To the editor:
Recently Li et al1 reported that circulating γδ T cells from tuberculin skin test (TST)-positive patients respond directly in vitro to early secreted antigenic target 6 (ESAT-6) protein from Mycobacterium tuberculosis by proliferating and by producing interferon γ (IFN-γ). We think that these results do not agree with the current model of γδ T-cell antigen (Ag) recognition and activation.
Human Vγ9Vδ2 γδ T cells represent 1% to 5% of circulating lymphocytes in adults and recognize nonpeptidic phosphoantigens, metabolites of isoprenoid pathway.2,3 Although most potent phosphoantigens, such as hydroxymethylbutyl-pyrophosphate (HMBP), are produced by infectious agents and recognized as nonself, others, such as isopentenyl pyrophosphate (IPP), are normal metabolites of mammalian mevalonate pathway, and their accumulation represents a danger signal from infected or transformed cells.4 A similar mechanism explains Vγ9Vδ2 response to aminobisphosphonates5 and alkyl amines.6 Due to small size, phosphoantigens are physically unable to cross-link T-cell receptors (TCRs), and a complex of apolipoprotein A (ApoA) and mitochondrial adenosine triphosphatase (ATPase) was proposed as a presenting molecule.7 However, the recognition mechanism of human Vγ9Vδ2 TCR remains incompletely defined. Other major histocompatibility complex (MHC) class I–like molecules may stimulate human γδ T cells: CD1c can present foreign lipids and glycolipids to Vδ1+ γδ T cells, a subset commonly found in humans in periphery.8
On the other hand, ESAT-6 protein is able to induce IFN-γ production by CD4 T lymphocytes from patients with active tuberculosis (TB) and latent TB infection (LTBI).9
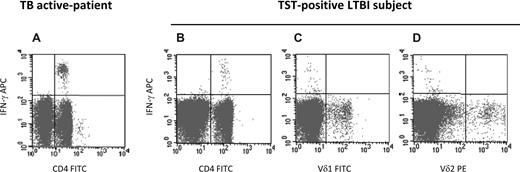
To evaluate γδ T cells' response to ESAT-6 protein, we tested 4 patients with active TB and 4 subjects with LTBI (positive to QuantiFERON TB Gold and TST). Figure 1 shows representative results from an active TB patient (Figure 1A) and a TST-positive LTBI subject (Figure 1B-D). As expected, CD4 T cells produce IFN-γ to ESAT-6 in active TB patients (Figure 1A) as well as in LTBI subjects (Figure 1B). Differently from Li et al,1 in the same conditions ESAT-6 failed to induce IFN-γ production from Vδ2 or Vδ1 γδ T cells (Figure 1C,D). Accordingly, IFN-γ response was not associated with CD4− T cells (Figure 1A,B).9
ESAT-6 protein induces αβ but not γδ T cells' response in active TB patients and TST-positive LTBI subjects. Peripheral blood mononuclear cells (PBMCs) from a representative active TB patient (A) and a representative TST-positive LTBI subject (B-D) were stimulated with ESAT-6 protein (5 μg/mL; Lionex, Braunschweig, Germany) in presence of anti-CD28 monoclonal antibody (1 μg/mL; BD Biosciences, San Jose, CA). To detect intracellular expression of IFN-γ, brefeldin-A (Sigma-Aldrich, St Louis, MO) at 10 μg/mL was used. After overnight stimulation, cells were stained with CD4-peridinin chlorophyll protein (PerCP), Vδ2-phycoerythrin (PE), Vδ1-fluorescein isothiocyanate (FITC), IFN-γ allophycocyanin (APC) anti–human conjugated monoclonal antibodies (BD Biosciences). For all staining procedures, an isotype-matched negative control was used. Data acquisition and analysis were performed using a FASCalibur flow cytometer and CellQuest software (both BD Biosciences).
ESAT-6 protein induces αβ but not γδ T cells' response in active TB patients and TST-positive LTBI subjects. Peripheral blood mononuclear cells (PBMCs) from a representative active TB patient (A) and a representative TST-positive LTBI subject (B-D) were stimulated with ESAT-6 protein (5 μg/mL; Lionex, Braunschweig, Germany) in presence of anti-CD28 monoclonal antibody (1 μg/mL; BD Biosciences, San Jose, CA). To detect intracellular expression of IFN-γ, brefeldin-A (Sigma-Aldrich, St Louis, MO) at 10 μg/mL was used. After overnight stimulation, cells were stained with CD4-peridinin chlorophyll protein (PerCP), Vδ2-phycoerythrin (PE), Vδ1-fluorescein isothiocyanate (FITC), IFN-γ allophycocyanin (APC) anti–human conjugated monoclonal antibodies (BD Biosciences). For all staining procedures, an isotype-matched negative control was used. Data acquisition and analysis were performed using a FASCalibur flow cytometer and CellQuest software (both BD Biosciences).
Since no direct specific response of γδ T cells to protein antigen, as described by Li et al,1 is known, the proposed γδ T-cell response to ESAT-6 should be confirmed by analyzing its dependence from a conserved protein antigen 3-dimensional conformation. Alternatively, other triggering agent(s) inducing activation of γδ T cells could be proposed. Since, according to Li et al,1 γδ T-cell response was linked to ESAT-6 in a dose-response manner, a contaminating phosphoantigen could be postulated in that particular ESAT-6 preparation; γδ T-cell response should disappear if more purified (or dialyzed) ESAT-6 protein is used. Therefore, human γδ T-cell direct response to soluble protein Ag is an unexpected result representing a new, direct nonprocessed surveillance route regarding extracellular proteins. This mechanism would be totally different from αβ T cells' response to protein Ag, which requires intracellular or extracellular processing and, respectively, MHC class I or II molecule presentation. If confirmed, γδ T cells could, once again, display a unique response capability. However, in our view, this possibility deserves a more accurate evaluation.
Authorship
This study was supported by grants from Italian Health Ministry.
Conflick-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rita Casetti, Laboratory of Cellular Immunology, National Institute for Infectious Diseases Lazzaro Spallanzani, IRCCS, Rome, Italy; e-mail: casetti@inmi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal