In this issue of Blood, Hallaert et al show that clinically approved c-Abl inhibitors reverse the protection against conventional chemotherapeutic drugs afforded to CLL cells from prolonged CD40 signaling.
Chronic lymphocytic leukemia (CLL), though well understood molecularly and functionally, essentially remains incurable. In vivo survival niches probably contribute to a surprisingly poor performance from drugs that seem very promising in vitro. Bone marrow stroma, blood-borne nurselike cells, and lymph node follicular dendritic cells are all culprits in the conspiracy.1 At the heart of their operations lies the induction of antiapoptotic proteins, including the “usual suspects” of Mcl-1, Bcl-2, and Bcl-xL. One avenue of attack is to target the survival proteins directly as recently described using BH3-neutralizing AT-101.2 In this issue of Blood, Hallaert et al have masterminded an alternative strategy that interrupts the signaling processes that promote CLL protection. To guide them toward the guilty pathway, a neat piece of detective work was employed.
First, they constructed a survival niche: CD154-transfected fibroblasts furnishing both CD40 stimulation and a range of stromal factors. CD40 ligation affords, in all manner of B cells, substantial protection to both default and deliberately provoked apoptosis, and CLL is no exception. In addition to confirming previously described CD40-driven antiapoptotic changes in CLL populations, the authors identified for the first time a reduction in the proapoptotic protein Bim-EL. It was known from other systems that ERK-signaling could alter levels of Bim-EL protein, and they went on to show that not only did CD154 stimulate ERK phosphorylation in CLL cells, but also that inhibiting ERK activity negated the ensuing Bim-EL decline. However, ERK proved an innocent bystander when considering resistance to therapeutic drugs. Thus, even with ERK incapacitated, fludarabine, bortezimob, and others were all powerless to outmaneuver the defenses bolstered by CD40 and stroma.
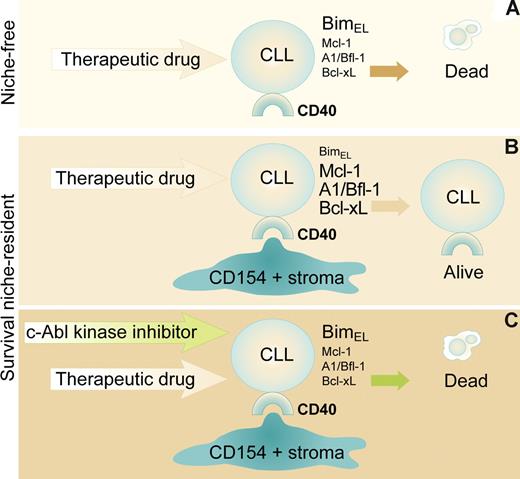
(A) CD40+ve CLL cells free of a survival niche (eg, in the circulation) constitutively expressing high levels of proapoptotic Bim-EL and low antiapoptotic Mcl-1, A1/BFl-1, and Bcl-xL successfully killed by therapeutic drugs. (B) Same cells receiving survival signals (here via CD154/stroma) down-regulate Bim-EL, up-regulate Mcl-1, A1/BFl-1, and Bcl-xL and are rendered resistant to therapeutic drugs. (C) Niche-protected CLL cells exposed to c-Abl kinase inhibitors reboot to niche-free levels of apoptosis regulators while restoring sensitivity to therapeutic drugs. Professional illustration by Paulette Dennis.
(A) CD40+ve CLL cells free of a survival niche (eg, in the circulation) constitutively expressing high levels of proapoptotic Bim-EL and low antiapoptotic Mcl-1, A1/BFl-1, and Bcl-xL successfully killed by therapeutic drugs. (B) Same cells receiving survival signals (here via CD154/stroma) down-regulate Bim-EL, up-regulate Mcl-1, A1/BFl-1, and Bcl-xL and are rendered resistant to therapeutic drugs. (C) Niche-protected CLL cells exposed to c-Abl kinase inhibitors reboot to niche-free levels of apoptosis regulators while restoring sensitivity to therapeutic drugs. Professional illustration by Paulette Dennis.
The authors next trained the spotlight on the increased Mcl-1 protein expression they had noted. This is when c-Abl began to audition for the leading role. Already a star of the Philadelphia chromosome, survival signaling via dysregulated Abl underpins pathobiology in chronic myeloid leukemia (CML). Not only Mcl-1 but also other antiapoptotic signatures from niche-protected CLL cells conformed to those emanating constitutively from BCR-Abl. With all the pieces now so elegantly in place, it does not require a Lieutenant Columbo to pronounce the denouement: c-Abl inhibitors used so successfully for treating CML likewise overcome conferred resistance to underperforming therapeutic drugs in CLL. Both widely used imatinib (gleevec) and even more so second-generation dasatinib (sprycel) rebooted CLL cells to drug sensitivity. This desired outcome was accompanied by a requisite reversal of the antiapoptotic profile imprinted by the survival niche. Neither IgVH mutational status nor p53 dysfunction tempered the success. As an added bonus, the authors were able to recapitulate findings on blood CLL cells with samples from lymph node biopsies.
This is an exciting advance. The data are persuasive and internally consistent. Questions do remain but not necessarily of a quality to warrant concern with respect to therapeutic options. The authors themselves confess to their model being “supraphysiological.” While B cells are constitutively programmed for CD154 receptivity, the opportunity for ligand delivery is, by necessity, fleeting. It would be interesting to learn the impact of short-term, low-level CD154 exposure on the outcomes monitored.3 Moreover, evidence for CD154 majorly contributing to in situ CLL survival is not convincing. Once more, reappraisal of the system, here replacing the transfected mouse fibroblasts with more natural accessory support, would be informative. The authors are equally open with respect to the targets of the “c-Abl inhibitors” within the context of CLL. Rationally designed to target the tyrosine kinase activity constitutive to BCR-Abl, imatinib and dasatinib are by no means Abl-specific. Though leaving open a mechanism-of-action in this new setting, the efficacy of these compounds (at least in vitro) remains. Defeating the complicity between hijacked extrinsic and autonomous intrinsic forces that currently limit therapeutic benefit in CLL may now be a step nearer.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■