Abstract
Dendritic cells (DCs) play a central role in initiating and polarizing the immune response. Therefore, DC maturation represents a key control point in the shift from innate to adaptive immunity. It is suspected that during pregnancy, hormones are critical factors that modulate changes reported to occur in maternal immunity. Here we examined the effect of 17-β-estradiol (E2) on the maturational response triggered by virus in human DCs and its influence on their ability to activate naive T cells. We developed an in vitro system to measure the response of DCs to virus infection with Newcastle disease virus (NDV) after a 24-hour E2 treatment. Using this system, we demonstrated that E2 pretreatment down-regulated the antiviral response to RNA viruses in DCs by profoundly suppressing type I interferon (IFN) synthesis and other important inflammatory products. In addition, the DCs capacity to stimulate naive CD4 T cells was also reduced. These results suggest an important role for E2 in suppressing the antiviral response and provide a mechanism for the reduced immunity to virus infection observed during pregnancy.
Introduction
Dendritic cells (DCs) are immunologic sentinels that function as crucial intermediates linking innate immunity to adaptive immunity. On exposure to microbial invaders, they undergo a maturational change that culminates in their migration to lymph nodes where they present microbe-derived peptides to specific T cells. The process of maturation is characterized by the up-regulation of surface molecules involved in the interaction with T cells and the release of numerous cytokines that direct T cell polarization.1,2 One mechanism for induction of DC maturation is through the interaction of toll-like receptors (TLRs) with invariant structures found on microbes known as pathogen-associated molecular patterns (PAMPs).3 However, recent studies have demonstrated that in response to RNA viruses, an internal sensing pathway is likely to play a more important role in virus sensing by classic dendritic cells (cDCs) but not with plasmacytoid dendritic cells (pDCs).4 In cDCs, cytosolic RNA helicases such as RIG-I and MDA5 respond to intermediates of virus replication and trigger DC maturation that is characterized by the release of inflammatory cytokines such as type I IFN, IL-12, TNF α, and IL-6 as well as numerous chemokines and antiviral effector molecules. Activation of the helicases induces the nuclear translocation of several factors crucial for transcription of the genes coding for DC maturation and the antiviral response.5-7
Steroid sex hormones increase throughout the 3 trimesters of pregnancy, reaching their highest levels during the last 3 months. 17-β-estradiol (E2), one of the most important of these hormones, reaches extremely high levels during the latter part of pregnancy and falls precipitously before parturition.8,9 It acts through binding to 2 known hormone receptors, estrogen receptors ERα and ERβ, which are expressed in specific tissues as well as in immune cells, including macrophages, CD8+ T cells, CD4+ T cells, B cells, monocytes, and DCs.10,11 Numerous interactions between the endocrine and the immune systems have been described.12-14 Several groups have shown that estrogen administration leads to clinical improvement in experimental autoimmune encephalomyelitis due to changes in DC function and the promotion of a Th2 immune response.15 In contrast, disease exacerbation in systemic lupus erythematosus (SLE) patients has also been reported during pregnancy.16
With regard to immunity to virus infection, Jilani et al reported that higher levels of sex steroid hormones during pregnancy correlate with the onset of fulminant viral hepatitis.17 Lilleri et al showed that the development of adaptive T-cell immunity in pregnant women infected with human cytomegalovirus (HCMV) appears to be a complex and slow process until a memory T-cell response develops.18 In the 1918 to 1919 Spanish flu epidemic, mortality associated with infection during pregnancy was reported to be more than 50%, with the greatest mortality risk reported in the latter half of pregnancy.19 Moreover, during the 1957 Asian Influenza pandemic, 50% of the women of child-bearing age who died were pregnant.20,21 Such observations argue that sex steroid hormones may modulate disease activity through their action on the immune system.22,23
Using a comprehensive system for evaluation of human DC activation previously developed in our laboratory,24 we evaluated the effects of E2 on the response of human DCs to virus infection. In addition, we measured the impact of this hormone on virus-infected DC stimulation of naive allogeneic CD4 T cells. Our data demonstrate that E2 down-regulates DCs' antiviral immune response, and also reduced the DCs' capacity to stimulate the naive CD4 T cells' immune response.
Methods
Viruses and cells
Recombinant Newcastle disease viruses (NDVs) NDV-B1 were generated from the B1 Hitchner avian vaccine strain as previously described.25 NDVs were titrated by immunofluorescence of Vero cells at 24 hours after infection, using the monoclonal antibody 7B1, which is specific for the NDV protein HN (5 μg/mL; Mount Sinai Hybridoma Shared Research Facility, New York, NY). Virus infections were performed in infection medium (Dulbecco modified Eagle medium [Invitrogen, Carlsbad, CA], 0.35% bovine serum albumin, 0.12% NaHCO3, 100 μg/mL penicillin-streptomycin). Vero cells were grown in tissue culture medium with 10% fetal calf serum [HyClone Logan, UT], 1 mM sodium pyruvate [Invitrogen], 2 mM glutamine [Invitrogen], and 50 μg/mL gentamicin [Invitrogen]. All cells were grown at 37°C in 7% CO2. Sendai Cantell (SeV) and influenza A (Flu) viruses were grown and purified as described.26,27 Briefly, SeV and Flu virus were grown at 37°C for 40 hours in 9-day-old embryonated eggs. Allantoic fluid was harvested, snap frozen, and stored at −80°C.
Isolation and culture of human DCs
Peripheral blood mononuclear cells (PBMCs) were isolated as previously described.24 Briefly, Ficoll density gradient centrifugation (Histopaque; Sigma-Aldrich, St Louis, MO) was performed with buffy coats of healthy donors (Mount Sinai Blood Donor Center and New York Blood Center).
CD14+ cells were immunomagnetically purified using anti–human CD14 antibody-labeled magnetic beads and iron-based Midimacs LS columns (Miltenyi Biotec, Auburn, CA). After elution from the columns, cells were plated (0.7 × 106 cells/mL) in DC medium (RPMI [Invitrogen], 4% Human Serum, 100 units/mL penicillin, and 100 μg/mL streptomycin [Invitrogen]) supplemented with 500 U/mL human granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ) and 1000 U/mL human interleukin-4 (IL-4; Peprotech) and incubated for 5 to 6 days at 37°C. Directly isolated myeloid DCs were immunologically purified using anti–human CD1c (BDCA-1) antibody-labeled magnetic beads (Miltenyi Biotec) and iron-based MiniMacs LS columns (Miltenyi Biotec). After elution from columns, cells were plated (0.7 × 106 cells/mL) and infected or pretreated with E2.
E2 treatment and DC infection
After 5 to 6 days in culture, 106 cells/mL were treated with E2 (Sigma-Aldrich, E4389, tested for cell culture and free of contaminants), at a concentration of 10 μg/mL, 1 μg/mL, and 0.1 μg/mL. After 24 hours of pretreatment, cells were infected with NDV-B1, SeV, and FluA at a multiplicity of infection (MOI) of 0.5 for 40 minutes in RPMI in the presence or not of E2. Cells were then plated in DC medium at 106 cells/mL for different time periods, depending on the experiment. Preliminary experiments using whole blood from either female or male donors showed comparable results. Following the same protocol of E2 pretreatment, DCs were also treated with LPS (0.5 μg/mL), poly I: poly C (250 ng/mL), R848 (3 μg/mL) from Invivogen (San Diego, CA) or with IFNβ (5000 U/mL) from Calbiochem (Gibbstown, NJ).
Immunofluorescence
For immunofluorescence, cells were washed with phosphate-buffered saline (PBS) containing 0.5% BSA, fixed with 3.7% formaldehyde for 10 minutes at RT, and permeabilized with 0.1% Triton X-100 for 5 minutes. Cells were incubated with ERα (Cell Signaling, Danvers, MA), followed by a goat anti–mouse-Ig antibody labeled with Alexa 488 (Invitrogen). After washing, samples were mounted with ProLong antifade reagent with DAPI (Invitrogen). Images were recorded with a Zeiss Axiophot2 microscope (Carl Zeiss, Thornwood, NY) coupled to a Retiga 2000R monochrome camera (QImaging, Tucson, AZ) controlled by QED Imaging software (QED, Delta, PA).
ELISAs
Enzyme-linked immunosorbent assays (ELISAs) for IFN-ß (Pestka Biomedical Laboratories, Piscataway, NJ) were used according to the manufacturer's instructions to quantify the cytokines in the DC supernatants. Plates were read in an ELISA reader from Biotek Instruments (Winooski, VT). ELISAs for IL-6, IL-8, IL-12 (p70) IP-10, IFNα, TNFα (Upstate Biotechnology, Billerica, Massachusetts) and IFNγ, IL-10, IL-13, IL-17 (Millipore, Billerica, Massachusetts) were performed as part of a multiplex assay following the manufacturers' protocol. Plates were read using a Luminex plate reader, and data were analyzed using software from Applied Cytometry Systems (Plano, TX).
Flow cytometry
Cells were stained with HLA-DR, CD11c, and CD14 to measure the purity of the working population according to the manufacturer's instructions (Beckman Coulter, Fullerton, CA, or BD-Pharmingen, Franklin Lakes, NJ). Costimulatory molecules were also analyzed by cell staining with CD80, CD86. The expression of each marker was determined by flow cytometry after gating on the lineage-specific marker, using a flow cytometer FC-500 from Beckman Coulter. Data were analyzed using CXP software (Beckman Coulter). Cytotoxicity of E2 was evaluated by propidium iodide (PI) staining (Invitrogen).
RNA extraction and quantitative real-time PCR from human DCs
RNA extraction and real-time polymerase chain reaction (RT-PCR) were performed as described before.24 Briefly, samples of 106 DCs differentially treated according to the experimental protocol were pelleted, and RNAs were isolated and treated with DNase using an Absolutely RNA RT-PCR miniprep kit (Stratagene, La Jolla, CA). RNAs were quantified using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). The yields of RNA were approximately 20 to 50 μg per sample. Quantitative (q)–RT-PCR was performed using a previously published SYBR green protocol with an ABI7900 HT thermal cycler (HT, Haarlem, The Netherlands).28 Each transcript in each sample was assayed 2 times, and the mean cycle threshold was used to calculate the x-fold change and control changes for each gene. Four housekeeping genes were used for global normalization in each experiment (actin, Rps11, glyceraldehyde-3-phosphate dehydrogenase, and tubulin genes).29 Data validity by modeling of reaction efficiencies and analysis of measurement precision was determined as described previously.28
Reporter gene assays
The reporter gene assay was performed as described before with slight modifications.30 Briefly, MCF-7 cells were transiently transfected with 2 μg IFNβ promoter reporter construct31 (provided by Dr D. Thanos, Columbia University, New York, NY), driving firefly luciferase production together with 0.2 μg pRL-TK (Promega, Madison, WI) constitutively expressing Renilla luciferase for normalization. To examine the role of retinoic acid–inducible gene I (RIG-I), 250 ng empty pCAGGS or pCAGGS expressing either RIG-I or the constitutively expressed RIG-IN (obtained from Dr Garcia Sastre, Mount Sinai School of Medicine) were added to the transfection mixture. Transfection was performed using Lipofectamine LTX (Invitrogen) according to the manufacturer's instructions. Twelve hours later, cells were pretreated or not with E2 for 24 hours and then infected with NDV-B1 or mock infected. Cell extracts were obtained 8 hours after infection and examined for expression of firefly and Renilla luciferase using a dual luciferase assay (Promega).
Viral RNA extraction and CIAP treatment
NDV-B1 grown from 60 10-day embryonated eggs was concentrated by ultracentrifugation at 23 000 rpm for 90 minutes. The pellet was resuspended in 1 mL PBS, and viral RNA was extracted using Trizol following the manufacturer's recommendations. Purified RNA was treated with 30 U Calf Intestine Alkaline Phosphatase (CIAP; Promega) in the presence of RNAse inhibitors (Invitrogen) for 3 hours at 37°C. Treated, mock-treated, and control human total RNA were repurified using the RNAeasy isolation kit (Roche, Nutley, NJ) and used to transfect cells.
Alloantigen stimulation of naive CD4 T cells by DCs
DCs treated with E2 (10 μg/mL) were infected with NDV-B1 (MOI 0.5) for 1 hour and cultured together with allogeneic naive CD4 T cells (naive CD4 isolation kit from Miltenyi Biotec) for 5 to 6 days in 48-well plates at a ratio of 1:5. After 3 days of coculture, IL-2 (10 ng/mL) was added to the culture medium. Supernatants from the cocultures were tested by ELISA for IFN-γ, IL-10, IL13, and IL-17 release, and T-cell proliferation was measured by Carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) labeling.
CSFE labeling
Purified naive CD4 T cells were resuspended in PBS 1× at 4 × 107 cells/mL. CFSE (Invitrogen) was added at a final concentration of 2.5 μM, and the cells were incubated for 30 minutes at 37°C. The cells were then washed 3 times with PBS and resuspended in Iscove Medium with 10% fetal bovine serum and culture with DCs. After 5 to 6 days of culture, cells were acquired with flow cytometer FC-500 from Beckman Coulter and analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistical analyses
The data were presented as mean plus or minus standard deviation (SD). Statistical analysis was performed by analysis of variance, and a P value less than .05 was considered significant.
Results
DCs express receptors for estrogen and respond to virus infection
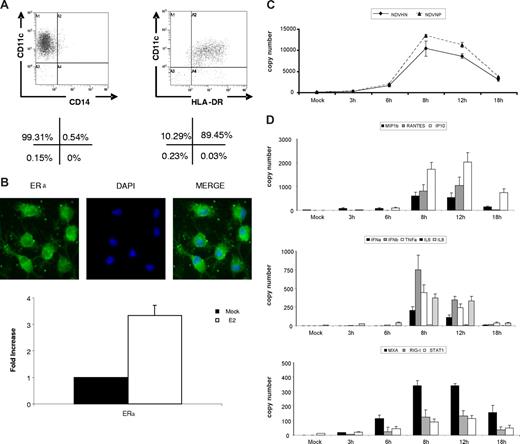
To develop a system for analysis of the impact of E2 on DC maturation, monocyte-derived DCs were generated from buffy coats from healthy human donors. The working cell population was evaluated for purity by flow cytometry after 5 to 6 days of culturing in GM-CSF and IL-4 and shown to be 99% plus or minus 1.27 CD11c+ CD14− and 89% plus or minus 3.27 CD11c+ HLADR+ (Figure 1A). These cells express estrogen receptors (ERs) as shown by immunofluorescent staining and by qRT-PCR (Figure 1B). In addition, these cells respond to E2 treatment by up-regulation of ERα, as has been previously described.32 When the DCs are infected with NDV-Bl, an avian paramyxovirus, viral replication peaks between 8 to 12 hours after infection as determined by qRT-PCR for NDV-B1 hemagglutinin-neuraminidase (HN) and NDV-B1 nucleoprotein (NP) mRNAs (Figure 1C). The DCs are triggered to mature efficiently in response to the virus infection as demonstrated by the expression of transcripts for chemokine genes (RANTES, MIP1β, IP-10), cytokine genes (IFNα/β, TNFα, IL-6, IL-8), and several IFN-related genes (STAT-1, MxA, RIG-I; Figure 1D) showing kinetics identical to that seen for the viral genes (8-12 hours).
NDV-B1 infection induced strong response in dendritic cells. CD14+ cells isolated from fresh blood were cultured for 5 days in the presence of IL-4 and GM-CSF to differentiate into dendritic cells (DCs). (A) Monocyte-derived DCs expressed a classic DC profile: CD11c+ HLADR+ CD14−. Percentages are representative of 5 independent experiments. (B) 40× original magnification of ERα immunofluorescence in immature DCs (top panel). Levels of ERα mRNA measure by RT-PCR in mock-treated cells and with 24-hour E2 treatment (bottom panel). Data are presented as fold increase over mock treated, mean plus or minus SD, and results are representative of 3 independent experiments. (C) Viral proteins (NDV HN and NDV NP) measured by qRT-PCR in DCs after 0, 3, 6, 8, 12, and 18 hours of NDV-B1 infection. Data are representative of 3 independent experiments. (D) Cytokines, chemokines, and viral gene expression in DCs after 0, 3, 6,8,12, and 18 hours of NDV-B1 infection measured by qRT-PCR. Results are representative of 3 independent experiments.
NDV-B1 infection induced strong response in dendritic cells. CD14+ cells isolated from fresh blood were cultured for 5 days in the presence of IL-4 and GM-CSF to differentiate into dendritic cells (DCs). (A) Monocyte-derived DCs expressed a classic DC profile: CD11c+ HLADR+ CD14−. Percentages are representative of 5 independent experiments. (B) 40× original magnification of ERα immunofluorescence in immature DCs (top panel). Levels of ERα mRNA measure by RT-PCR in mock-treated cells and with 24-hour E2 treatment (bottom panel). Data are presented as fold increase over mock treated, mean plus or minus SD, and results are representative of 3 independent experiments. (C) Viral proteins (NDV HN and NDV NP) measured by qRT-PCR in DCs after 0, 3, 6, 8, 12, and 18 hours of NDV-B1 infection. Data are representative of 3 independent experiments. (D) Cytokines, chemokines, and viral gene expression in DCs after 0, 3, 6,8,12, and 18 hours of NDV-B1 infection measured by qRT-PCR. Results are representative of 3 independent experiments.
E2 inhibits the activation of DCs by NDV-B1
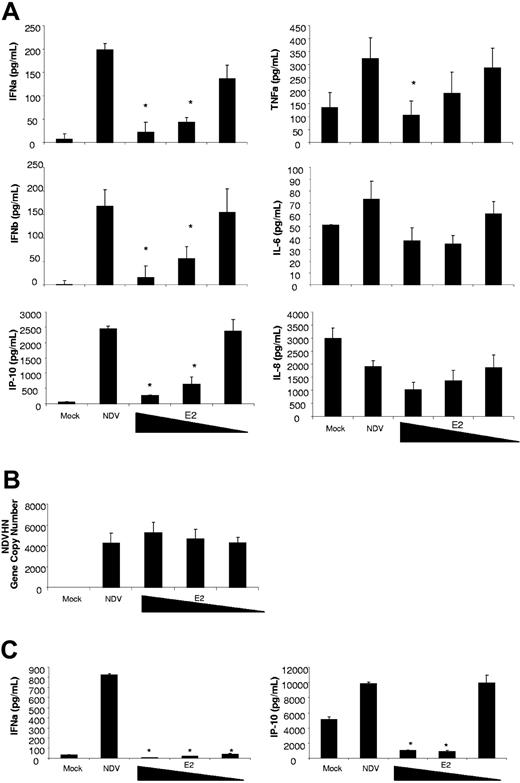
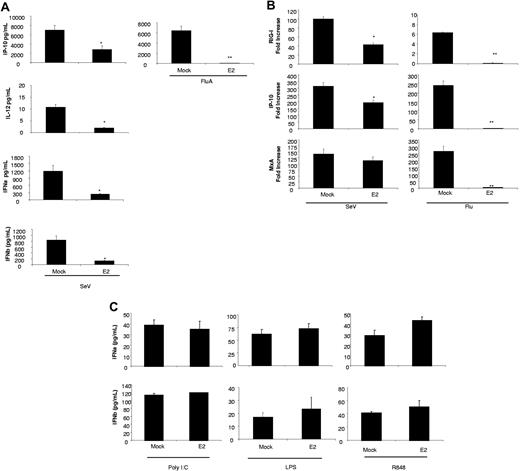
To evaluate the effect of E2 on DC maturation, DCs were pretreated for 24 hours with E2 (10, 1, 0.1 μg/mL) and infected with NDV-B1 for 9 hours (MOI 0.5) without removing the E2. The release of antiviral and proinflammatory cytokines was measured by multiplex-ELISA as is shown in Figure 2A. The results show a concentration-dependent reduction on the release of the antiviral proteins IFNα/β and IP-10 in those cells treated with E2. E2 administration significantly reduced the release of the proinflammatory cytokine TNFα at the highest concentration, while no statistically significant differences were observed with the release of IL-6 and IL-8 (Figure 2A). The reduction in cytokine release did not result from a diminution in the virus stimulus as the E2 had no impact on virus replication (Figure 2B). The E2 was not toxic to the cells as PI showed no relevant levels of cell death induced by any of the treatments (data not shown).
E2 suppresses the antiviral response induced by NDV-B1 in DCs. (A) Measurement of cytokines in supernatants from DCs pretreated for 24 hours with E2 (10, 1, 0.1 g/mL) and infected for 9 hours with NDV-B1 (MOI 0.5) in the continued presence of E2. Data are presented as mean plus or minus SD of triplicate samples from 5 independent experiments.*P < .001 versus NDV-B1–infected DCs. (B) NDV-B1 replication after 9 hours of infection was measured in all the experimental conditions by NDVHN gene expression (qRT-PCR). Data are presented as mean plus or minus SD from 3 independent experiments. (C) ELISA of myeloid DC supernatants, directly isolated from fresh blood pretreated for 24 hours with E2 (10, 1, 0.1 μg/mL) and infected with NDV-B1 (MOI 0.5) for 9 hours in the continued presence of E2. Data are presented as mean plus or minus SD from 3 independent experiments.*P < .001 versus NDV-B1–infected myeloid DCs.
E2 suppresses the antiviral response induced by NDV-B1 in DCs. (A) Measurement of cytokines in supernatants from DCs pretreated for 24 hours with E2 (10, 1, 0.1 g/mL) and infected for 9 hours with NDV-B1 (MOI 0.5) in the continued presence of E2. Data are presented as mean plus or minus SD of triplicate samples from 5 independent experiments.*P < .001 versus NDV-B1–infected DCs. (B) NDV-B1 replication after 9 hours of infection was measured in all the experimental conditions by NDVHN gene expression (qRT-PCR). Data are presented as mean plus or minus SD from 3 independent experiments. (C) ELISA of myeloid DC supernatants, directly isolated from fresh blood pretreated for 24 hours with E2 (10, 1, 0.1 μg/mL) and infected with NDV-B1 (MOI 0.5) for 9 hours in the continued presence of E2. Data are presented as mean plus or minus SD from 3 independent experiments.*P < .001 versus NDV-B1–infected myeloid DCs.
To ensure that the monocyte-derived DCs accurately reflected those found in vivo, myeloid DCs (mDCs) were directly isolated from fresh blood, pretreated with E2 for 24 hours, and infected for 9 hours with NDV-B1 in the presence of E2. Similar to the observations with monocyte-derived DCs, E2 pretreatment inhibited the release of antiviral proteins in freshly isolated human DCs (Figure 2C).
How long a pretreatment period is needed to establish E2-triggered inhibition of the response of DCs to virus infection?
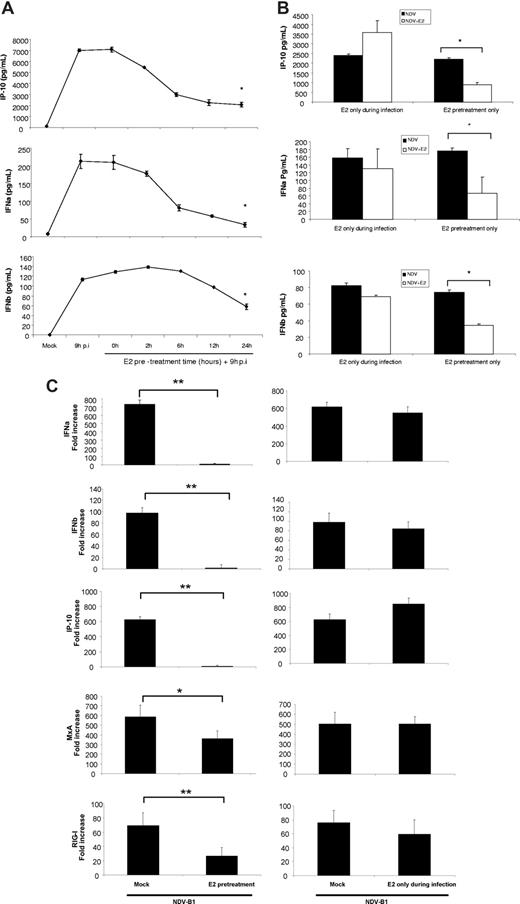
To determine the length of pretreatment with E2 necessary to observe this effect, DCs were pretreated with 10 ug/mL E2 for 0, 2, 6, 12, and 24 hours before infection with NDV-B1 for 9 hours in the continued presence of E2. The results from the E2 pretreatment kinetic show a time-dependent down-regulation of the antiviral response in DCs by E2, where the 24-hour pretreatment provided the most significant down-regulation (Figure 3A). Preincubation is essential because E2 added concurrently with the virus (0 hours of pretreatment) had no impact on the levels of IFNα/β and IP-10 release (Figure 3A). Moreover, when E2 was washed out of the culture before infection with virus (E2 pretreatment only), the pretreated DCs still showed a significant reduction in the release of antiviral proteins compared with infected cells (Figure 3B). These results demonstrate the need for pretreatment of the cells with E2 to observe an inhibition of antiviral response in DCs.
E2 pretreatment is necessary for the antiviral response down-regulation in DCs. (A) Measurement of IFNα/β and IP-10 levels by ELISA. E2 (10 μg/mL) pretreatment kinetic (0, 2, 6, 12, 24 hours) of DCs later infected for 9 hours with NDV-B1 in the presence of E2. Data are presented as mean plus or minus SD from 4 independent experiments. *P < .05 versus NDV-B1–infected DCs. (B) Measurement of IFNα/β and IP-10 by ELISA. DCs pretreated with 10 μg/mL E2 and infected for 9 hours without E2 in the medium (E2 pretreatment only) or DCs with no E2 pretreatment but infected in the presence of E2 for 9 hours with NDV-B1 (E2 only during infection). Data are presented as mean plus or minus SD from 4 independent experiments. *P < .05 versus NDV-B1–infected cells. (C) Type I IFN and IFN-responsive gene expression measured by qRT-PCR. DCs pretreated for 24 hours and infected for 9 hours with NDV-B1 in the presence of E2 (E2 pretreatment) and DCs without E2 pretreatment infected with NDV-B1 in the presence of E2 (E2 only during infection). Data are presented as mean plus or minus SD from 4 independent experiments. **P < .001 or *P < .05 versus NDV-B1–infected DCs.
E2 pretreatment is necessary for the antiviral response down-regulation in DCs. (A) Measurement of IFNα/β and IP-10 levels by ELISA. E2 (10 μg/mL) pretreatment kinetic (0, 2, 6, 12, 24 hours) of DCs later infected for 9 hours with NDV-B1 in the presence of E2. Data are presented as mean plus or minus SD from 4 independent experiments. *P < .05 versus NDV-B1–infected DCs. (B) Measurement of IFNα/β and IP-10 by ELISA. DCs pretreated with 10 μg/mL E2 and infected for 9 hours without E2 in the medium (E2 pretreatment only) or DCs with no E2 pretreatment but infected in the presence of E2 for 9 hours with NDV-B1 (E2 only during infection). Data are presented as mean plus or minus SD from 4 independent experiments. *P < .05 versus NDV-B1–infected cells. (C) Type I IFN and IFN-responsive gene expression measured by qRT-PCR. DCs pretreated for 24 hours and infected for 9 hours with NDV-B1 in the presence of E2 (E2 pretreatment) and DCs without E2 pretreatment infected with NDV-B1 in the presence of E2 (E2 only during infection). Data are presented as mean plus or minus SD from 4 independent experiments. **P < .001 or *P < .05 versus NDV-B1–infected DCs.
Virus infection triggers a significant increase in the transcription of IFNα/β and the IFN-inducible genes: IP-10, RIG-I, and MxA in DCs after 9 hours of infection with NDV-B1 (Figure 3C). However, 24 hours of pretreatment followed by infection of these cells in the presence of E2 (E2 pretreatment) drastically inhibits their transcription (Figure 3C left panel). In contrast, infection in the presence of E2 without pretreatment (E2 only during infection) had no effect on transcription of IFN or IFN-regulated genes (Figure 3C right panel). Thus, E2 potently inhibits the transcription of genes coding for type I IFN and IFN-activated proteins, but only if the DCs are pretreated with E2.
E2 did not affect the signaling pathway induced by IFNβ treatment
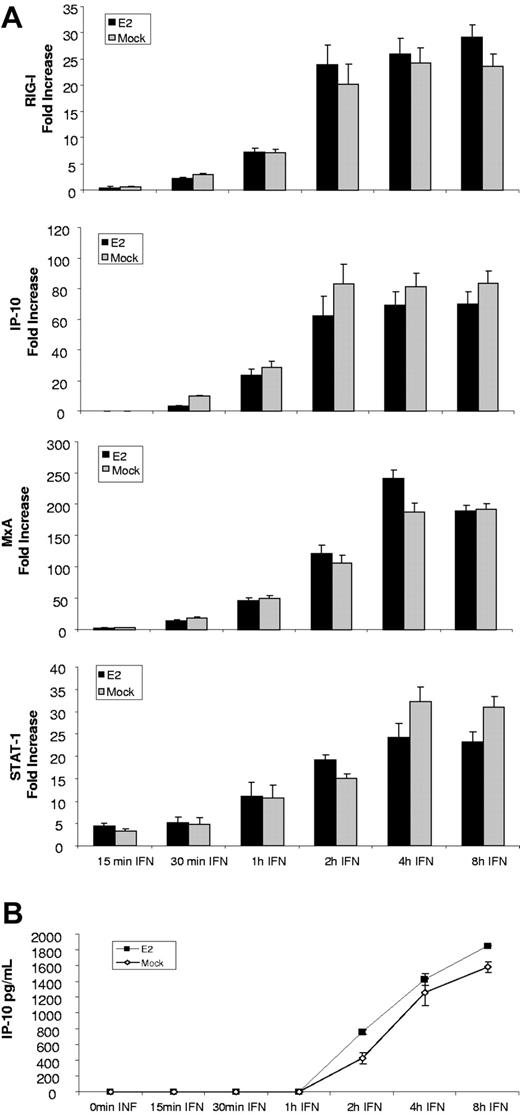
Several of the genes examined in DCs can be directly triggered by IFN signaling while others are enhanced by type I IFN binding to its receptor. Thus, the inhibitory effect of E2 on DCs could be caused by a direct effect on the transcription of the antiviral and inflammatory genes or by a blockade of signaling events. To assess the effect of E2 on IFNβ signaling, DCs were pretreated with E2 for 24 hours and then treated with IFNβ (5000 U/mL) for 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours in the presence of E2. Transcription of the genes was measured by qRT-PCR. The results presented in Figure 4A show that E2 did not affect the expression of genes involved in IFN signaling such as STAT-1, nor the IFN-responsive genes IP-10, RIG-I, and MxA. IP-10 release was also measured by ELISA and the results (Figure 4B) confirm the qRT-PCR data, showing that E2 had no effect on the secretion of this IFN-dependent chemokine. These results indicate that E2 is not suppressing DC activation by inhibiting the IFN-signaling pathway, and suggest that the inhibition of IFN as well as IFN-related events is most likely impacting the synthesis of antiviral proteins triggered by virus replication.
E2 did not modify the signaling induced by IFNβ. (A) Expression of genes involved in type I IFN signaling and IFN-inducible genes measured by qRT-PCR in DCs pretreated for 24 hours with E2 and treated for 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours with 5000 U/mL IFNβ. Data are presented as mean plus or minus SD of 4 independent experiments. (B) IP-10 expression measure by ELISA in DCs pretreated for 24 hours with E2 and treated for 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours with 5000 U/mL IFNβ. Data are presented as mean plus or minus SD from 4 independent experiments.
E2 did not modify the signaling induced by IFNβ. (A) Expression of genes involved in type I IFN signaling and IFN-inducible genes measured by qRT-PCR in DCs pretreated for 24 hours with E2 and treated for 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours with 5000 U/mL IFNβ. Data are presented as mean plus or minus SD of 4 independent experiments. (B) IP-10 expression measure by ELISA in DCs pretreated for 24 hours with E2 and treated for 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours with 5000 U/mL IFNβ. Data are presented as mean plus or minus SD from 4 independent experiments.
The E2 inhibitory effect can be observed with other RNA viruses but not with TLR3, 4, or 7/8 ligands
NDV-B1 is a potent trigger of DC maturation24 that depends on IFN release for complete maturation. Influenza virus contains a powerful immune antagonist, NS1,33 that makes it an extremely weak activator of DCs.24 Sendai virus (SeV) is the best activator of DCs that we have tested and the activation is independent of IFN signaling.34 In addition, SeV is the only virus that we have tested that triggers IL-12 release directly.27 Therefore, we tested the ability of E2 to inhibit the antiviral response mediated by DCs in response to Influenza and SeV as characterized by the release of IP-10, IFNα/β, and IL-12.
E2 treatment significantly down-regulates the antiviral response induced in DCs by both SeV and Influenza. The data presented in Figure 5A demonstrate a significant down-regulation of IFNα/β, IP-10, and IL-12 in those cells infected with SeV after E2 pretreatment. In addition, even though Influenza infection did not induce either type I IFN nor IL-12, E2 pretreatment drastically reduced the secretion of IP-10 induced by this infection in DCs. SeV and Influenza induce an increase in RIG-I transcription as well as in other IFN-related genes (MxA and IP-10). This induction was also reduced by E2 pretreatment (Figure 5B).
E2 down-regulates the antiviral response induced by RNA viruses but not by TLR agonist. (A) Cytokine levels measures by ELISA from DCs pretreated for 24 hours with 10 μg/mL E2 and infected with Influenza A (Flu) or SeV at an MOI of 0.5 for 9 hours. Data are presented as mean plus or minus SD from 4 independent experiments.*P < .05 or **P < .001 versus infected DCs. (B) RIG-I, IP-10, and MxA levels measured by qRT-PCR from DCs pretreated for 24 hours with 10 μg/mL E2 and infected with Influenza A (Flu) or SeV at an MOI of 0.5 for 9 hours. Data are presented as mean plus or minus SD from 4 independent experiments. *P < .05 or **P < .001 versus infected DCs. (C) Cytokine levels measured by ELISA from DCs pretreated for 24 hours with 10 μg/mL E2 before TLR 3, 4, and 7/8 activation.
E2 down-regulates the antiviral response induced by RNA viruses but not by TLR agonist. (A) Cytokine levels measures by ELISA from DCs pretreated for 24 hours with 10 μg/mL E2 and infected with Influenza A (Flu) or SeV at an MOI of 0.5 for 9 hours. Data are presented as mean plus or minus SD from 4 independent experiments.*P < .05 or **P < .001 versus infected DCs. (B) RIG-I, IP-10, and MxA levels measured by qRT-PCR from DCs pretreated for 24 hours with 10 μg/mL E2 and infected with Influenza A (Flu) or SeV at an MOI of 0.5 for 9 hours. Data are presented as mean plus or minus SD from 4 independent experiments. *P < .05 or **P < .001 versus infected DCs. (C) Cytokine levels measured by ELISA from DCs pretreated for 24 hours with 10 μg/mL E2 before TLR 3, 4, and 7/8 activation.
Immature DCs express TLR3, TLR4, and TLR 7/8. To analyze the effect of E2 on these signaling pathways, we pretreated DCs with E2 before treating them with TLR ligands; poly I:poly C (TLR 3), LPS (TLR 4), and R848 (TLR 7/8). In contrast to the results for RNA viruses, E2 pretreatment did not down-regulates the activation induced by TLR agonist in DCs (Figure 5C). Thus, E2 inhibits activation of DCs to RNA viruses but not to TLR agonists.
E2 inhibits activation of IFNβ promoter and RIG-I
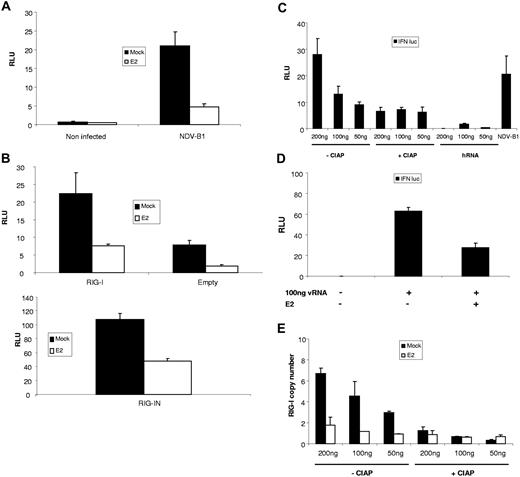
In DCs one of the earliest events in virus-triggered activation is synthesis of IFNβ. To specifically dissect the mechanism by which E2 suppresses DC activation, estrogen-sensitive MCF-7 cells were transfected with an IFNβ promoter reporter construct driving firefly luciferase and subsequently infected with NDV-B1. The results shown in Figure 6A suggest that E2 inhibits synthesis of IFNβ through inhibition of promoter activation. Moreover, NDV-B1 activation of IFNβ occurs through stimulation of the RIG-I pathway.35 Indeed, Figure 6B (top panel) shows that in our system, overexpressing RIG-I during NDV-B1 infection does increase IFNβ transcription, and that E2 pretreatment inhibited this signal. Finally, the expression of a constitutively active RIG-I, RIG-IN induced IFNβ transcription in the absence of viral stimulation. Figure 6B (bottom panel) shows that this signal is also down-regulated by E2 pretreatment, suggesting that E2 inhibits the RIG-I signaling pathway and, as a consequence, IFNβ transcription.
E2 inhibit DC maturation triggered by 5′ triphosphate viral RNA. MCF-7 cells were transfected with IFNβ promoter reporter construct driving firefly luciferase(A) alone or (B) along with a plasmid expressing RIG-I (top panel), RIG IN (bottom panel), or an empty plasmid. Cells were then pretreated or not with 10 μg/mL E2 and 24 hours later and infected with NDV-B1 (MOI = 0.5) for 6 to 8 hours (top panel) or just pretreated for 24 hours with E2 (bottom panel). The Relative Luciferase Units (RLUs) were measured 6 to 8 hours after NDV-B1 infection and RIG-IN transfection, respectively. (C) Cells were cotransfected with NDV genomic RNA (50 ng, 100 ng, 200 ng), treated or not with CIAP, or transected with irrelevant human mRNA (50 ng, 100 ng, 200 ng) along with IFNβ promoter reporter. As a control for the activation of the IFNβ promoter, cells were also infected with NDV-B1 for 6 hours. Cells pretreated or not with E2 were: (D) cotransfected with 100 ng viral RNA along with IFNβ promoter reporter for 6 to 8 hours or (E) transfected with viral RNA (50, 100, 200 ng) treated or not treated with CIAP. RIG-I expression was measured by RTqPCR 6 hours after infection. Results are presented as mean plus or minus SD and are representative of 3 experiments.
E2 inhibit DC maturation triggered by 5′ triphosphate viral RNA. MCF-7 cells were transfected with IFNβ promoter reporter construct driving firefly luciferase(A) alone or (B) along with a plasmid expressing RIG-I (top panel), RIG IN (bottom panel), or an empty plasmid. Cells were then pretreated or not with 10 μg/mL E2 and 24 hours later and infected with NDV-B1 (MOI = 0.5) for 6 to 8 hours (top panel) or just pretreated for 24 hours with E2 (bottom panel). The Relative Luciferase Units (RLUs) were measured 6 to 8 hours after NDV-B1 infection and RIG-IN transfection, respectively. (C) Cells were cotransfected with NDV genomic RNA (50 ng, 100 ng, 200 ng), treated or not with CIAP, or transected with irrelevant human mRNA (50 ng, 100 ng, 200 ng) along with IFNβ promoter reporter. As a control for the activation of the IFNβ promoter, cells were also infected with NDV-B1 for 6 hours. Cells pretreated or not with E2 were: (D) cotransfected with 100 ng viral RNA along with IFNβ promoter reporter for 6 to 8 hours or (E) transfected with viral RNA (50, 100, 200 ng) treated or not treated with CIAP. RIG-I expression was measured by RTqPCR 6 hours after infection. Results are presented as mean plus or minus SD and are representative of 3 experiments.
E2 inhibits DC maturation trigger by 5′ triphosphate viral RNA
The cytoplasmic helicase RIG-I senses virus infection by binding to uncapped viral 5′triphosphates. Figure 6C shows that treatment of NDV-B1 genomic RNA with Intestine Alkaline Phosphatase (CIAP) eliminated the stimulatory ability of NDV-B1 RNA, measured by IFNβ promoter reporter construct driving firefly luciferase (Figure 6C). These data demonstrate that RIG-I is the main sensor of transfected NDV-B1 RNA. Interestingly, pretreatment of cells with E2 impaired the ability of transfected NDV-B1 RNA to stimulate the IFNβ promoter (Figure 6D) and the expression of RIG-I (Figure 6E), suggesting that E2 treatment has a negative effect on the RIG-I signaling pathway.
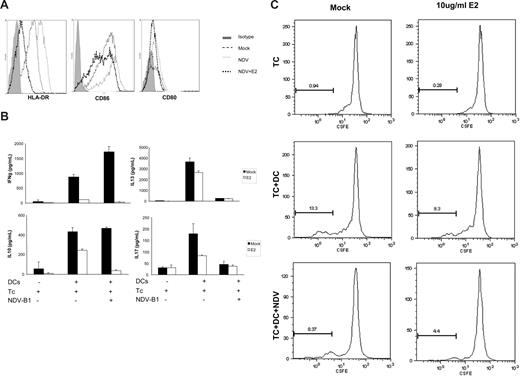
E2 inhibits up-regulation of MHC II and costimulatory molecules and abrogates DCs' capacity to stimulate naive CD4 T cells
One of the hallmarks of DC maturation is the up-regulation of MHC II and costimulatory molecules essential for interacting with T cells and inducing their proliferation. NDV-B1 infection up-regulates these molecules on DCs. However, pretreatment with E2 reduces the up-regulation of CD80 and CD86 and HLA-DR expression after NDV-B1 infection (Figure 7A). Increased expression of these molecules is partially responsible for the observation that mature DCs efficiently stimulate naive T cells.24 Thus, we analyzed the ability of virus-infected DCs, treated or not treated with E2, to stimulate allogeneic naive CD4 T cells. DCs that were treated with 10 ug/mL E2 were infected with NDV-B1 and cocultured with allogeneic naive CD4 T cells for 5 to 6 days. At that time, the release of cytokines by CD4 naive T cells was measured by multiplex ELISA. The results in Figure 7B demonstrate the ability of E2 treatment to down-regulate the secretion of IFNγ and IL-10 either in mock-infected cells or NDV-B1–infected cells. On the other hand, viral infection drastically down-regulates IL-13 (Th2) and IL-17 (Th17) compared with the levels observed in mock-infected cells. E2 treatment reduced the secretion of these cytokines in mock-infected cells.
E2 reduces T-cell priming by DCs. (A) Costimulatory molecule expression (HLADR, CD86, and CD80) measured by FACS. DCs were mock treated, infected with NDV-B1, or pretreated for 24 hours with E2 before infection. Results are representative of 3 independent experiments. (B) DCs treated or not for 24 hours with 10 μg/mL E2 were infected for 1 hour with NDV-B1 at an MOI of 0.5. Afterward, DCs were incubated with naive CD4 T cells from an allogeneic donor at a ratio of 1:5 for 5 to 6 days. Supernatants were harvested and tested by ELISA for the release of IFN-γ, IL-10, IL-13, and IL-17. Data presented as mean plus or minus SD and are representative of 2 independent experiments. (C) Naive CD4 T cells labeled with CSFE (2.5 μM) were incubated with DCs infected or mock infected in the presence or absence of E2 for 5 to 6 days. Naive CD4 T-cell proliferation was measured by FACS. Results were representative of 2 independent experiments.
E2 reduces T-cell priming by DCs. (A) Costimulatory molecule expression (HLADR, CD86, and CD80) measured by FACS. DCs were mock treated, infected with NDV-B1, or pretreated for 24 hours with E2 before infection. Results are representative of 3 independent experiments. (B) DCs treated or not for 24 hours with 10 μg/mL E2 were infected for 1 hour with NDV-B1 at an MOI of 0.5. Afterward, DCs were incubated with naive CD4 T cells from an allogeneic donor at a ratio of 1:5 for 5 to 6 days. Supernatants were harvested and tested by ELISA for the release of IFN-γ, IL-10, IL-13, and IL-17. Data presented as mean plus or minus SD and are representative of 2 independent experiments. (C) Naive CD4 T cells labeled with CSFE (2.5 μM) were incubated with DCs infected or mock infected in the presence or absence of E2 for 5 to 6 days. Naive CD4 T-cell proliferation was measured by FACS. Results were representative of 2 independent experiments.
Figure 7C shows that, after 5 to 6 days of coculture, naive CD4 T cells proliferated in response to allogenic DCs, and this proliferation was significantly down-regulated in the presence of E2. Taken together, these results suggest that E2 treatment reduces the capacity of DCs to stimulate naive CD4 T cells by reducing the proliferative capacity of theses cell as well as down-regulating the secretion of several cytokines.
Discussion
Sex-associated hormones have been shown to exert a powerful effect on various immune system functions.36,37 Even the monthly hormonal changes associated with the estrous cycle have been reported to impact immunity.38 Variation in hormone production may explain observed differences in the incidence of autoimmune disease between men and women.39 However, the most drastic changes in female hormone levels occur during pregnancy. Estrogen and progesterone rise throughout the course of pregnancy reaching extremely high concentrations in the third trimester.8,9 There are many reports of increased susceptibility to infectious agents associated with pregnancy, suggesting an important role of pregnancy-associated hormones in this process.40-42 During the 1918 to 1919 Spanish flu and the 1957 Asian Influenza pandemic, pregnant women were much more likely than the general population to succumb to these infections, with the greatest mortality risk reported in the latter half of pregnancy.19-21 These observations suggest that pandemic influenza might be much more impacted by changes in immunity during pregnancy than yearly epidemic influenza infection.
The immune system is arbitrarily divided into innate and adaptive branches. Innate immune mechanisms (phagocytosis and the type I IFN system, among others) function to prevent the establishment of infection and, if infection occurs, to slow its spread. Clearance of the infection is usually accomplished by the adaptive system that includes cytokine producing CD4 cells, cytotoxic CD8 cells, and neutralizing antibodies.
DCs play a key role in bridging innate and adaptive immunity. The activation of DCs by exposure to pathogens triggers a maturational shift that converts them into efficient antigen-presenting cells capable of activating naive T cells and secreting cytokines that dictate the polarity of the response.1,2 During virus infection, DCs can be stimulated either through TLRs or RIG-I like receptors (RLRs). However, the evidence suggests that for classic DCs, the internal RLR pathways represents the primary activating mechanism.35 With respect to DCs, evidence suggests that the pathway for type I IFN production and DC maturation are linked and are probably activated by the same receptors, RLR.6 These receptors are RNA helicases that have been shown to have specificity for RNA intermediates made during virus replication. In response to virus infection, the hallmarks of cDC activation are the production of type I IFN, inflammatory cytokines, chemokines, and T-cell interacting molecules.35
Because DCs are key cells involved in the initiation, direction, and control of adaptive immune responses, any impact that hormones such as estrogen have on their functions might have profound effects on immune responsiveness. While there is no definitive information on the mechanism by which steroid hormones are affecting the immune system, several reports have demonstrated some degree of regulation of DC function by E2.43,44
Here we have shown that E2, the main secreted estrogen, inhibits the activation of DCs by viral infection. Data from our group have demonstrated that E2 pretreatment regulates antiviral response in DCs either from male or female donors (data not shown). DC activation by NDV-B1 infection was determined by measuring transcription of a panel of genes involved in antiviral response as well as secretion of proteins essential for the antiviral immune response. Using this system, we demonstrated that E2 inhibits the synthesis of type I IFN and reduced expression of inflammatory cytokines such TNFα, IL-12, and chemokines such as IP-10. This inhibitory effect was observed with both monocyte-derived DCs and those isolated directly from blood. In addition, we demonstrated that this effect is optimal if the DCs are preincubated with E2 for 24 hours. This long time lag may suggest that E2 pretreatment may be leading to the production of new proteins, to changes in gene accessibility, or to alterations in the activity of cellular proteins that may influence the DCs' ability to mature in response to viral infection.
E2 appears to have its strongest impact on the IFN pathway. These significant effects of E2 on DCs' antiviral response suggest several mechanisms by which this hormone may exerts it effect. The triggering of the type I IFN pathway by viral infection involves the activation of a paracrine-autocrine loop responsible for the IFN signal amplification after pathogen recognition.45 In regard to this sequence of events, E2 may be acting at the IFN induction level, IFN signaling level, or upstream of IFN. Our results demonstrate that E2 did not affect the type I IFN signaling pathway. Based on the evidence that demonstrates that RNA viruses trigger type I IFN primarily through RLR pathways35,46 in classic DCs, we hypothesized that these cellular helicases may represent a target of the E2 inhibition. Indeed, we have observed E2 inhibition of RIG-I activation in response to virus infection and of constitutively active RIG-I (RIG-IN). Moreover, we demonstrated that E2 pretreatment impaired the ability of the RIG-I ligand 5′ triphosphate viral RNA47 to trigger DC maturation by down-regulating the activation of the IFNβ promoter and the expression of RIG-I to the basal levels present in cells transfected with viral RNA treated with CIAP.
Previous reports suggested that E2-pretreated DCs increase their response to TLR stimulation.48 In contrast to what we observed with viral stimulation, we showed that E2 pretreatment does not affect the synthesis of IFNα/β in response to TLR agonists, suggesting that the target of E2 in DCs is the internal pathway for viral sensing involving RIG-I but not TLRs.
SeV is the best inducer of DC maturation and type I IFN induction that we have tested. Our laboratory has demonstrated that DC maturation and activation, as well as type I IFN release by SeV infection, is triggered through RLR.34 When SeV is used for activation, E2 inhibits both the IFN production and inflammatory cytokines such IL-12. Similar results were obtained when the DCs' maturation was induced by influenza virus infection. The reduction of these cytokines might be explained if we consider that the pathway for type I IFN production and DC maturation could be linked. In this way, the regulation of RIG-I or other elements of this pathway by E2, in addition to inhibiting the type I IFN system, might also be regulating the maturation of the cells by viral infection.
The concentration of E2 that suppressed the activation of DCs in our system is 5- to 10-fold higher than the average level of E2 in blood of a pregnant woman in her third trimester. Paharkova-Vatchkova et al, using murine experimental models, demonstrated that E2 promotes the differentiation of DCs from precursors in the bone marrow.49 Preliminary results from our group using our human monocyte derived DCs system demonstrated that 24 hours of E2 did not induce DC differentiation (data not shown). However, E2 represents 1 of 3 types of estrogen found in the blood of pregnant women. Moreover, estrogen receptors are found on many cell types, and a contribution from other factors released by those cells may also impact the DCs.
It is not readily apparent why inhibition of type I IFN and DC maturation is necessary during pregnancy. There is, however, significant data that suggest that inflammatory Th1 cytokines such as IFNγ are elevated in many adverse pregnancies.50 Cocultures of E2-treated DCs infected with virus or mock-infected and naive CD4 T cells showed a profound reduction in the release of Th1-related cytokines. Moreover, Th2 and Th17 cytokines were also down-regulated by E2 treatment in mock-infected cells. Surprisingly, NDV-B1 infection significantly reduced the secretion of these cytokines. This clearly correlates with a decrease in the proliferation levels of naive CD4 T cells cocultured with infected DCs in the presence of E2. Taken together these results suggest that E2 significantly down-regulates the activation of naive CD4 T cells. In addition, NDV-B1 infection stimulates the secretion of Th1-characteristic cytokines while significantly reducing IL-17 (Th17) and IL-13 (Th2). Interestingly, there is a precipitous drop in E2 levels immediately before parturition. If indeed there is an inhibition of inflammatory responses before this point, it may be that an inflammatory state may be needed for successful birth.
These results demonstrate that E2 interferes with RNA virus–triggered DC maturation and type I IFN production through RLR (RIG-I). Because DCs represent a key intermediary bridging innate and adaptive immunity, inhibition of this transition may have profound effects with regard to susceptibility and recovery from virus infection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Dr D. Thanos for kindly providing the IFN-β promoter reporter construct, Dr A. Garcia Sastre and E. Nistal-Villan for the RIG-IN plasmids, B. Moltedo for collaborating in the allogenic coculture setup, and Dr V. Abrahams for kindly providing the ERα primers. We would also like to thanks the Quantitative PCR Shared Resource Facility at the Mount Sinai School of Medicine.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases grants N01-A1-50028 and U19 A1062623 (Center for Investigating Viral Immunity and Antagonism).
National Institutes of Health
Authorship
Contribution: M.M.E. designed and performed research, analyzed data, and wrote the manuscript; T.K. performed research and wrote the manuscript; E.R. performed research; A.F.S. designed research and wrote the manuscript; C.B.L. designed and performed research and wrote the manuscript; and T.M.M. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Thomas M. Moran, Professor of Microbiology, Microbiology Department, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029; e-mail: thomas.moran@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal