Abstract
Cytotoxic T lymphocytes (CTLs) and natural killer cells help control infections and tumors via a killing activity that is mediated by the release of cytotoxic granules. Granule secretion at the synapse formed between the CTL and the target cell leads to apoptosis of the latter. This process involves polarization of the CTL's secretory machinery and cytotoxic granules. The small GTPase Rab27a and the hMunc13-4 protein have been shown to be required for both granule maturation and granule docking and priming at the immunologic synapse. Using a tandem affinity purification technique, we identified a previously unknown hematopoietic form of Slp2a (Slp2a-hem) and determined that it is a specific effector of the active form of Rab27a. This interaction occurs in vivo in primary CTLs. We have shown that (1) Rab27a recruits Slp2a-hem on vesicular structures in peripheral CTLs and (2) following CTL-target cell conjugate formation, the Slp2a-hem/Rab27a complex colocalizes with perforin-containing granules at the immunologic synapse, where it binds to the plasma membrane through its C2 domains. The overexpression of a dominant-negative form of Slp2a-hem markedly impaired exocytosis of cytotoxic granules—indicating that Slp2a is required for cytotoxic granule docking at the immunologic synapse.
Introduction
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are able to kill virus-infected cells and tumor cells via the polarized secretion of perforin- and granzyme-containing cytotoxic granules at the immunologic synapse formed between the CTL/NK effector and the target cell.1,2 In CTLs, the interaction of the T-cell receptor (TCR) with an antigen-presenting target cell induces the rapid polarization of the microtubule-organizing center (MTOC); the contact of centrosome with the plasma membrane is followed by the secretion of cytotoxic granules at the immunologic synapse.3-6 We recently reported that functional maturation of perforin-containing granules involves fusion with a Rab27a- and hMunc13-4–dependent “exocytic compartment.” This fusion step occurs close to the plasma membrane, at the immunologic synapse.7
Rab27a and hMunc13-4 have been shown to be essential effectors of the granule exocytosis machinery. Defects in either of the 2 proteins leads to functional impairment of the granule-dependent cytotoxic pathway; this results in several inherited conditions, which all feature unbalanced expansion and activation of CD8 T lymphocytes and uncontrolled macrophage activation.8,9 Defects in Rab27a (a member of the large family of small GTPases involved in intracellular vesicular transport) lead to the condition known as Griscelli syndrome type 2 (GS2)10 in humans and the ashen phenotype in mice.11 Like other Ras-related proteins, Rab proteins cycle between a GDP-bound (“off”) and a GTP-bound (“on”) form.12,13 Even though cytotoxic granules from Rab27a-deficient lymphocytes do polarize with the MTOC toward the target cell, docking of granules and subsequent release of their contents at the immunologic synapse is markedly impaired.14-16 hMunc13-4, a protein that is defective in patients with familial hemophagocytic lymphohistiocytosis type 3 (FHL3) and in Jinx mice,17 controls the cytotoxic granule priming step, which may also require association with Rab27a.16,18,19 In addition, hMunc13-4 has been shown to regulate assembly of recycling and late endosomal structures, leading to the formation of an endosomal exocytic compartment that fuses with perforin-containing granules at the immunologic synapse7 and licenses them for exocytosis.
Several effectors of the GTP-bound form of Rab27a have been described. They fall into 3 families: the Slp (synaptotagmin-like protein) family, the Slac2 (Slp lacking C2 domains) family, and the RBD (Rab3-binding domain) family.20-25 All members of these families share an amino-terminal Rab27-binding domain (the “Slp-homology domain”: SHD), which directly interacts with Rab27a in its GTP-bound form. Slp and RBD family proteins also contain 2 C2 domains (putative phospholipid or protein interaction sites) at the carboxy terminus, whereas Slac2 members have a myosin- or actin-binding domain instead. This structural difference suggests that Rab27a-binding proteins have distinct functions. Slp4-a/granuphilin-a has been implicated in the tethering of insulin granules at the plasma membrane by interaction with both Rab27a and the SNARE protein syntaxin-1a.26,27 Conversely, Slac2-a/Mlph and Slac2-c/MyRIP link Rab27a to molecular motors (MyoVa or MyoVIIa) that are responsible for melanosome transport in melanocytes and in retinal pigment epithelium cells, respectively.28-31 Slp2a has also been shown to regulate peripheral melanosome distribution and cell shape in melanocytes.32 Both processes require the phosphatidylserine-binding ability of Slp2a's C2A domain, whereas the control of melanosome distribution depends only on the Rab27a-binding activity of Slp2a's SHD domain.32 Furthermore, by studying Slp2a-deficient mice, a role of Slp2a in the docking and the secretion of mucus granules of gastric surface cells has been evidenced.33 Recently, Slp2a has also been shown to promote the recruitment of glucagon-containing granules to the plasma membrane in pancreatic α cells—a process that requires the ability of Slp2a's C2A domain to bind phosphatidylserine phospholipids in the absence of calcium.34
Although the critical role of the Slp family members in the tethering of secretory granules at the plasma membrane in different cell types is now widely accepted, their involvement in recruitment for docking at the immunologic synapse of cytotoxic granules remains ill defined. Here, we used a tandem affinity purification35,36 (TAP) expression system to identify specific effectors that interact with Rab27a in cytotoxic cells. This procedure enabled us to isolate a new isoform of Slp2a (Slp2a-hem) as a specific effector of the active form of Rab27a in the YT NK cell line and demonstrate its involvement in cytotoxic granule exocytosis.
Methods
The Rab27a-TAP-tag construct
A Rab27a-TAP-tag construct containing 2 protein A IgG-binding domains, a tobacco etch virus (TEV) cleavage site, and a calmodulin-binding peptide (CBP) sequence downstream of the TEV cleavage site was generated as previously described.7 The active (Q78L) and inactive (T23N) mutants of Rab27a37 in fusion with TAP-tag were then cloned into a pIRESneo vector (Clontech, Palo Alto, CA).7
Slp2a sequencing and cloning
The human Slp2a-hem isoform sequence was obtained as follows: cDNA was prepared from YT and lymphokine-activated killer (LAK) cell mRNA using Superscript II and Random primers (Invitrogen, Frederick, MD) according to the manufacturer's protocol. Multiple fragments of Slp2a-hem cDNA were amplified by polymerase chain reaction (PCR) and sequenced (primer sequences available upon request). A long PCR fragment spanning the SHD to the C2 domains of Slp2a isoforms was also amplified and sequenced using the following primers: F-5′-AGGCCATCATGAAGGTTT-3′ and R-5′-TGGCGACTTTTCCTACTTG-3′. Comparison of the compiled sequence with the published KIAA1597 one revealed a deletion of exon 10 and an insertion of a 120-bp fragment encoded by part of intron 12 according to the intron/exon structure described in ENSEMBL exon view ENST00000316356.38 We defined the inserted sequence as exon 12, which implies that the following exons numbers are shifted by one according to ENSEMBL exon view. The sequence of the Slp2a hematopoietic (Slp2a-hem) isoform is published in GenBank under the accession number EU035829.39 The human Slp2a-hem isoform sequence was cloned as follows: human Slp2a (KIAA1597) cDNA cloned into the fj09819 vector was kindly provided by Dr T. Nagase (Kazusa DNA Research Institute, Kisarazu, Japan). The fragment encoding Slp2a exons 10, 12, 13, and 14 (specific for the Slp2a-hem isoform) was cloned into pCRII-TOPO using the following primers: forward 5′-CTGCTGATGAACTGTCTCA-3′ and reverse 5′-AATTTAGATGACTTCCCCTAG-3′. Full-length Slp2a-hem was constructed by replacing the domain between the Bsa1 and Kpn1 restriction sites of Slp2a KIAA1597 (including the sequence encoded by exons 10, 11, 12, and 14) by the Slp2a-hem–specific sequence. Full-length Slp2a-hem was amplified by PCR using the following primers: forward 5′-GCCTCGAGTCAAAATGATTGACTTAAGC-3′ and reverse 5′-GCGTCGACTCATTGGGCCCATTTGGAAATCTTGGCAA-3′, and then subcloned into pEGFP-C1 (Clontech). Slp2a's SHD domain was amplified using the following primers: forward 5′-GGAATTCTTCAAAATGATTGACTTAAGCTTC-3′ and reverse 5′-GCGGATCCACCTCGAGTGCCCCATTTTCTCTGTCTTT-3′. The Slp2a C2 domains were amplified using a 5′-GCGAATTCATGGGAAATATTCAGTTTGCA-3′ forward primer and the reverse primer used to amplify full-length Slp2a. These fragments were then cloned into pEGFP-C2 (Clontech). The Slp2a-hem SHD mutant (E11A, R32A) was generated by PCR with the QuikChange Multi site-directed mutagenesis kit (Stratagene, La Jolla, CA) using pEGFP-C1-Slp2a-hem and the following primers: forward 5′-CTGACTGAAGAGGCACAAGAGGCCATC-3′ (E11A) and forward 5′-GGCCGAAGAAGAGGCAGTCAGACATTTGCC-3′ (R32A). The full-length Rab27a was cloned in fusion with the monomeric fluorescence protein red (DsRed-Rab27a; Clontech).
The PESTFIND program was used to determine the location of PEST domains in the Slp2a sequence (European Molecular Biology Network; https://emb1.bcc.univie.ac.at/toolbox/pestfind/pestfind-analysis-webtool.htm).
Slp2a expression analysis
Slp2a-hem and KIAA1597 expression was analyzed by PCR in a panel of tissue cDNAs (Human MTC panel I; Clontech), in a panel of blood cell cDNAs (Human blood fraction MTC panel; Clontech), and in purified human NK cell cDNA, using the primers designed to amplify specifically Slp2a isoforms. The same forward primer was used with the 2 reverse primers targeting exon 13 for Slp2a-hem and exon 11 for KIAA1597 (Slp2a forward 5′-AATGGGCTGCATTCTCATTC-3′, Slp2a-hem reverse 5′-CAGGGGAGCTGCTAAGATTG-3′, KIAA1597 reverse 5′-TGGCTTCTGATCTGGTTTCTC-3′). β-Actin–specific primers (forward 5′-TACCACTGGCATCGTGATGGACT-3′ and reverse 5′-TCCTTCTGCATCCTGTCGGCAAT-3′) were used for normalization.
Cell lines, cell culture, and transfection
Peripheral blood lymphocytes (PBLs) were isolated from whole blood from controls and from Griscelli syndrome patients. P1 carries 2 heterozygote RAB27A missense mutations (L70P and A76V) and P2 carries a homozygous RAB27A nonsense mutation (Q118X). Samples were obtained from patients and controls who had granted their written informed consent in accordance with standard practice at the French National Institute for Health and Medical Research (Inserm) and the Declaration of Helsinki. LAK-T cells were generated as previously described.7 The 293T cell line was transfected using the FuGENE 6 liposomal transfection reagent (Roche Diagnostics, Indianapolis, IN). The YT human NK cell line and LAK-T cells were transfected using the Amaxa nucleofection technique (Amaxa Biosystems, Gaithersburg, MD) in accordance with the manufacturer's protocols. YT cell lines stably expressing TAP-tag-Q78L or TAP-tag-T23N were maintained in presence of geneticin (GIBCO, Carlsbad, CA).
TAP-tag purification
The TAP-tag purification was performed as previously described.35,36 Briefly, cells were lysed in IPP150 lysis buffer, the lysate was centrifuged at 25 000g for 10 minutes, and the supernatant was ultracentrifuged at 100 000g for 1 hour. The supernatant was rotated with IgG matrix (Amersham Biotech, Piscataway, NJ). The IgG beads were washed twice with binding buffer and then once with TEV cleavage buffer. The beads were resuspended in TEV cleavage buffer plus 100 IU TEV protease (Life Technologies, Bethesda, MD) and rotated for 2 hours at 16°C. The TEV eluate recovered from the IgG beads was adjusted to calmodulin-binding buffer and rotated for 1 hour at 4°C with calmodulin beads (Stratagene). The beads were then washed, recovered in sample buffer, and loaded onto SDS–polyacrylamide gel (Bio-Rad, Hercules, CA). Proteins were detected by Coomassie blue staining.
Mass spectrometry and protein identification
After separation by SDS–polyacrylamide gel electrophoresis (PAGE), bands were excised from the Coomassie blue–stained gel and analyzed as previously described.40 The liquid chromatography (LC) system was directly coupled to a mass spectrometer (QTOF Ultima; Waters, Milford, MA). Mass spectrometry (MS) and MS/MS data were acquired and processed automatically using MassLynx 4.0 software (Waters). Database searching was performed using MASCOT 2.1 software (Matrix Science, Boston, MA). Two databases were used: an in-house list of well-known contaminants (keratins and trypsin) and an updated compilation of the SwissProt41 and TrEMBL42 databases.
Immunoprecipitation and immunoblotting
LAK-T cells were lysed in presence of 22 μg of NaF and 45 μg of NaVO4 per 10 mL lysis buffer. An anti-Rab27a antibody (Transduction Laboratories, Lexington, KY) was used for immunoprecipitation. Immune complexes were harvested using protein G (Sigma, St Louis, MO). Proteins were loaded on SDS–polyacrylamide gel and transferred onto an Immobilon membrane (Millipore, Billerica, MA). YT cells (stably transfected or not with mutant Rab27a) were lysed as described for LAK-T cells.
Antibodies used for immunoblotting
A polyclonal rabbit anti-Slp2a SHD was generated by NeoMPS (Strasbourg, France). The following antibodies were used for immunoprecipitation (IP) and immunoblotting: rabbit polyclonal anti-Slp2a, rabbit polyclonal anti–hMunc13-4,16 anti-Rab27a (IgG2a clone 3; Transduction Laboratories), anti–phosphatidylinositol-3-OH kinase (PI (3)K) P85 (rabbit antiserum; 06-497; Upstate Biotechnology, Lake Placid, NY), and protein A-HRP linked (Amersham Biotech).
Immunofluorescence microscopy
The cell conjugate formation was performed as previously described.7 The following antibodies and labels were used: purified mouse monoclonal antihuman perforin (deltaG9; Pharmingen, San Diego, CA), Alexa Fluor 555–conjugated goat anti–mouse IgG F(ab′)2 secondary antibody (A21424; Molecular Probes, Eugene, OR), and Texas Red (Molecular Probes).
A LSM 5 PASCAL machine (Zeiss, Heidelberg, Germany) was used for confocal microscopy. Eight-bit images were acquired with a Plan-Neofluor 100× oil objective with 1.3 numerical aperture. Image sets were processed with the Zeiss LSM Image Browser or Adobe Photoshop version CS (Adobe Systems, San Jose, CA). Fluorescence was quantified with Metamorph software version 6.3 R2 (Universal Imaging, Ypsilanti, MI). For each marker comparison, 4 to 5 cells were analyzed, each with 15 to 20 images of the plane, representative of more than 100 cells. At the immunologic synapse, the extent of colocalization was estimated by computation of the Pearson coefficient for at least 10 cells, each with 10 images of planes.43
Degranulation assay
The degranulation assay for N-alpha-benzyloxycarbonyl-l-lysine thiobenzyl ester (BLT) esterase (granzyme A) activity was performed as previously described.10 Statistical analysis using a Student t test was performed with Prism 3 software (GraphPad Software, La Jolla, CA). Statistical significance was defined as a P value less than .05.
Results
Interaction of Slp2a with Rab27a in cytotoxic cells
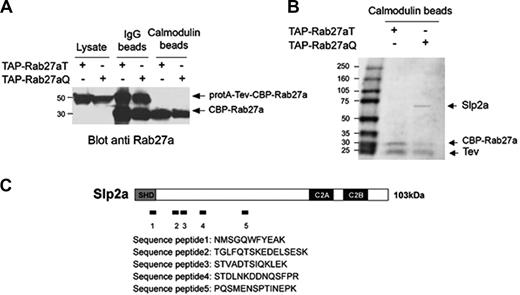
To isolate effector molecules that interact with Rab27a in cytotoxic cells, we generated 2 stable YT cell lines expressing either an active (Q78L) or an inactive (T23N) form of Rab27a for targeting distinct ligands. Both mutants were fused to a tag composed of the IgG-binding domains from Staphylococcus aureus protein A, a cleavage site for the TEV protease, and a calmodulin-binding peptide (CBP), to perform TAP. This method enables the rapid purification of protein complexes under native conditions in 2 successive affinity column steps.35,36 Full tag forms of Rab27a mutants migrate with an approximate size of 50 kDa and the TEV-cleaved tag runs as a 30-kDa protein (Figure 1A). A single band around 60 kDa was specifically copurified with the active Rab27a mutant (Figure 1B). Following protein digestion, mass spectrometry (MS) analysis identified peptides corresponding to the Slp2a protein (Figure 1C).
Tandem affinity purification of the Rab27a/Slp2a complex. (A) TAP-Rab27aT (an inactive mutant) and TAP-Rab27aQ (an active mutant) were purified from 2 stable YT cell lines expressing these constructs. Two successive affinity purification steps were performed using IgG beads and then calmodulin beads. Total lysates, the IgG bead fraction, and the calmodulin bead fraction were separated by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto an Immobilon membrane. Western blot analysis of TAP-tagged Rab27a mutants was revealed with anti-Rab27a. (B) A fraction of protein purified with TAP-Rab27aT and TAP-Rab27aQ after the TAP procedure was separated using 4% to 20% SDS-PAGE and visualized by Coomassie blue staining. (C) Schematic representation of the Slp2a domains used to localize the peptides identified by mass spectrometry. The peptide sequences are shown.
Tandem affinity purification of the Rab27a/Slp2a complex. (A) TAP-Rab27aT (an inactive mutant) and TAP-Rab27aQ (an active mutant) were purified from 2 stable YT cell lines expressing these constructs. Two successive affinity purification steps were performed using IgG beads and then calmodulin beads. Total lysates, the IgG bead fraction, and the calmodulin bead fraction were separated by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto an Immobilon membrane. Western blot analysis of TAP-tagged Rab27a mutants was revealed with anti-Rab27a. (B) A fraction of protein purified with TAP-Rab27aT and TAP-Rab27aQ after the TAP procedure was separated using 4% to 20% SDS-PAGE and visualized by Coomassie blue staining. (C) Schematic representation of the Slp2a domains used to localize the peptides identified by mass spectrometry. The peptide sequences are shown.
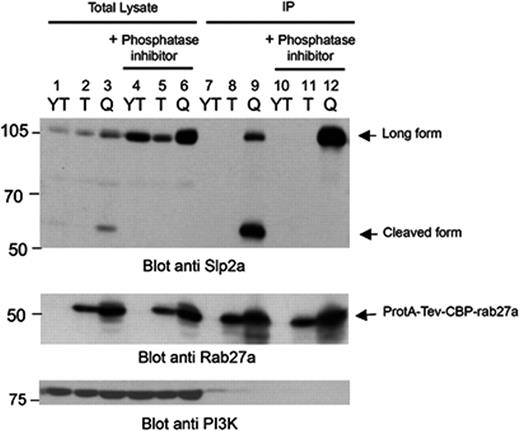
Slp2a (a member of the Slp family) is encoded by SYTL2. It contains an SHD domain in the N-terminal region and tandem C2 domains at the C terminus (Figure 1C). Full-length Slp2a has an expected molecular weight of 103 kDa. Since the molecular weight of the Slp2a protein that copurified with Rab27a in the YT cell line was lower than expected, we sought to determine whether this discrepancy was due to protein degradation or the expression of a shortened isoform of Slp2a in cytotoxic cells. We generated a rabbit antibody against the Slp2a SHD domain and used it to analyze the endogenously expressed Slp2a in the YT cell line. Both full-length and shorter Slp2a products (60 kDa) were detected in lysates and were immunoprecipitated from YT cells transfected with the active Rab27a mutant (Figure 2 lanes 3 and 9). These proteins were also detected in untransfected YT cells and in YT cells transfected with the inactive form of Rab27a following a longer exposure time (Figure 2 lanes 1,2 and data not shown). Thus, a much higher expression of Slp2a was detected in the YT cell line overexpressing the active form of Rab27a—suggesting that Rab27a regulates the stability or the expression of Slp2a. When YT cell lines were lysed in the presence of phosphatase inhibitors, only the full-length Slp2a protein was detected (Figure 2 lanes 4-6 and 9). Thus, as previously described for 2 other Rab27a effectors (ie, Slac2-a/melanophilin and Slac2-c/MyRIP44 ), Slp2a appears to be extremely sensitive to proteolysis—a process that is phosphatase dependent. We conclude that the 60-kDa Slp2a protein product that was TAP-purified with the active form of Rab27a results from phosphatase-dependent proteolytic cleavage of the full-length Slp2a protein, which is endogenously expressed in YT cytotoxic cells. Phosphatase inhibitors were therefore used at the cell lysis stage in subsequent experiments. These results show that the full-length form of Slp2a is expressed in cytotoxic cells and that it interacts with the active form of Rab27a.
Detection of a proteolytic cleavage product of endogenous full-length Slp2a in the absence of phosphatase inhibitors. YT cell lines expressing the TAP-active form of Rab27a (Q) or the TAP-inactive form of Rab27a (T) and untransfected YTs (YT) were lysed in the presence or absence of phosphatase inhibitor. Lysates were then immunoprecipitated with IgG beads. Lysates and precipitates (IPs) were analyzed by Western blot for the presence of cleaved or full-length forms of Slp2a using a rabbit antibody raised against the Slp2a SHD domain. Equally, the presence of TAP mutants Rab27a was detected using an anti-Rab27a. Western blot analysis of PI3K was used as a loading control.
Detection of a proteolytic cleavage product of endogenous full-length Slp2a in the absence of phosphatase inhibitors. YT cell lines expressing the TAP-active form of Rab27a (Q) or the TAP-inactive form of Rab27a (T) and untransfected YTs (YT) were lysed in the presence or absence of phosphatase inhibitor. Lysates were then immunoprecipitated with IgG beads. Lysates and precipitates (IPs) were analyzed by Western blot for the presence of cleaved or full-length forms of Slp2a using a rabbit antibody raised against the Slp2a SHD domain. Equally, the presence of TAP mutants Rab27a was detected using an anti-Rab27a. Western blot analysis of PI3K was used as a loading control.
Tissue distribution of Slp2a isoforms
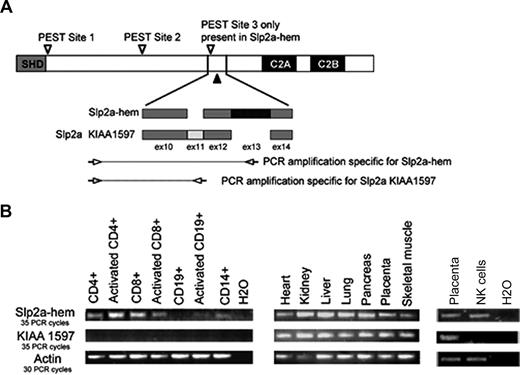
Sequencing of the full-length Slp2a cDNA isolated from both YT cells and lymphokine-activated killer T (LAK-T) cells revealed a high degree of sequence homology between this newly described form of Slp2a (which we have named “Slp2a-hem” for “hematopoietic cells”) and the previously described KIAA1597 form, present in the fj09819 image clone. These sequences differ only in one region, which is located between the coding sequences of exons 10 and 14 as a result of alternative splicing of the Slp2a mRNA. As shown in Figure 3A, Slp2a-hem uses exons 10, 12, 13, and 14, whereas exons 10, 11, 12, and 14 are present in KIAA1597. It was found that the PCR product spanning the SHD to the C2 domains of Slp2a cDNA from YT cells and LAK-T cells corresponds to Slp2a-hem as shown by sequencing. No other isoform of Slp2 (including Slp2a KIAA1597) was detected by sequencing. It is noteworthy that the other members of the Slp2 family (Slp2b-f) lack the N-terminal region of Slp2a, including the Rab27a-binding SHD region (Figure 3A).
Tissue expression of Slp2a isoforms. (A) A schematic representation of the exon combinations (exons 10 to 14) that define 2 splicing isoforms of Slp2a (KIAA1597 and Slp2a-hem). The location of 3 putative PEST-like sequences (▽) and the phosphatase-sensitive region of Slp2a-hem leading to the cleaved form (▲) are shown. The locations of the PCR primers used to specifically amplify each Slp2a isoform are indicated by arrows. (B) RT-PCR analysis, using commercial cDNA panels from nonhematopoietic and hematopoietic tissues, and cDNA from purified human NK cells. The couples of primers shown in panel A were used to amplify each Slp2a isoforms in hematopoietic and nonhematopoietic cDNA panels. Actin expression was used for normalization.
Tissue expression of Slp2a isoforms. (A) A schematic representation of the exon combinations (exons 10 to 14) that define 2 splicing isoforms of Slp2a (KIAA1597 and Slp2a-hem). The location of 3 putative PEST-like sequences (▽) and the phosphatase-sensitive region of Slp2a-hem leading to the cleaved form (▲) are shown. The locations of the PCR primers used to specifically amplify each Slp2a isoform are indicated by arrows. (B) RT-PCR analysis, using commercial cDNA panels from nonhematopoietic and hematopoietic tissues, and cDNA from purified human NK cells. The couples of primers shown in panel A were used to amplify each Slp2a isoforms in hematopoietic and nonhematopoietic cDNA panels. Actin expression was used for normalization.
Analysis of Slp2a sequences with the PESTFIND program predicted 3 PEST sequences (ie, potential signals for protein degradation: site 1: residues 105-149; site 2: residues 365-382; and site 3: residues 534-552; Figure 3A). It is noteworthy that the first 2 sequences are also present in the Slp2a isoforms, whereas the third is generated by exon 11 splicing and is present in Slp2a-hem only (Figure 3A).
We then investigated Slp2a isoform expression patterns in various human tissues. To specifically amplify Slp2a-hem and KIAA1597, we used primers located in the Slp2a N-terminal specific region and in exon 13 or 11, respectively (Figure 3A). Only Slp2a-hem was detected in hematopoietic cells, with a strong expression in CD4+ and CD8+ T lymphocytes, a lower expression in CD14+ monocytes and CD19+ B lymphocytes, and a variation in signal intensity following cell activation (Figure 3B). Similarly, Slp2a-hem was the only isoform detected in NK cells. A broadly Slp2a-hem expression was also detected in all nonhematopoietic tissues (Figure 3B). In contrast, KIAA1597 was not detectable in hematopoietic cells, although it was strongly expressed in all the nonhematopoietic tissues analyzed. Taken as a whole, these results demonstrate the existence of a new Slp2a-splicing product (Slp2a-hem) that is the only isoform of Slp2a expressed in hematopoietic cell lineages.
Rab27a interacts with and stabilizes Slp2a-hem in primary CTLs
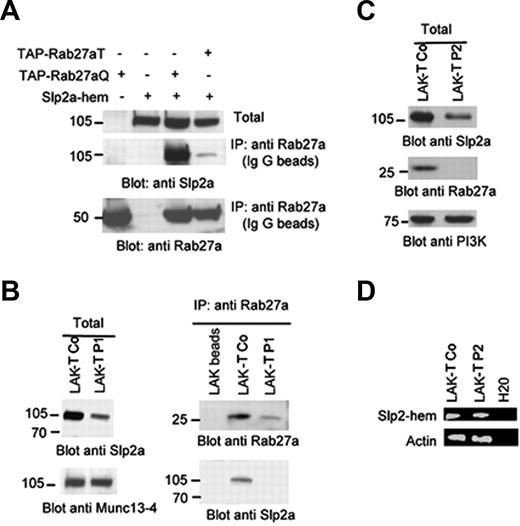
To confirm that Slp2a-hem is able to interact with Rab27a, we coexpressed it with either the active form of Rab27a fused with the TAP tag (TAP-Rab27aQ) or with the inactive form (TAP-Rab27aT) in the 293T cell line. As shown in Figure 4A, IgG immunoprecipitation revealed a strong interaction between Slp2a-hem and the active Rab27a mutant. In contrast, only very faint coimmunoprecipitation of the Slp2a-hem with the inactive Rab27a mutant was observed.
The hematopoietic form of Slp2a interacts with Rab27a in transfected 293T cells and in primary T cells. (A) The TAP-active (TAP-Rab27aQ) or TAP-inactive form of Rab27a (TAP-Rab27aT) was cotransfected in 293T cells with the hematopoietic form of Slp2a. Cell lysates were immunoprecipitated with IgG beads. Coprecipitated Rab27a TAP mutants and Slp2a-hem were examined using immunoblot analysis with anti-Rab27a and anti-Slp2a. (B) Primary CTLs (LAK-T Co) from a control patient and a GS2 patient 1 (LAK-T P1) were lysed in the presence of phosphatase inhibitors. Cell lysates were immunoprecipitated with the beads alone (LAK-T beads) or with a monoclonal anti-Rab27a. The immunoblots were analyzed using anti-Rab27a, anti-Slp2a, and anti–Munc13-4. (C) Total cell lysates of primary CTLs from a control (Lak-T Co) and a GS2 patient with Rab27a nonsense mutation (Lak-T P2) analyzed by Western blotting. Blot was probed with anti-Rab27a and anti-Slp2a. Western blot analysis of PI3K was used as a loading control. (D) RT-PCR analysis of Slp2a-hem–specific transcript from a control and GS2 patient P2 (Lak-T P2). Actin expression was used for normalization.
The hematopoietic form of Slp2a interacts with Rab27a in transfected 293T cells and in primary T cells. (A) The TAP-active (TAP-Rab27aQ) or TAP-inactive form of Rab27a (TAP-Rab27aT) was cotransfected in 293T cells with the hematopoietic form of Slp2a. Cell lysates were immunoprecipitated with IgG beads. Coprecipitated Rab27a TAP mutants and Slp2a-hem were examined using immunoblot analysis with anti-Rab27a and anti-Slp2a. (B) Primary CTLs (LAK-T Co) from a control patient and a GS2 patient 1 (LAK-T P1) were lysed in the presence of phosphatase inhibitors. Cell lysates were immunoprecipitated with the beads alone (LAK-T beads) or with a monoclonal anti-Rab27a. The immunoblots were analyzed using anti-Rab27a, anti-Slp2a, and anti–Munc13-4. (C) Total cell lysates of primary CTLs from a control (Lak-T Co) and a GS2 patient with Rab27a nonsense mutation (Lak-T P2) analyzed by Western blotting. Blot was probed with anti-Rab27a and anti-Slp2a. Western blot analysis of PI3K was used as a loading control. (D) RT-PCR analysis of Slp2a-hem–specific transcript from a control and GS2 patient P2 (Lak-T P2). Actin expression was used for normalization.
We next determined whether the Slp2a-hem/Rab27a association occurs in primary T cells. Lysates of cytotoxic lymphocytes (total) were subjected to immunoprecipitation with anti-Rab27a (Figure 4B). We found that Slp2a-hem was indeed coimmunoprecipitated with a Rab27a antibody. In contrast, the mutant Rab27a proteins expressed in LAK-T cells from a GS2 patient (Lak-T P1) did not coimmunoprecipitate with Slp2a-hem (Figure 4B). The 2 heterozygous RAB27A missense mutations identified in this patient led to the substitution of critical residues (L70P and A76V) located near Rab27a's nucleotide-binding site and are predicted to affect Rab effector binding. As shown in Figure 4B, these mutations impair Rab27a mutant protein stability and Slp2a-hem protein expression—thus confirming data obtained with transfected YT cell lines (Figure 2). A reduced intensity of Slp2a-hem protein expression was also detected in cells from a second GS2 patient totally deficient for Rab27a protein expression (Lak-T P2) (Figure 4C), whereas Slp2a-hem transcript level in P2 Lak-T cells remained similar to the one in control (Figure 4D). These results indicate that the active form of Rab27a specifically and directly interacts with Slp2a-hem, in primary CTLs, and that this protein association can be observed prior to engagement with target cells (ie, before the triggering of cytotoxic granule exocytosis). In addition, we found that Rab27a is required for the stabilization of Slp2a-hem.
Subcellular localization of Slp2a-hem in CTLs
We have previously shown that in unstimulated CTLs, the vesicular distribution of Rab27a differs from that of granules containing perforin and granzymes.7 To investigate the subcellular localization of Slp2a-hem, we transiently expressed a full-length green fluorescent protein (GFP)–tagged Slp2a-hem in LAK-T cells. First, Slp2a-hem colocalization with perforin staining was assessed. As shown in Figure 5A, the subcellular localization of GFP-Slp2a-hem was distinct from that of perforin (Slp2a-hem/perforin, 0.8% ± 0.5% overlap; Figure 5A). Slp2a-hem was found to be distributed between both cytosol and vesicular structures. To determine whether Slp2a-hem is present in the same secretory vesicles as Rab27a, constructs encoding GFP-Slp2a-hem and Dsred-Rab27a were coexpressed in LAK-T cells. We indeed found strong overlap of green and red fluorescence signals (Slp2a-hem/Rab27a, 74.5% ± 2.2% overlap; Figure 5B). Furthermore, the vesicular distribution of Slp2a-hem was broadened when this protein was coexpressed with the monomeric fluorescence protein red fused to Rab27a (DsRed-Rab27a), since both proteins are recruited on the same vesicular structures. Taken as a whole, these results indicate that Slp2a-hem and Rab27a interact in CTLs and colocalize on the same vesicular structures (which are distinct from the perforin-containing compartment). Given that the vesicular distribution of Slp2a-hem was intensified by overexpression of Rab27a, we assessed whether the perforin-containing granules could now localize to the same structures in this setting. When GFP-Slp2a-hem and unlabeled Flag-Rab27a were coexpressed in LAK-T cells that were stained for perforin, there was no significant fluorescence overlap (GFP-Slp2a-hem/perforin, 9.3% ± 3% overlap; Figure 5C top panel). Similarly, coexpression of unlabeled Slp2a-hem and GFP-Rab27a did not induce overlap with perforin staining (GFP-Rab27a/perforin, 8.2% ± 4.5% overlap; Figure 5C bottom panel).
Slp2a-hem and Rab27a colocalize on the same vesicular structures, and the latter is distinct from perforin-containing granules. (A) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-hem and stained with a perforin monoclonal antibody (red). (B) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-hem and DsRed-Rab27a. (C) Confocal microscopy of wild-type LAK-T cells transfected with either GFP-Slp2a-hem and unlabeled Rab27a (top panel) or GFP-Rab27a and unlabeled Slp2a-hem (bottom panel). Fixed cells were then stained with an antiperforin (red). (D) Confocal microscopy of GS2 (Rab27a-deficient) LAK-T cells transfected with GFP-Slp2a-hem and labeled with an antiperforin (red) (top panel). Confocal microscopy of GS2 LAK-T cells cotransfected with GFP-Slp2a-hem and DsRed-Rab27a (bottom panel). Scale bars represent 5 μm. Data are representative of at least 4 independent experiments.
Slp2a-hem and Rab27a colocalize on the same vesicular structures, and the latter is distinct from perforin-containing granules. (A) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-hem and stained with a perforin monoclonal antibody (red). (B) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-hem and DsRed-Rab27a. (C) Confocal microscopy of wild-type LAK-T cells transfected with either GFP-Slp2a-hem and unlabeled Rab27a (top panel) or GFP-Rab27a and unlabeled Slp2a-hem (bottom panel). Fixed cells were then stained with an antiperforin (red). (D) Confocal microscopy of GS2 (Rab27a-deficient) LAK-T cells transfected with GFP-Slp2a-hem and labeled with an antiperforin (red) (top panel). Confocal microscopy of GS2 LAK-T cells cotransfected with GFP-Slp2a-hem and DsRed-Rab27a (bottom panel). Scale bars represent 5 μm. Data are representative of at least 4 independent experiments.
To more directly assess the importance of Rab27a in recruiting Slp2a-hem to vesicular structures, we assessed Slp2a-hem localization in Rab27a-deficient cytotoxic cells from a GS2 patient. In Rab27a-deficient CTLs, GFP-Slp2a-hem was absent from the vesicular structures and, instead, was distributed throughout the cytoplasm (Slp2a-hem/perforin, 8.3% ± 3% overlap; Figure 5D top panel). Transient expression of DsRed-tagged Rab27a in GS2 patient cells restored the vesicular distribution of GFP-Slp2a-hem, with both proteins fully colocalizing on vesicular structures (Slp2a-hem/Rab27a, 72.4% ± 2.3% overlap; Figure 5D bottom panel).
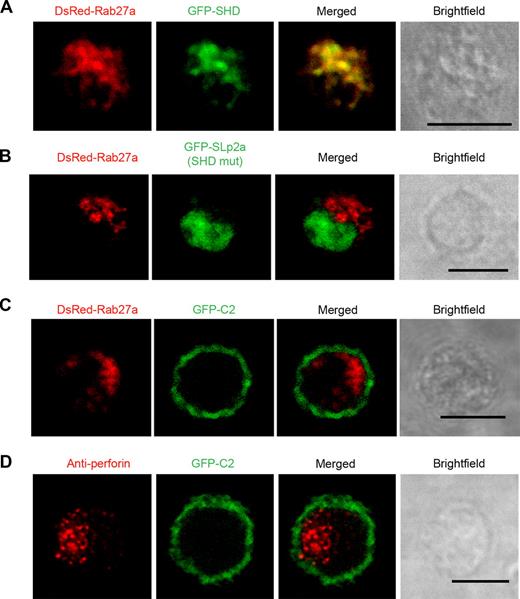
The Slp and the Slac2 families have been shown to interact with Rab27a through their SHD domain. To determine whether Slp2a-hem localizes on the Rab27a(+) vesicular structures through an SHD/Rab27a interaction, the Slp2a SHD domain was fused to a GFP and coexpressed in LAK-T cells along with DsRed-Rab27a. As shown in Figure 6A, both constructs were found on the same granules—strongly suggesting that Slp2a-hem is recruited to Rab27a(+) vesicles via its SHD domain (Slp2a-SHD/Rab27a, 78% ± 6.5% overlap; Figure 6A). We next asked whether the recruitment of Slp2a-hem on Rab27a(+) vesicles indeed requires an interaction between Slp2a-hem and Rab27a. We generated 2 point mutations in the Slp2a SHD domain (Slp2a-SHDmut) unabling it to bind to Rab27a. Coexpression of GFP-Slp2a-SHDmut and DsRed-Rab27a revealed mislocalization of the mutant protein, which was diffusely distributed in the cell (Slp2a-SHDmut/Rab27a, 8.5% ± 5.5% overlap; Figure 6B). These data indicate that Slp2a-hem needs to interact with Rab27a via its SHD domain to be recruited to the Rab27a(+) vesicular structures. In addition, we found that isolated Slp2a-C2 domains were associated with the plasma membrane independently of Rab27a and the vesicular structures (Slp2a-C2/Rab27a, 1.4% ± 0.7% overlap; Figure 6C). Slp2a-C2 domains localized at the plasma membrane, regardless of Rab27a expression—as observed in Rab27a-deficient T cells (Slp2a-C2/perforin, 0.2% ± 0.2% overlap; Figure 6D). Taken as a whole, these results demonstrate that the presence of Rab27a is required for the vesicular localization of Slp2a-hem in CTLs.
Slp2a recruitment on vesicular structures requires Rab27a. (A) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-SHD and DsRed-Rab27a. (B) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-SHDmut and Dsred-Rab27a. (C) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-C2 and DsRed-Rab27a. (D) Confocal microscopy of GS2 LAK-T cells transfected with GFP-Slp2a-C2 and labeled with an antiperforin (red). Scale bars represent 5 μm. Data are representative of at least 3 independent experiments.
Slp2a recruitment on vesicular structures requires Rab27a. (A) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-SHD and DsRed-Rab27a. (B) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-SHDmut and Dsred-Rab27a. (C) Confocal microscopy of wild-type LAK-T cells transfected with GFP-Slp2a-C2 and DsRed-Rab27a. (D) Confocal microscopy of GS2 LAK-T cells transfected with GFP-Slp2a-C2 and labeled with an antiperforin (red). Scale bars represent 5 μm. Data are representative of at least 3 independent experiments.
Slp2a-hem drives the docking of Rab27a-associated granules at the CTL-target cell contact zone
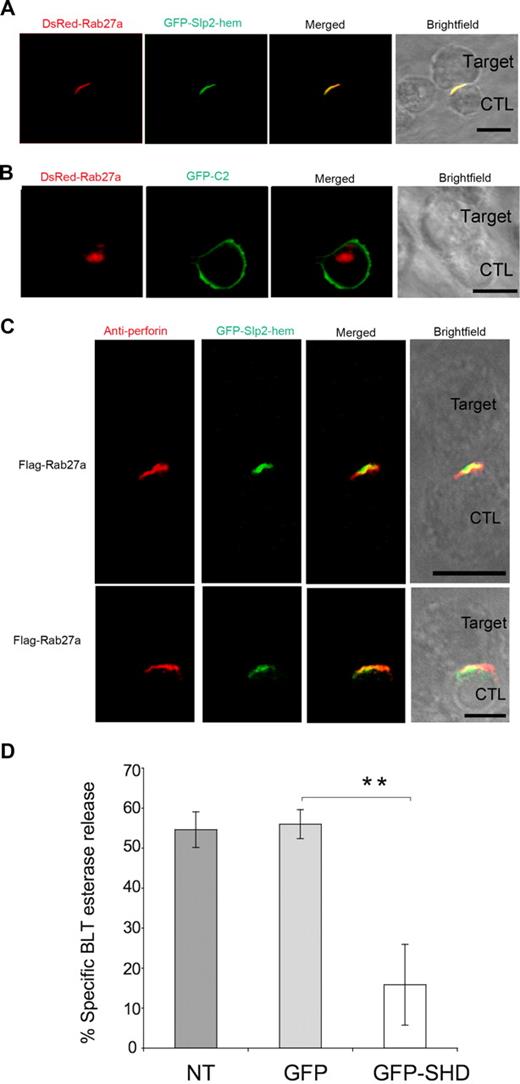
Based on these findings, we postulated that Slp2a-hem should redistribute together with Rab27a with the perforin-containing vesicles upon CTL activation. We therefore investigated whether Slp2a-hem and Rab27a proteins polarized and colocalized with the perforin-containing granules at the contact zone between conjugated cytotoxic lymphocytes and target cells. Conjugate cell formation indeed led to the polarization of “exocytic vesicles” labeled by both Rab27a and Slp2a-hem at the site of cell-cell contact (Figure 7A). A similar labeling pattern was observed in more than 80% of analyzed conjugates. In contrast, the Slp2a-C2 domain expressed in cytotoxic cells was not targeted to the cell contact zone and, in this setting, polarized Rab27a-labeled vesicles remained at a distance from the cell-cell contact zone (Figure 7B). This finding strongly supports the view that by interacting with Rab27a, Slp2a-hem enables vesicle docking at the plasma membrane within the cell-cell contact zone. To determine whether Rab27a/Slp2a-hem exocytic vesicles readily encounter perforin-containing granules at the immunologic synapse, we analyzed the colocalization of perforin with GFP-Slp2a-hem, along with unlabeled Rab27a. As shown in Figure 7C, perforin-containing granules at the cell-cell contact site colocalized with Slp2a-hem on a small area defined as the secretory zone. We then quantitatively analyzed the pixel intensity at the immunologic synapse for each combination (Figure 7C) and calculated the Pearson correlation coefficient (r) for the 2 colors. As a positive control for colocalization, we visualized a monoclonal antiperforin antibody combined with a mixture of secondary anti-immunoglobulin antibodies tagged with 2 distinct fluorophores. This yielded an r value of 0.85. We found a strong correlation for Slp2a-hem and perforin staining when unlabeled Rab27a was coexpressed (0.5 > r > 0.8). Our data suggest that Slp2a-hem is carried on Rab27a vesicles at the immunologic synapse and constitutes another component of the lytic machinery involved in the docking step of the exocytic vesicles that fuse with perforin-containing granules to form mature cytotoxic granules.
Slp2a-hem is recruited at the CTL-target cell contact zone and a dominant-negative form of Slp2a decreases cytotoxic granule exocytosis. (A) Microscopic analysis of wild-type LAK-T cells transfected with GFP-Slp2a-hem and DsRed-Rab27a and conjugated with L1210 target cells. (B) Microscopic analysis of wild-type LAK-T cells transfected with GFP-Slp2a C2 and DsRed-Rab27a and conjugated with L1210 target cells. (C) Confocal microscopy of wild-type LAK cells transfected with GFP-Slp2a-hem and unlabeled Rab27a and conjugated with L1210 target cells. Cells were then fixed, permeabilized, and stained with an antiperforin. Two different conjugates are shown. Scale bars represent 5 μm. Data are representative of 5 (A,B) or 4 (C) independent experiments. (D) The release of cytotoxic granule BLT esterase activity from transfected LAK-T cells is shown. Untransfected LAK-T cells (NT) or those transfected with GFP alone (GFP), or SHD of Slp2a tagged with GFP (GFP-SHD) were incubated on anti-CD3–coated plates for 4 hours. Cell supernatants were collected and assayed by enzyme-linked immunosorbent assay (ELISA) for serine esterase. Data are the mean of 3 independent experiments. Error bars show standard deviation among experiments. Transfection efficiency for each construct was between 40% and 50% as determined by FACScan analysis of GFP production (data not shown). **P < .01 (bracketed comparison).
Slp2a-hem is recruited at the CTL-target cell contact zone and a dominant-negative form of Slp2a decreases cytotoxic granule exocytosis. (A) Microscopic analysis of wild-type LAK-T cells transfected with GFP-Slp2a-hem and DsRed-Rab27a and conjugated with L1210 target cells. (B) Microscopic analysis of wild-type LAK-T cells transfected with GFP-Slp2a C2 and DsRed-Rab27a and conjugated with L1210 target cells. (C) Confocal microscopy of wild-type LAK cells transfected with GFP-Slp2a-hem and unlabeled Rab27a and conjugated with L1210 target cells. Cells were then fixed, permeabilized, and stained with an antiperforin. Two different conjugates are shown. Scale bars represent 5 μm. Data are representative of 5 (A,B) or 4 (C) independent experiments. (D) The release of cytotoxic granule BLT esterase activity from transfected LAK-T cells is shown. Untransfected LAK-T cells (NT) or those transfected with GFP alone (GFP), or SHD of Slp2a tagged with GFP (GFP-SHD) were incubated on anti-CD3–coated plates for 4 hours. Cell supernatants were collected and assayed by enzyme-linked immunosorbent assay (ELISA) for serine esterase. Data are the mean of 3 independent experiments. Error bars show standard deviation among experiments. Transfection efficiency for each construct was between 40% and 50% as determined by FACScan analysis of GFP production (data not shown). **P < .01 (bracketed comparison).
Expression of a dominant-negative form of Slp2a-hem impairs cytotoxic granule release
The role of Slp2a-hem in the process of the CTL's exocytic lytic machinery was further demonstrated by overexpressing a dominant-negative domain of Slp2a-hem. To observe the role of a dominant-negative Slp2a-hem on cytotoxic granule exocytosis, LAK-T cells were transfected with the Slp2a-hem SHD domain or the full-length protein fused to GFP. The transfectants were assayed for their ability to release granzyme A into the medium upon activation. After 4 hours of stimulation with a coated anti-CD3 antibody, between 45% and 55% of CD8 T cells transfected with pEGFP vector alone were able to degranulate granzyme A–containing cytotoxic granules, just as in untransfected LAK-T cells (NT) (Figure 7D). In contrast, LAK-T cells transfected with the dominant-negative Slp2a-hem exhibited an impaired degranulation capacity, since only 15% were able to release granzyme A (Figure 7D). Interestingly, the C2 domain of Slp2a-hem does not impair lytic granule exocytosis, indicating that the C terminus part of Slp2a-hem cannot exert a dominant-negative effect (data not shown). These findings support the hypothesis whereby Slp2a-hem is an effector of Rab27a contributing to the cytotoxic function of lymphocytes. However, knockdown of Slp2a by shRNA was not sufficient to impair lytic granule secretion (data not shown), suggesting that additional Rab27a effector(s) expressed in CTL can complement Slp2a-hem function.
Discussion
Our previous work identified a regulated maturation of the cytotoxic granule exocytosis pathway in CTLs.7 This maturation of lytic vesicles is triggered by the CTL's recognition of the target cell, which leads to fusion between the perforin-containing granules and the exocytic vesicles, an endosomal compartment carrying effectors of the exocytic machinery, including Rab27a and hMunc13-4. This fusion occurs close to the immunologic synapse. In the present study, we identified a new isoform of Slp2a as a critical effector of Rab27a. Slp2a-hem is carried by the exocytic vesicles and takes part in cytotoxic granule exocytosis in lymphocytes. The TAP method enables us to isolate a specific hematopoietic form of Slp2a that interacts with the active form of Rab27a in CTLs. We evidenced the incorporation of an alternative exon into the coding sequence within the linker region between Slp2a's SHD and the C2 domains; Slp2a-hem is characterized by combination of exons 10, 12, 13, and 14 in the alternatively spliced region. We have demonstrated that Slp2a-hem is preferentially expressed in hematopoietic cell lineages and in the brain. Studies of transfected heterologous cells and primary T cells confirmed a constitutive association of Slp2a-hem with Rab27a. Interestingly, by overexpressing Rab27a mutants in the YT NK cell line and by using Rab27a-deficient cells from a GS2 patient, we found that stable expression of Slp2a-hem requires Rab27a expression. In CTLs, Slp2a-hem is recruited on exocytic vesicles via its SHD domain and has to be associated with Rab27a to function in the cytotoxic granule exocytosis pathway. The Slp2a-hem(+) exocytic vesicles are polarized toward the immunologic synapse, where they partially colocalize with perforin-containing granules in a small secretion area. This process appears to be dependent on the expression of a competent Slp2a-hem molecule. Although Slp2a-SHD construct expressed in CTLs impairs lytic granule release by blocking Rab27a function, knockdown of Slp2a did not. This result suggests that in CTLs, the defect in Slp2a-hem could be overcome by another Rab27a effector(s). A very recent report supports this assumption by describing the weak expression of Slp1 in human CTLs.45 In addition, this work shows that both Slp2a and Slp1-deficient CTLs from gene-targeted mice have normal cytotoxic ability.45 These 2 Rab27a effectors could actually mutually compensate for this function. Slp2a was also characterized in this work as a component of the secretory lysosome exocytosis machinery of murine and human CTLs. However, in contrast to the human Slp2a-hem isoform herein characterized, which targets Rab27a-associated vesicles in unconjugated cells, the reported murine and human Slp2a isoforms directly, when overexpressed, target the plasma membrane of unconjugated CTLs.45 This discrepancy may be explained by the use of different Slp2a isoforms in each report. The human Slp2a isoform tested by Holt et al45 was not described, but the murine one lacks 2 exons indicated as 2SII and 2SIII. Such a difference in exon combination of Slp2a may differentially affect protease sensitivity of the protein and its degradation. Indeed, the exon combination of Slp2a-hem creates a third PEST domain and dramatically increases instability of Slp2a-hem in the absence of interaction with Rab27a, likely preventing plasma membrane localization of unconjugated Slp2a-hem.
Rab27a is known to be involved in the secretory granule process in several cell types, regulating vesicle trafficking along actin filaments and docking events at the plasma membrane. Each of these functions depends on distinct families of Rab27a effectors. Slac2 family members that are devoid of C2 domains (as Mlph/Slac2-a and MyRIP/Slac-2c) link Rab27a with the molecular motors Myosin Va and Myosin VIIa, respectively, and drive active transport of vesicles along actin filaments.29,30,46 The 2 C2 domains present in the Slp family members enable phospholipid binding at the plasma membrane and thus confer this family of Rab27a effectors with a role in the secretory machinery at the vesicle docking step.47 For instance, transient expression of Slp4-a/granuphilin-a significantly increases the number of insulin granules docked to the plasma membrane in pancreatic β cells,48,49 whereas Slp2a targets glucagon granules to the plasma membrane in pancreatic α cells. The same docking function has been described for Rabphilin, which is structurally identical to the Slp members.50 Similarly, we observed that the C2 domains of Slp2a-hem are able to target the plasma membrane in CTLs. Membrane association of Slp2a (which shares the same C2 region as Slp2a-hem) was previously shown to depend on the ability of its C2A domain to bind phosphatidylserine and phosphatidylinositol-4,5-bisphosphate at the plasma membrane.32,34 In unconjugated CTLs, Slp2a-hem does not mediate docking of exocytic vesicles to the plasma membrane, despite the membrane localization of its isolated C2 domains. Thus, cell activation upon conjugate formation is a prerequisite for the docking step of Slp2a-hem–bearing exocytic vesicles at the immunologic synapse. The triggering of CTLs by target recognition induces the movement of exocytic vesicles that carry the secretory machinery (including Rab27a, hMunc13-4, and also Slp2a-hem) to the site of secretion to allow cytotoxic granule exocytosis.7
How are Rab27a-bearing exocytic vesicles transported to the CTL's immunologic synapse? Although no additional effector of Rab27a has been found through the TAP procedure, it is still not clear whether in CTLs, other effectors than Slp2a-hem, likely of the Slac-2 family, are expressed and could be involved in a terminal transport on actin filaments as this has been described for melanosomes within melanocytes. Recent studies, however, have shown that recognition of a target by the CTL induces the clustering of exocytic vesicles around the MTOC that is polarized toward the target, and that this process occurs independently of Rab27a.4,7 Next, the centrosome contacts the plasma membrane and enables the direct actin-independent delivery of granules at the secretion site.5 Thus, in CTLs, the mechanism of granule secretion may not require the regulated transport of exocytic vesicles along actin filaments.
Slp2a-hem could mediate the docking of exocytic vesicles with the plasma membrane at the immunologic synapse prior to fusion with perforin-containing granules at the immunologic synapse. In such a case, Slp2a-hem function could be viewed as a way of stabilizing exocytic vesicles in the secretory area and thus favoring fusion with perforin-containing granules. Alternatively, Slp2a-hem could be involved in the exocytosis of competent cytotoxic granules that have already fused with exocytic vesicles by mediating contact with the plasma membrane. Further experiments are under way to test these different hypotheses.
The finding that Slp2a-hem is extremely sensitive to phosphatase-dependent proteolysis activity is intriguing. We found that the splicing of exon 11 in the Slp2a-hem creates a third predicted PEST domain that is present only in this isoform. Interestingly, the phosphatase-sensitive region of Slp2a-hem lies within this alternative splicing region—suggesting that the third PEST sequence could be responsible for generation of the cleaved form. Use of this characteristic of Slp2a-hem in the regulated secretory pathway for cytotoxic granules is an attractive hypothesis. Previous work in yeast has shown that the directional movement of vacuoles along actin filaments into the bud cell requires the assembly of the Myo2p-Vac17p-Vac8p transport complex.51 Disassembly of this transport complex (used to prevent vacuoles returning to the mother cell) occurs through the degradation of Vac17p (mediated through its PEST domain). In melanocytes, another tripartite protein complex (MyoVa-Mlph/Slac2-a-Rab27a) involving the Rab27a effector Mlph/Slac2-a is required for melanosome transport along actin filaments.30 As with Vac17p, the presence of PEST sequences within Mlph/Slac2-a regulates transport complex disassembly via Mlph/Slac2-a degradation.44 By analogy with these transport models, one could envisage that disassembly of the Rab27a-Slp2a-hem docking complex occurs through the rapid degradation of Slp2a-hem. Complex disassembly may enable alternative interactions of Rab27a with other partners (such as Slp1 or hMunc13-4) and thus allow exocytic vesicles to either fuse into mature cytotoxic granules or reach the priming step and proceed further toward exocytosis. Although the precise function of Slp2a-hem in the tightly regulated cytotoxic granule exocytosis pathway remains to be fully understood, the present work clearly demonstrates that the hematopoietic isoform of Slp2a is an effector of Rab27a function in cytotoxic granule exocytosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Takahiro Nagase (Kazua DNA Research Institute, Chiba, Japan) for providing KIAA1597 cDNA clone. We acknowledge Perrine Knapnougel and Stéphanie Certain for their technical assistance.
This work was supported by grants from Inserm (Paris, France), the Agence Nationale de la Recherche (ANR-05-MIM-010 and BLAN06-3 145379, Paris, France), the Fondation pour la recherche Médicale (Equipe labélisée FRM 2007, DEQ20061107930, Paris, France), and the joint action of Ministère de l'Education Nationale et de la Recherche (BCMS103). M.M.M. was supported by a doctoral fellowship from the Ministère de l'éducation Nationale, de la Recherche et de la Technologie, and the Association pour la Recherche sur le Cancer (ARC).
Authorship
Contribution: G.M., M.M.M., J.M.L., E.D., R.A., N.L., J.G., and M.C. performed experiments; G.M. and M.M.M. analyzed results and made figures; N.M. conducted the sampling and clinical study of GS2 patients; G.M. and G.S.B. designed the research; and G.M., A.F., and G.S.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: G. de Saint Basile, Inserm, Unité U768, Laboratoire du Développement Normal et Pathologique du Système Immunitaire, Paris, F-75015, France; e-mail: sbasile@necker.fr.
References
Author notes
*G.M. and M.M.M. contributed equally to this work.