Correlating with the down-regulation of DvL-2, there was a significant reduction in proliferation at 48 hours, as assessed by the MTS assay (Figure 5B). A substantial degree of cell proliferation was evident even after treatment with DvL-2 siRNA, and we believe this is due to that fact that the DvL-2 inhibition was far from being complete, and that other mechanisms (such as the Akt signaling pathway) likely continue to promote cell growth. With the same experimental conditions, we detected the occurrence of apoptosis, as evidenced by the dose-dependent increase in cleaved caspase-3 in the treated cells but not the negative control cells (Figure 5C). Similarly, as illustrated in Figure 6A, in vitro treatment with quercetin showed a significant and dose-dependent reduction in the number of viable cells at 48 hours, as assessed by the MTS assay (left panel) and trypan blue exclusion test (right panel). We also have performed similar experiments using sFRPs. No significant difference was found, probably because the continuous production of Wnts by MCL cells overwhelmed the sFRPs added in vitro. As illustrated in Figure 6B, cell-cycle analysis by flow cytometry showed that MCL cells treated by quercetin induced G0/G1 cell-cycle arrest as well as an increased proportion of the sub-G0 fraction.
Biologic effect of WNT pathway inhibition by DvL-2 targeting.(A) Treatment of Jeko-1 cells with siRNA for DvL-2 for 24 hours showed a dramatic decrease in the protein expression of DvL-2. (B) Wnt pathway inhibition by DvL-2 induced a significant decrease in Jeko-1 cell growth as measured by MTS assay. Error bars indicate SD. (C) Treatments of Jeko-1 cells for 48 hours with DvL-2 siRNA demonstrate a dose-dependent cleavage of caspase-3.
Biologic effect of WNT pathway inhibition by DvL-2 targeting.(A) Treatment of Jeko-1 cells with siRNA for DvL-2 for 24 hours showed a dramatic decrease in the protein expression of DvL-2. (B) Wnt pathway inhibition by DvL-2 induced a significant decrease in Jeko-1 cell growth as measured by MTS assay. Error bars indicate SD. (C) Treatments of Jeko-1 cells for 48 hours with DvL-2 siRNA demonstrate a dose-dependent cleavage of caspase-3.
Quercetin induce apoptosis in MCL. (A) MTS assay (left panel) and trypan blue exclusion test (right panel) were performed to assess the biologic effects of quercetin on MCL cell lines. All 3 cell lines showed a dose-dependent decrease in cell growth with quercetin treatment. Negative controls in all experiments were treated with the highest volume of DMSO used in the treated group. Results from the treated group are normalized to those of the negative controls. (B) Cell-cycle analysis by flow cytometry was performed using Mino cells with or without quercetin treatment. M1 represents the Go/G1 phase; M2, the S phase; M3/4, the G2/M phase; and M5, the subG0 apoptotic cell population. Compared with cells with quercetin treatment, treated cells showed a decrease in the proportion of cells in the S phase as well as the G2/M phase. In addition, there was a dramatic increase in the size of the subG0 cell population, in keeping with the occurrence of apoptosis. (C) All 3 MCL cell lines showed dose-dependent down-regulation of cyclin D1 after quercetin treatment at 24 hours. Negative controls were treated with DMSO in the same volumes used in the treatment group. (D) All 3 MCL cell lines showed dose-dependent down-regulation of Bcl-2 and Bcl-XL after quercetin treatment at 24 hours. Negative controls were treated with DMSO in the same volumes used in the treatment group. (E) All 3 MCL cell lines showed expression of cleaved caspases-3, -7, and -9, as well as PARP, after quercetin treatment at 24 hours. Negative controls were treated with DMSO in the same volumes used in the treatment group. Error bars indicate SD.
Quercetin induce apoptosis in MCL. (A) MTS assay (left panel) and trypan blue exclusion test (right panel) were performed to assess the biologic effects of quercetin on MCL cell lines. All 3 cell lines showed a dose-dependent decrease in cell growth with quercetin treatment. Negative controls in all experiments were treated with the highest volume of DMSO used in the treated group. Results from the treated group are normalized to those of the negative controls. (B) Cell-cycle analysis by flow cytometry was performed using Mino cells with or without quercetin treatment. M1 represents the Go/G1 phase; M2, the S phase; M3/4, the G2/M phase; and M5, the subG0 apoptotic cell population. Compared with cells with quercetin treatment, treated cells showed a decrease in the proportion of cells in the S phase as well as the G2/M phase. In addition, there was a dramatic increase in the size of the subG0 cell population, in keeping with the occurrence of apoptosis. (C) All 3 MCL cell lines showed dose-dependent down-regulation of cyclin D1 after quercetin treatment at 24 hours. Negative controls were treated with DMSO in the same volumes used in the treatment group. (D) All 3 MCL cell lines showed dose-dependent down-regulation of Bcl-2 and Bcl-XL after quercetin treatment at 24 hours. Negative controls were treated with DMSO in the same volumes used in the treatment group. (E) All 3 MCL cell lines showed expression of cleaved caspases-3, -7, and -9, as well as PARP, after quercetin treatment at 24 hours. Negative controls were treated with DMSO in the same volumes used in the treatment group. Error bars indicate SD.
In view of the importance of cyclin D1 in MCL and its link to the GSK3β, we investigated if the expression of cyclin D1 is altered with inhibition of WCP. Western blot analysis was performed using lysates from MCL cell lines treated with quercetin at 24 hours. As shown in Figure 6C, we observed a significant decrease in cyclin D1 in all 3 MCL cell lines examined. We also determined if this down-regulation of cyclin D1 was due to a reduction at the transcriptional level. Thus, we performed qRT-PCR to assess the expression of cyclin D1 mRNA. Jeko-1 cells treated with quercetin at 20 μM for 24 hours showed a 3.6-fold decrease in cyclin D1 (P < .05, Student t test). At 40 μM quercetin, a 10.2-fold decrease in cyclin D1 was noted (P < .05). These results correlate well with the cyclin D1 protein levels illustrated in Figure 6C and support that the mechanism underlying the quercetin-induced decrease in cyclin D1 is transcriptional.
To further characterize the apoptosis induced by WCP inhibition, we assessed the expression of 2 antiapoptotic proteins including Bcl-XL and Bcl-2. As shown in Figure 6D, these 2 antiapoptotic proteins were down-regulated in MCL cells treated with quercetin. As shown in Figure 6E, the occurrence of apoptosis induced by quercetin involved cleavages of PARP, caspase-3, caspase-7, and caspase-9. The occurrence of apoptosis in quercetin-treated MCL cells was also further supported by morphologic examination (not shown).
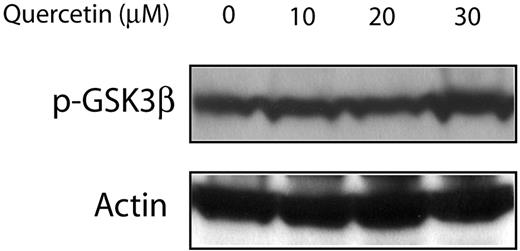
Since quercetin was also reported to be an inhibitor of the PI3K/Akt pathway, which is also a known regulator of GSK3β, we assessed if the quercetin effects on MCL are mediated via PI3K/Akt or directly on β-catenin. As shown in Figure 7, no detectable change was seen in the expression levels of p-GSK3β. Thus, these findings support that the observed biologic effects are directly mediated upon β-catenin.
Treatment of Jeko-1 cells with quercetin had no detectable effect on the GSK3β phosphorylation status. Jeko-1 cells were treated with different concentrations of quercetin. Treatment with the diluent served only as the negative control.
Treatment of Jeko-1 cells with quercetin had no detectable effect on the GSK3β phosphorylation status. Jeko-1 cells were treated with different concentrations of quercetin. Treatment with the diluent served only as the negative control.
Discussion
WCP is involved in the transduction of various extracellular stimuli that regulate a wide range of fundamental cellular processes including cell proliferation and apoptosis.14 The oncogenic potential of WCP has been illustrated in several human cancers, most notably epithelial cancers.14 The importance of WCP in hematopoietic malignancies is not as extensively studied. More recently, β-catenin activation, coupled with GSK3β inactivation, has been demonstrated in chronic myeloid leukemia in blast crisis and precursor B-cell acute lymphoblastic leukemia.27
For the first time, we have provided evidence of WCP activation in MCL cell lines and tumors. To support this conclusion, we have demonstrated that β-catenin is localized to the nucleus and transcriptionally active in all 3 MCL cell lines examined. The finding of nuclear staining of β-catenin in approximately half of the MCL tumors supports that activation of β-catenin is a relatively frequent aberration in MCL. As discussed in the Introduction, β-catenin is regulated by the destruction complex in which GSK3β is one of the key components. When GSK3β is inactivated, β-catenin is released from the destruction complex and allowed to accumulate in the cytoplasm and migrate to the nucleus. In keeping with this concept, we found strong expression p-GSK3β in all MCL cell lines examined and approximately half of the MCL tumors. Importantly, expression of nuclear β-catenin significantly correlates with that of p-GSK3β in the 64 MCL tumors examined, strongly suggesting a functional link between GSK3β inactivation and the expression of nuclear β-catenin in vivo.
The fact that we identified evidence of WCP activation in all 3 MCL cell lines examined but only half of MCL tumors is likely related to the use of different techniques. For the cell lines, we used Western blots and TOP/FOP, both of which are more sensitive than immunohistochemistry that was used to assess the paraffin-embedded MCL tumors. Nevertheless, we also have considered the possibility that a subset of MCL tumors is truly negative for WCP activation. Of note, virtually all MCL cell lines were established from the blastoid variant, which may have a higher frequency of WCP activation compared with the classical variant seen in the vast majority of our archival MCL cases. Further studies including a higher number of blastoid MCL cases will be needed to explore this issue.
With regard to the mechanism of GSK3β inactivation and phosphorylation, it has been recently suggested that the PI3K/Akt signaling pathway may play a role. Multiple publications have reported Akt activation in MCL, particularly those of the blastoid variant.11 In one study, GSK3β was examined in 3 typical MCL cases; 2 of these 3 cases are strongly positive, even in the absence of detectable pAkt.11 These findings support the concept that Akt is likely not the only means by which GSK3β is inactivated in MCL. Our results from this study suggest that WCP may well be the alterative mechanism by which GSK3β is inactivated. Correlating with this concept, WCP has been reported to induce phosphorylation of GSK3β independent of PI3K/Akt in other cell types.28-32
To support that WCP stimulation directly contributes to GSK3β inactivation in MCL, we assessed the effects on GSK3β after WCP is inhibited using sFRPs (naturally occurring decoys for the Wnt receptors). The use of sFRPs and their inhibitory effects on the phosphorylation/activation status of GSK3β has been previously described in other biologic systems.24,28,33-35 In MCL, blockade of WCP by sFRP1 or sFPR4 resulted in a significant decrease in p-GSK3β. This observation is consistent with some of the published results generated in different cell types and experimental systems.34-36 These findings strongly support that the phosphorylation/inactivation of GSK3β is directly attributed to WCP stimulation. Our studies using siRNA to inhibit DvL-2 revealed results that are consistent with this concept. Specifically, down-regulation of DvL-2 triggered apoptosis and led to a significant reduction in cell proliferation (Figure 5).
Our oligonucleotide microarray data generated using 3 MCL cell lines and 3 tumor samples revealed that several Wnt members are consistently expressed. Since our main purpose is to obtain the expression profile of various genes in WCP in MCL, we did not include any normal lymphoid tissues for comparison. Based on the CT values, we have observed that the most abundantly expressed Wnt members in MCL are Wnt3 and Wnt10A, both of which have been implicated in oncogenesis in other cell types.37-41 Furthermore, MCL cells expressed several frizzled receptors as well as LRP5, and thus, functional Wnt receptors are likely expressed. Lastly, we also detected consistent expression of TCF/LEF, a protein complex required for the full transcriptional functions of β-catenin (Table 1).
In this study, we have provided the first evidence that β-catenin is biologically significant in MCL, as blockade of WCP using quercetin or siRNA knockdown of DvL-2 decreased cell growth. Our results are consistent with those described in other experimental models; degradation of DvL-2 has been shown to block WCP.42 In the quercetin experiments, apoptosis was triggered, with involvement of activation of caspases-3, -7, and -9. Quercetin has been reported to be a potent inhibitor of β-catenin that is independent of GSK3β.25,26 Nevertheless, it has also been reported to be an inhibitor of the PI3K/Akt pathway.43 The lack of detectable change in the levels of p-GSK3β or pAkt after quercetin treatment supports the concept that the quercetin effects in MCL cells were not dependent on PI3K/Akt or GSK3β. Instead, we believe that the quercetin effects are via direct β-catenin inactivation, as previously described in other models.26 In keeping with this concept, we found that addition of quercetin to MCL cell lines dramatically decreased cyclin D1 expression, a known downstream target of β-catenin.44
After the submission of this current paper, we completed a comprehensive clinicopathologic study with a focus on correlating p-GSK3β with various clinical parameters including survival. We found that p-GSK3β significantly correlated with a worse clinical outcome in a cohort of 83 MCL patients. Using this larger cohort, we also have confirmed the positive correlation between p-GSK3β and nuclear β-catenin (Randy Chung, A.C.P., M.A., H.A., Sunita Ghosh, P.G., and R.L., Biological and clinical significance of GSK3β inactivation in mantle cell lymphoma, manuscript submitted).
In conclusion, for the first time, we demonstrate that WCP is constitutively active in approximately half of MCL tumors, and we have provided evidence that these aberrations promote cell growth in MCL. Our findings suggest that WCP may be a potentially useful therapeutic target for these tumors.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research grants from the Alberta Cancer Foundation (Edmonton, AB) and the Canadian Cancer Society (Toronto, ON) (R.L.). P.G. is a recipient of the Lymphoma Research Foundation of Canada (Mississauga, ON) Fellowship Award. H.M.A. is a recipient of the Clinical Scientist award from the National Institutes of Health (Bethesda, MD) (CA114395) and the University of Texas M. D. Anderson (Houston, TX) Physician Scientist Program Award.
National Institutes of Health
Authorship
Contribution: P.G. and R.L. designed the experiments; P.G., M.A., H.A., and J.D.B. performed all experimental procedures; P.G., M.A., A.C.P., and R.L. analyzed the data; H.M.A. and R.L. reviewed the immunostaining results; and P.G. and R.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raymond Lai, Department of Laboratory Medicine and Pathology, Cross Cancer Institute and University of Alberta, 11560 University Avenue, Edmonton, AB, Canada T6G 1Z2; e-mail: raymondmail_65@yahoo.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal