Abstract
Interleukin-15 (IL-15) is crucial for the development of naive and memory CD8 T cells and is delivered through a mechanism called transpresentation. Previous studies showed that memory CD8 T cells require IL-15 transpresentation by an as yet unknown cell of hematopoietic origin. We hypothesized that dendritic cells (DCs) transpresent IL-15 to CD8 T cells, and we examined this by developing a transgenic model that limits IL-15 transpresentation to DCs. In this study, IL-15 transpresentation by DCs had little effect on restoring naive CD8 T cells but contributed to the development of memory-phenotype CD8 T cells. The generation of virus-specific, memory CD8 T cells was partially supported by IL-15Rα+ DCs through the preferential enhancement of a subset of KLRG-1+CD27− CD8 T cells. In contrast, these DCs were largely sufficient in driving normal homeostatic proliferation of established memory CD8 T cells, suggesting that memory CD8 T cells grow more dependent on IL-15 transpresentation by DCs. Overall, our study clearly supports a role for DCs in memory CD8 T-cell homeostasis but also provides evidence that other hematopoietic cells are involved in this function. The identification of DCs fulfilling this role will enable future studies to better focus on mechanisms regulating T-cell homeostasis.
Introduction
The generation and maintenance of memory CD8 T cells is regulated by multiple mechanisms that involve both early programming of memory T-cell precursors as well as a continuous supply of external signals.1,2 Whereas the events and signals that program memory precursors are not well understood, interleukin-15 (IL-15) has clearly been shown to drive the generation and maintenance of memory CD8 T cells.3,4 The recent evidence that IL-15 is delivered through the mechanism of transpresentation via IL-15Rα by cell-cell contact5 opens up many questions in the field of memory CD8 T-cell homeostasis, the foremost being the identity of the cell transpresenting IL-15 to memory CD8 T cells.
To better understand how IL-15 transpresentation is regulated, the identity of the cell type mediating IL-15 transpresentation must be known. Studies attempting to address this have found that either parenchymal or hematopoietic cells mediate transpresentation, depending on the responding lymphocyte.6,7 This finding is seminal as it shows that cells can be in an IL-15Rα+ environment and remain ignorant to IL-15. Considering that IL-15Rα is ubiquitously expressed, it is surprising that the responding cell has such strict requirements for a specific cell to transpresent IL-15.8 In contrast to IL-15Rα expression, IL-15 protein is not believed to be expressed by many IL-15Rα–expressing cells (ie, T cells); however, expression of IL-15 has been difficult to determine as reagents detecting IL-15 protein have been limited. As such, multiple studies using functional readouts have found that coexpression of IL-15 and IL-15Rα is integral for a cell to transpresent IL-15.9-11 These observations emphasize that the identity of an IL-15 transpresenting cell may not simply be implied by the expression of IL-15Rα but rather is better determined through functional analysis.
For memory CD8 T cells, in vivo studies found that IL-15Rα–expressing hematopoietic cells are the dominant cell types driving homeostatic proliferation.7,12 The hematopoietic cells providing the IL-15 signal are RAG-1–independent, indicating that IL-15 transpresentation is not mediated by either T or B lymphocytes.9 As DCs, monocytes, and macrophages have been shown to express IL-15Rα protein and are capable of inducing IL-15,13,14 these cells are potential mediators of IL-15 transpresentation. Indeed, bone marrow (BM)–derived DCs and some monocytic cell lines can transpresent IL-15 in vitro5 ; however, whether their analogous in vivo counterparts transpresent IL-15 to CD8 T cells is not known. Since IL-15 transpresentation requires cell-cell interactions and DCs have a natural propensity for T-cell interactions, DCs are a prime candidate to transpresent IL-15 to memory CD8 T cells. Therefore, the goal of this study was to examine the contribution of DCs in transpresentation of IL-15 to CD8 T cells.
In this study, we demonstrate that DCs transpresent IL-15 to CD8 T cells driving specific functions during differentiation. Although IL-15 is important for development of naive CD8 T cells and intraepithelial lymphocytes (IELs),15,16 exclusive IL-15 transpresentation by DCs failed to restore these populations. DCs did, however, preferentially promote the survival of KLRG-1+CD27− cells during the development of virus-specific memory CD8 T cells. Furthermore, DCs were highly efficient in driving homeostatic proliferation of memory CD8 T cells. Collectively, our data identify a previously unknown role of DCs in the differentiation and homeostasis of memory CD8 T cells; however, the inability of DCs to restore all IL-15 functions indicates that other cell types participate in transpresenting IL-15 to memory CD8 T cells.
Methods
Mice
C57BL/6J (CD45.2) and C57BL/6J (Thy1.1+) were purchased from The Jackson Laboratory (Bar Harbor, ME) and C57BL/6J (CD45.1) mice were purchased from the National Cancer Institute (NCI; Frederick, MD). IL-15Rα−/− mice16 were generously provided by A. Ma (University of California at San Francisco) and backcrossed to C57BL/6 mice 15 generations, and IL-15−/− mice15 were purchased from Taconic Farms (Germantown, NY). The full-length murine IL-15Rα cDNA was cloned by polymerase chain reaction (PCR) from cDNA generated from a C57BL/6 spleen RNA using TA Topo cloning kit (Invitrogen, Carlsbad, CA). The IL-15Rα cDNA was then subcloned downstream of the CD11c promoter by ligating into the pCD11c-pDOI-5 vector17 (graciously provided by Thomas Brocker, Institute for Immunology, Ludwig-Maximilian University, Munich, Germany) at the EcoRI site. The final 7.4-kb CD11c-IL-15Rα fragment was removed from the vector backbone by XhoI and NotI digestion and used for microinjection into C57BL/6 pronuclei for generation of transgenic mice. Microinjection was performed by the Genetically Engineered Mouse Facility at the University of Texas M. D. Anderson Cancer Center. Tg-positive mice were identified by PCR of tail DNA and bred to IL-15Rα−/− background. All mice were maintained under specific pathogen–free conditions at the M. D. Anderson Cancer Center in accordance with its Institutional Animal Care and Use Committee guidelines. Bone marrow chimeras were generated as previously described.7
Flow cytometric analysis
Lymphocytes were isolated from various tissues as previously described.6,7 IL-15Rα and IL-15 were detected with goat anti–IL-15Rα-biotin (R&D Systems, Minneapolis, MN) and rabbit anti–IL-15 biotin (Peprotech, Rocky Hill, NJ), respectively, followed by streptavidin-APC (Jackson ImmunoResearch Laboratories, West Grove, PA). Background staining was determined by staining analogous populations from either IL-15Rα−/− or IL-15−/− mice or with a biotinylated Ig control (Jackson ImmunoResearch Laboratories). Prior to staining for IL-15 and IL-15Rα, cells were preincubated with Fc block (BD Biosciences, San Jose, CA). The following monoclonal antibodies (mAbs) were purchased from either BD Biosciences, eBioscience (San Diego, CA), or BioLegend (San Diego, CA): CD44, CD62L, CD8α, CD19, CD3, DX5, CD4, CD11b, CD11c, CD127, Thy1.1, KLRG-1, CD27, and MHC class II (I-Ab). For stimulation with lipopolysaccharide (LPS), cells were isolated from spleen and cultured at 10 × 106/mL in RPMI containing 2.5 mM HEPES, 5.5 × 10−5 M 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin, 5 mM glutamine, and 10% fetal bovine serum (FBS; complete medium [CM]) at 37°C with 5% CO2 either in the presence or absence of 1 μg/mL LPS (Sigma-Aldrich, St Louis, MO) for 24 hours. For studies of a viral immune response, mice were infected intravenously with 105 pfu (vesicular stomatitis virus [VSV], Indiana strain) and VSV-specific CD8 T cells were detected using an H-2Kb tetramer (National Institutes of Health [NIH] tetramer facility) loaded with the VSV nucleoprotein epitope (RGYVYQGL) as previously described.7 Flow cytometric data were acquired with an LSRII (BD Biosciences) or FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Treestar, Ashland, OR). Lymphocyte percentages and total cell numbers were calculated and evaluated using the Student t test. Values of P less than .05 were considered statistically significant.
Analysis of homeostatic proliferation
Proliferation of endogenous cells was assessed by BrdU incorporation. VSV-infected mice were given BrdU in their drinking water (0.8 mg/mL) for 5 to 6 weeks. Cells were stained for surface markers as usual followed by staining using the BrdU Flow Kit (BD Biosciences) according to manufacturer's instructions. For transfer studies, C57BL/6 (Thy1.1) mice were infected intravenously with 105 pfu VSV and boosted at least 30 days after infection with VSV-New Jersey. Spleens were harvested at least 30 days after most recent infection. Splenic CD8 T cells were negatively enriched using the Dynal CD8 Enrichment Kit (Dynal Biotech, Oslo, Norway) according to the manufacturer's instructions, labeled with 2 mM carboxyfluorescein succinimidyl ester (CFSE), and injected intravenously into various recipients (approximately 10 × 106 cells/mouse). Enriched cells were found to be 75% to 85% CD8+, 4% to 8% NK1.1, and less than 1% to 2% CD11c, B220, CD4.
Results
CD11c promoter drives IL-15Rα expression preferentially by DCs
Since DCs are highly specialized for T-cell interactions and stimulation as well as express IL-15 and IL-15Rα,14,18,19 we examined the contribution of IL-15 transpresentation by DCs on CD8 T-cell development and the generation of memory CD8 T cells. To target IL-15Rα to DCs, a transgenic mouse model was generated in which IL-15Rα expression is driven by the murine CD11c promoter, a well-described promoter with a high specificity to DCs.17 We chose to control IL-15Rα protein expression rather than IL-15 to address specifically the role of IL-15 transpresentation. To eliminate IL-15Rα expression from all other cells, the CD11c-IL-15Rα Tg mice were bred to the IL-15Rα−/− background. Once backcrossed, CD11c-specific expression of cell surface IL-15Rα protein was assessed using immunofluorescence staining and flow cytometry.
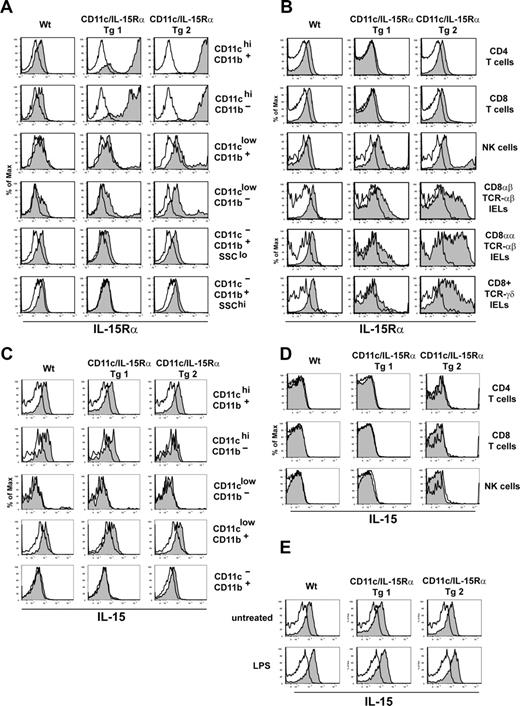
Using this approach, 2 founders were identified that expressed IL-15Rα by CD11c+ cells: one founder line with IL-15Rα restricted to CD11c+ cells and a second founder line with expression spilling over onto CD11c− hematopoietic cells. Overall, the CD11c-driven expression of IL-15Rα closely correlated with the endogenous expression of CD11c, resulting in a model where DCs are the dominant cell type expressing IL-15Rα (ie, Tg line 1). Analysis of transgenic IL-15Rα expression showed that IL-15Rα was highly expressed by lineage-negative, CD19, CD3, DX5/ CD11b+ CD11chi, and CD11b−CD8+CD11chi DCs and was moderate in lin−CD11clo (putative DC precursors and pDCs) in both Tg founder lines (Figure 1A). On CD11c− cells, which include monocytes (CD11b+SSClo), granulocytes (CD11b+SSChi), and T cells, Tg-1 had no expression of IL-15Rα whereas Tg-2 had levels similar to that of wild-type (Wt) cells (Figure 1A,B). As natural killer (NK) cells and IELs express CD11c, each of these cell types expressed IL-15Rα in both Tg founder lines at varying levels (Figure 1B). Whereas previous studies have shown that IL-15Rα is expressed at a low level on CD11c+ cells,20 a comparison of IL-15Rα expression by DC subsets directly ex vivo had not been described before. Interestingly, this analysis demonstrates that IL-15Rα expression is relatively low and homogenous among DC subsets and other myeloid cells (Figure 1A).
Expression of IL-15 and IL-15Rα in CD11c-IL-15Rα Tg mice. (A) Histograms show IL-15Rα expression on myeloid subsets in Wt, CD11c-IL-15Rα Tg-1 and Tg-2, and IL-15Rα−/− mice after gating on lineage-negative (CD3, CD19, DX5) splenocytes. Filled histograms indicate IL-15Rα staining on indicated populations, open histograms represent staining of the same population from IL-15Rα−/− mice. (B) Histograms show IL-15Rα staining on the respective lymphoid populations: top 3 panels are CD4+TCRαβ+, CD8+TCRαβ+, NK1.1+-TCRαβ− cells from the spleen; bottom 3 panels show respective IEL populations. (C,D) Splenocytes (107) from Wt, CD11c/IL-15Rα Tg-1, Tg-2, and IL-15−/− mice were cultured overnight in CM prior to staining. Filled histogram indicates IL-15 staining, the open histogram represents staining of the same cells from IL-15−/− mice. Histograms in panel C were first gated on lineage-negative cells as in panel A. (E) Splenocytes were treated as in panel C, in the absence or presence of 1 μg/mL LPS. IL-15 staining was determined after gating on CD11chi CD11b+ cells.
Expression of IL-15 and IL-15Rα in CD11c-IL-15Rα Tg mice. (A) Histograms show IL-15Rα expression on myeloid subsets in Wt, CD11c-IL-15Rα Tg-1 and Tg-2, and IL-15Rα−/− mice after gating on lineage-negative (CD3, CD19, DX5) splenocytes. Filled histograms indicate IL-15Rα staining on indicated populations, open histograms represent staining of the same population from IL-15Rα−/− mice. (B) Histograms show IL-15Rα staining on the respective lymphoid populations: top 3 panels are CD4+TCRαβ+, CD8+TCRαβ+, NK1.1+-TCRαβ− cells from the spleen; bottom 3 panels show respective IEL populations. (C,D) Splenocytes (107) from Wt, CD11c/IL-15Rα Tg-1, Tg-2, and IL-15−/− mice were cultured overnight in CM prior to staining. Filled histogram indicates IL-15 staining, the open histogram represents staining of the same cells from IL-15−/− mice. Histograms in panel C were first gated on lineage-negative cells as in panel A. (E) Splenocytes were treated as in panel C, in the absence or presence of 1 μg/mL LPS. IL-15 staining was determined after gating on CD11chi CD11b+ cells.
A similar technique was used to measure IL-15 on the cell surface using a recently available antibody. On lineage-negative myeloid cells, cell-surface IL-15 was detected on CD11b+CD11c+ and CD8+CD11b−CD11c+ DCs as well as on CD11cloCD11b+ cells at similar levels in both Wt, Tg-1, and Tg-2 cells, although was not present on CD11cloCD11b− pDCs (Figure 1C). In Wt and Tg-2 but not Tg-1 mice, IL-15 expression was observed at a lower level on CD11b+CD11c− cells (monocytes and granulocytes; Figure 1C) displaying the potential for cells other than DCs to transpresent IL-15. Among lymphocytes, neither CD4+TCRβ+, CD8+TCRβ+, NK1.1+TCRβ−, or CD19+ cells isolated from Wt or either CD11c-IL-15Rα Tg mice expressed IL-15 on the cell surface (Figure 1D, data not shown). In response to LPS stimulation, the amount of IL-15 up-regulated was similar in DCs of both Wt and Tg mice (Figure 1E) suggesting that, in spite of elevated IL-15Rα on Tg DCs, IL-15 expression is not coordinately enhanced. Altogether, DCs, putative DC precursors, and monocytes/granulocytes express cell-surface IL-15 in Wt mice; however, since IL-15 transpresentation requires both IL-15 and IL-15Rα expression, DCs are likely the only cell transpresenting IL-15 in the CD11c-IL-15Rα Tg-1 mice.
Naive and memory-phenotype CD8 T cells are partially recovered in the presence of IL-15Rα+ DCs
IL-15Rα−/− mice have reduced numbers of peripheral CD8 T cells, with the deficiency in memory-phenotype CD8 T cells affected more than the naive CD8 T cells.16 Decreased numbers of naive CD8 T cells in the absence of IL-15Rα are due predominantly to the decreased survival of naive CD8 T cells, as only minor effects on the thymic CD8 T cells are observed.21 To determine if CD11c-driven IL-15Rα affects thymic T-cell development, thymocytes were analyzed from the CD11c-IL-15Rα Tg mice and compared with IL-15Rα−/− and Wt mice. The proportions of the major thymocyte subsets (CD4−CD8−, CD4+CD8+, CD4+, and CD8+ thymocytes) were similar in all groups of mice (Figure 2A and data not shown), indicating that transgenic expression of IL-15Rα does not cause major disruptions in thymic T-cell development.
Phenotype of CD8 T cells as a function of CD11c-driven IL-15Rα expression. Lymphocytes from thymus, spleen (SP), lymph node (LN), lung (LG), and bone marrow (BM) were isolated from the mice indicated (6-8 weeks old) and stained for cell-surface markers to identify differences in T-cell populations. (A) Representative flow cytometry plots from thymus and spleen. (B) The percentage of CD8 T cells along with the proportion of naive (CD44low) and memory-phenotype (CD44hi) in various tissues. Results are averages with n = 10 mice/group for Tg-1. For Tg-2, n = 8 for spleen and n = 2 for other tissues. § represents a significant difference between naive T cells in Tg and Wt (P < .05), whereas * indicates differences between memory phenotype in Tg and Wt (P < .05). (C) The number of each IEL population recovered from small intestine of the indicated mice. Error bars represent standard deviation (SD) from a representative experiment; values from Tg mice are not significantly different than those from IL-15Rα−/− mice. (D) Total numbers of myeloid populations in the spleen of the indicated mice. Error bars represent SD, n = 2, for one representative experiment.
Phenotype of CD8 T cells as a function of CD11c-driven IL-15Rα expression. Lymphocytes from thymus, spleen (SP), lymph node (LN), lung (LG), and bone marrow (BM) were isolated from the mice indicated (6-8 weeks old) and stained for cell-surface markers to identify differences in T-cell populations. (A) Representative flow cytometry plots from thymus and spleen. (B) The percentage of CD8 T cells along with the proportion of naive (CD44low) and memory-phenotype (CD44hi) in various tissues. Results are averages with n = 10 mice/group for Tg-1. For Tg-2, n = 8 for spleen and n = 2 for other tissues. § represents a significant difference between naive T cells in Tg and Wt (P < .05), whereas * indicates differences between memory phenotype in Tg and Wt (P < .05). (C) The number of each IEL population recovered from small intestine of the indicated mice. Error bars represent standard deviation (SD) from a representative experiment; values from Tg mice are not significantly different than those from IL-15Rα−/− mice. (D) Total numbers of myeloid populations in the spleen of the indicated mice. Error bars represent SD, n = 2, for one representative experiment.
Upon extending our analysis to peripheral tissues, differences in the CD8 T cells between the different groups of mice were apparent. As previously reported, the percentage of CD8 T cells in the IL-15Rα−/− mice is decreased by approximately 50% compared with Wt mice,16 a trend observed not only in the spleen but also in the lymph node, lung, and bone marrow (Figure 2A,B). In both CD11c-IL-15Rα Tg lines, the percentages of splenic CD8 T cells were increased compared with those observed in the IL-15Rα−/− mice (∼9% and 11% compared with only ∼6%), but were lower than in the Wt mice (∼12%; Figure 2A,B). This trend was seen consistently in the other tissues analyzed. Since the total CD8 T-cell population is comprised of both naive and memory-phenotype CD8 T cells and IL-15 preferentially affects the memory CD8 T cells,19 the effects of the restricted IL-15Rα expression on these 2 subpopulations was measured. For the naive CD8 T cells (CD44low), the presence of IL-15Rα+ DCs in Tg-1 increased the percentage of naive CD8 T cells from approximately 50%, as observed in IL-15Rα−/− mice, to between 62% and 71% of Wt values, depending on the tissue (Figure 2B); however, this effect was more dramatic in the Tg-2 line where the percentages of naive T cells were almost similar to levels seen in Wt mice. Among total CD8 T cells, percentages of memory-phenotype (CD44hi) CD8 T cells in both CD11c-IL-15Rα Tg mice approached parity with Wt levels in all tissues examined, ranging from 90% to 100% of Wt levels (Figure 2B). Although the percentage of memory CD8 T cells is almost recovered, a deficiency in the total levels of memory CD8 T cells still exists as a function of the deficit in naive CD8 T cells in CD11c-IL-15Rα Tg mice (Figure 2B). Altogether, these findings show that the CD11c-IL-15Rα transgenic environment partially restored the deficiencies in total CD8 T cells observed in the IL-15Rα mice with memory-phenotype CD8 T cells affected more than naive CD8 T cells.
Intestinal IELs, specifically TCRγδ and CD8ααTCRαβ cells, depend heavily on IL-15 for their normal development.16 To determine whether CD11c-driven expression of IL-15Rα+ affects IEL development, IELs were isolated from each group of mice and the respective populations were compared. Similar to IL-15Rα−/− mice, CD11c-IL-15Rα Tg-1 and Tg-2 mice were similarly deficient in TCRγδ and CD8ααTCRαβ cells whereas conventional CD8αβTCRαβ cells were present in normal numbers (Figure 2C). While there are clearly IL-15Rα+ cells present in the intestines, the inability of these cells to recover the defects in IEL development exemplifies the importance of cell specificity in responsiveness to transpresented IL-15.
Since IL-15Rα expression by DCs could indirectly affect CD8 T-cell development by altering the development and normal function of DCs, general characteristics of DCs were examined. Among lineage-negative cells, no differences in the number or distribution of various DC subsets were observed in spleen (Figure 2D) among the 4 groups of mice. In addition, MHC class II expression by CD11chiDCs was not different (data not shown). Altogether, elevated IL-15Rα expression did not alter the development or maturation of DCs.
IL-15Rα expression by DCs contributes to the generation of virus-specific memory CD8 T cells
We next measured the generation and maintenance of antigen-specific memory CD8 T cells in response to an acute viral infection in our transgenic system. In separate experiments, Wt, CD11c-IL-15Rα Tg-1, Tg-2, and IL-15Rα−/− mice were infected with 105 pfu VSV and the levels of N-tetramer+ CD8 T cells in the peripheral blood were measured at various times after infection. During the expansion phase, no detectable differences in the percent of VSV-specific T cells were observed between the groups of mice (Figure 3A), which is consistent with previous studies showing IL-15Rα is not required for normal Ag-specific CD8 T-cell expansion.3,4 Following the contraction phase (30 days after infection), the percentage of VSV-specific CD8 T cells present in the blood was increased in the CD11c-IL-15RαTg-1 mice compared with IL-15Rα−/− mice, but was lower than that of Wt mice (Figure 3A); this was in contrast to Tg-2 mice, which had levels similar to Wt mice (Figure 3B). During the memory phase, both CD11c-IL-15Rα Tg mice were able to maintain levels of VSV-specific CD8 T cells similar to Wt mice (Figure 3A,B). The discrepancy between the 2 Tg mice is best explained by the leaky expression of IL-15Rα expression onto CD11c− cells, therefore suggesting that transpresentation by cells other than DCs provide IL-15 to CD8 T cells during the contraction phase.
Role of IL-15Rα+ DCs in a viral immune response. The kinetics of an antiviral CD8 T-cell response was compared among different groups of mice by measuring the percent of VSV-specific CD8 T cells present in the peripheral blood as determined by N-tetramer staining: (A) CD11c-IL-15Rα Tg-1, (B) CD11c-IL-15Rα Tg-2. (C,D) Dot plots show the percent of N-tetramer+ cells versus CD44 expression after gating on CD8+ cells present in SP, LN, and LG 25 weeks after infection in Tg-1 (C) and Tg-2 (D). (E) Graphs show total numbers of N-tetramer+ cells recovered from the various tissues in Wt, Tg-1, and IL-15Rα−/− mice. Error bars represent SD, n = 2. Numbers above the bars represent percent of Wt mice. (F) KLRG-1 and CD27 expression on N-tetramer+ CD8 T cells in peripheral blood at indicated times after infection. (G) Histograms show CD62L expression by N-tetramer+ CD8 T cells in various tissues 25 weeks after infection.
Role of IL-15Rα+ DCs in a viral immune response. The kinetics of an antiviral CD8 T-cell response was compared among different groups of mice by measuring the percent of VSV-specific CD8 T cells present in the peripheral blood as determined by N-tetramer staining: (A) CD11c-IL-15Rα Tg-1, (B) CD11c-IL-15Rα Tg-2. (C,D) Dot plots show the percent of N-tetramer+ cells versus CD44 expression after gating on CD8+ cells present in SP, LN, and LG 25 weeks after infection in Tg-1 (C) and Tg-2 (D). (E) Graphs show total numbers of N-tetramer+ cells recovered from the various tissues in Wt, Tg-1, and IL-15Rα−/− mice. Error bars represent SD, n = 2. Numbers above the bars represent percent of Wt mice. (F) KLRG-1 and CD27 expression on N-tetramer+ CD8 T cells in peripheral blood at indicated times after infection. (G) Histograms show CD62L expression by N-tetramer+ CD8 T cells in various tissues 25 weeks after infection.
To determine whether tissue-specific effects were evident, the level of VSV-specific CD8 T cells was measured in the spleen, lymph nodes, and lung. In all the tissues analyzed, the percentage of VSV-specific memory CD8 T cells in CD11c-IL-15Rα Tg-1 was greater than in the IL-15Rα−/− mice, but less than in the Wt mice (Figure 3C). When IL-15Rα is present on both CD11c+ and CD11c− cells (Tg-2), the percentage of viral-specific CD8 T cells in the tissues was similar to that of Wt mice (Figure 3D). As expected, due to preexisting defects in total CD8 T cells, total numbers of Ag-specific CD8 T cells were also deficient (Figure 3E and data not shown). Overall, the effect of transpresentation by DCs in different tissues was commensurate with the trends observed in the blood, indicating no tissue-specific effects.
CD27, KLRG-1, IL-7Rα, and CD62L are cell-surface markers that have been used to define subpopulations of effector and memory CD8 T cells.22-24 During the transition from effector to memory T cell, CD27+ KLRG-1−, IL-7Rαhi CD8 T cells have been identified as those having a greater potential to differentiate into memory T cells. In contrast, KLRG-1+CD27−IL-7Rα− cells are thought to represent short-lived effectors that rely temporarily on IL-15 for their survival.22 Therefore, to determine whether IL-15Rα+ DCs alter the differentiation of these subsets, expression of CD27, KLRG-1, and IL-7Rα by antigen-specific CD8 T cells was analyzed at various times after infection in the CD11c-IL-15Rα Tg-1 mice. We chose to use the combination of CD27 and KLRG-1, as CD27−KLRG-1+ cells were more easily defined than IL-7Rα−KLRG-1+ cells. At the peak of the expansion (7 days after infection), no differences in the proportion of VSV-specific CD8 T cells defined by KRLG-1 and CD27 were observed between the different groups (Figure 3F). Interestingly, from 19 to 60 days after infection, the VSV-specific CD8 T cells in the IL-15Rα–deficient mice were predominantly CD27+KLRG-1−, whereas both CD27+KLRG-1− and CD27−KLRG-1+ populations were abundant at similar levels in both the CD11c-IL-15Rα and Wt mice (Figure 3F). Considering that all memory CD8 T cells use IL-15, our findings suggest that IL-15Rα+ DCs preferentially drive the survival of short-lived CD27− KLRG-1+ memory T cells, whereas other IL-15Rα+ cells may promote development of long-lived memory CD8 T cells.
Whereas KLRG-1, CD27, and IL-7Rα identify T-cell subsets during the transition from the effector phase to the memory phase, high CD62L expression on a subset of memory T cells is associated with the ability to migrate to lymphoid tissues and an enhanced self-renewal known as central memory.25,26 To determine whether CD11c-directed expression of IL-15Rα affects the generation of central and effector memory subsets, CD62L expression on VSV-specific memory CD8 T cells was examined. Depending on the tissue site of isolation, VSV-specific memory CD8 T cells had a similar pattern of CD62L expression among all groups of mice (Figure 3G and data not shown). This suggests that IL-15Rα did not impact the proportion of memory CD8 T-cell subsets, as defined by CD62L expression, that arise during an acute viral infection.
IL-15Rα+ DCs drive homeostatic proliferation of memory CD8 T cells
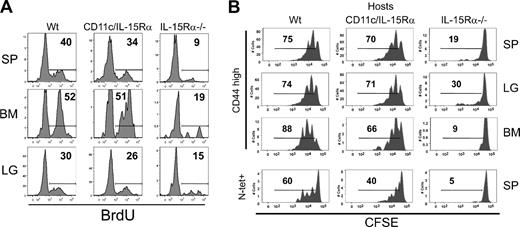
Whereas we demonstrated that IL-15Rα+ DCs alone were not completely sufficient in generating normal levels of memory CD8 T cells, it was unclear whether IL-15Rα+ DCs could still drive normal homeostatic proliferation. To examine the homeostatic proliferation of endogenous Ag-specific memory CD8 T cells, VSV-infected mice from each group (Wt, CD11c-IL-15Rα Tg-1, and IL-15Rα−/−) were given BrdU in their drinking water at 40 days after infection for 4 weeks. After this course of treatment, BrdU incorporation of the VSV-specific memory CD8 T cells in various tissues was measured. In all the tissues analyzed, VSV-specific CD8 T cells incorporated similar levels of BrdU in CD11c-IL-15Rα Tg-1 and Wt mice (Figure 4A). In contrast, VSV-specific memory CD8 T cells had little BrdU incorporation in the IL-15Rα−/− mice. Although the extent of BrdU incorporation was slightly different among tissues, the effect of the transgenic IL-15Rα+ by DCs drove almost normal levels of cell division. The similar BrdU incorporation in VSV-specific memory CD8 T cells between Wt and CD11c-IL-15Rα Tg-1 mice is evidence that IL-15Rα+ DCs are pivotal in mediating homeostatic proliferation of memory CD8 T cells.
Homeostatic proliferation of memory CD8 T cells. (A) Histograms show the percentage of VSV-N–specific memory CD8 T cells that have incorporated BrdU. (B) Spleen cells from normal, CD45.1+, VSV-infected mice were enriched for CD8 T cells, labeled with CFSE, and transferred to various CD45.2+ hosts. Two months after transfer, lymphocytes from SP, LG, and BM were analyzed for CFSE intensity. Top panels show CFSE dilution in CD45.1+ CD44hi CD8+ T cells (memory-phenotype) in various tissues. Bottom row depicts CFSE dilution in splenic N-tetramer+ cells.
Homeostatic proliferation of memory CD8 T cells. (A) Histograms show the percentage of VSV-N–specific memory CD8 T cells that have incorporated BrdU. (B) Spleen cells from normal, CD45.1+, VSV-infected mice were enriched for CD8 T cells, labeled with CFSE, and transferred to various CD45.2+ hosts. Two months after transfer, lymphocytes from SP, LG, and BM were analyzed for CFSE intensity. Top panels show CFSE dilution in CD45.1+ CD44hi CD8+ T cells (memory-phenotype) in various tissues. Bottom row depicts CFSE dilution in splenic N-tetramer+ cells.
The possibility exists that the ability of memory CD8 T cells to undergo homeostatic proliferation is affected earlier in the immune response by the transgenic expression of IL-15Rα. We therefore examined the proliferation of VSV-specific memory CD8 T cells generated in a normal environment. CD8 T cells were enriched from congenic Wt mice (CD45.1+) previously infected with VSV, CFSE-labeled, and adoptively transferred into CD11c-IL-15Rα Tg-1, Wt, and IL-15Rα−/− mice (all CD45.2+). Approximately 2 months later, CFSE dilution of the donor CD8 T cells was measured. In both Tg and Wt mice, the memory-phenotype CD8 T cells divided 2 to 3 times in the spleen, lung, and BM in a similar manner (Figure 4B). Similar results were observed with the CD11c-IL-15Rα Tg-2 line (data not shown). In contrast, the IL-15Rα−/− host was unable to support division of the memory-phenotype CD8 T cells (Figure 4B). Among the VSV-specific memory CD8 T cells and memory CD8 T cells in the BM, both the Wt and Tg mice were able to induce cell division; however, cell division was greater in the Wt than in the Tg hosts (Figure 4B). Overall, the exclusive expression of IL-15Rα to DCs provides a sufficient signal for memory CD8 T cells to proliferate under homeostatic conditions.
IL-15Rα+ DCs are not the only hematopoietic cells participating in homeostatic proliferation of memory CD8 T cells
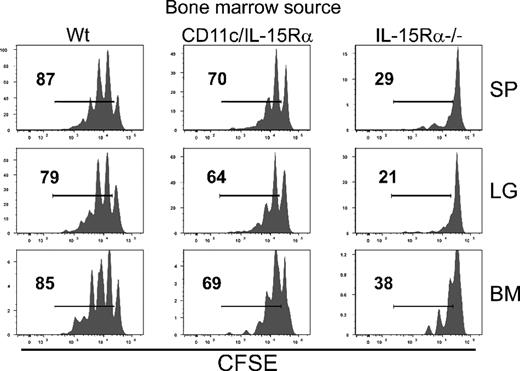
The homeostatic proliferation of memory CD8 T cells was almost completely recovered in mice that express IL-15Rα exclusively by CD11c+ cells (Figure 4B), indicating that other cell types transpresent IL-15 to memory CD8 T cells. Since our previous studies showed that IL-15Rα by parenchymal cells could play a minor role in homeostatic proliferation,7 we wanted to determine if a host containing IL-15Rα+ DCs along with IL-15Rα+ parenchymal cells would be sufficient to completely restore the homeostatic proliferation of memory CD8 T cells. Therefore, 3 groups of BM chimeras were generated with each group containing different combinations of IL-15Rα on either DCs and/or parenchymal cells: (1) Wt BM in Wt hosts (IL-15Rα expressed by all cells); (2) CD11c-IL-15RTg-1 in Wt hosts (IL-15Rα expressed by DCs and parenchymal cells); (3) IL-15Rα−/− BM in Wt hosts (IL-15Rα expressed only by parenchymal cells). After reconstitution, CD8 T cells (∼10 × 106) from a cohort of VSV-infected Thy1.1+ mice (at least 40 days after infection) were isolated, labeled with CFSE, and transferred into the various groups of chimeras. Six weeks later, homeostatic proliferation of the donor memory CD8 T cells was assessed by measuring CFSE dilution. Memory CD8 T cells divided 1 to 3 times in the Wt chimeras (Wt into Wt), representing the normal level of homeostatic proliferation over this time period (Figure 5). In the chimeras containing IL-15Rα+ DCs along with IL-15Rα+ parenchymal cells (CD11c-IL-15RαTg-1 into Wt), the memory CD8 T cells divided but not to same extent as in the Wt chimeras (Figure 5). Little cell division of memory CD8 T cells was observed in the chimeras containing IL-15Rα only in the parenchyma (IL-15Rα−/− into Wt; Figure 5). These differences indicate that homeostatic proliferation of memory CD8 T cells is not completely restored in the presence of IL-15Rα+ DCs and parenchymal cells and suggest that IL-15Rα+ hematopoietic cells other than DCs participate in the response.
Contribution of CD11c− hematopoietic cells in memory CD8 T-cell homeostasis. BM cells were isolated from IL-15Rα−/−, CD11c-IL-15RαTg-1, and Wt mice (all CD45.2+) and transferred into lethally irradiated Wt hosts (CD45.1+). CD8 T cells were isolated from VSV-infected Thy1.1+ cells, labeled with CFSE, and transferred into the various BM chimeras after lymphocyte reconstitution (between 8 and 12 weeks after BM transfer). Six weeks after transfer, CFSE dilution of donor cells was measured.
Contribution of CD11c− hematopoietic cells in memory CD8 T-cell homeostasis. BM cells were isolated from IL-15Rα−/−, CD11c-IL-15RαTg-1, and Wt mice (all CD45.2+) and transferred into lethally irradiated Wt hosts (CD45.1+). CD8 T cells were isolated from VSV-infected Thy1.1+ cells, labeled with CFSE, and transferred into the various BM chimeras after lymphocyte reconstitution (between 8 and 12 weeks after BM transfer). Six weeks after transfer, CFSE dilution of donor cells was measured.
Discussion
Generating a model with DC-restricted IL-15Rα expression has allowed us to demonstrate that DCs are not only important for presenting antigen for T-cell stimulation but also for presenting IL-15 during later stages of CD8 T-cell differentiation. This function of DCs in memory CD8 T-cell homeostasis had been speculated but never demonstrated. Our study has also demonstrated that IL-15 transpresentation by DCs has specific roles during CD8 T-cell differentiation in that DCs were most effective at inducing homeostatic proliferation of established memory CD8 T cells, while having less of a role in generating memory CD8 T cells and the homeostasis of naive CD8 T cells. In contrast, transpresentation by DCs had no effect on IEL development. In concurrent analysis of our model, we have also found that IL-15 transpresentation by DCs heavily influenced NK-cell development while only minimally affecting NK T-cell development (E.F.C. and K.S.S., manuscript in preparation). Altogether, our study has shown that cell specificity is pivotal in dictating IL-15 responsiveness by transpresentation and that DCs are an integral cell type transpresenting IL-15 to memory CD8 T cells.
The enhanced ability of DCs to transpresent IL-15 as differentiation progresses could be an indication that developing memory CD8 T cells become more accessible to DCs and preferentially receive IL-15 signals in a DC-enriched environment. This is contradictory to studies touting BM as the predominant site for memory CD8 T homeostasis, as BM is a site with a very low frequency of DCs. The importance of BM in providing homeostatic signals is based on the presence of memory CD8 T cells undergoing a higher rate of cell division,27-29 which could be explained by preferential migration of actively dividing central memory CD8 T cells. If BM is the predominant site for memory CD8 T homeostasis, we would predict an exaggerated defect in homeostatic proliferation of memory CD8 T cells in the BM of our Tg model, where only DCs transpresent IL-15. On the contrary, homeostatic proliferation of memory CD8 T cells was restored rather efficiently in the BM of the CD11c-IL-15Rα Tg mice, suggesting that memory CD8 T cells received adequate IL-15 signals elsewhere and then preferentially migrated to the BM. Moreover, memory CD8 T cells that are sequestered in the spleen and lymph node with FTY720 treatment undergo normal homeostatic proliferation despite this tissue restriction.30 This, along with our findings, suggests that memory CD8 T cells efficiently receive homeostatic signals in many tissues besides the BM.
In addition to the specific effects on the homeostatic proliferation of established memory CD8 T cells, DCs appeared to have a distinct role in generating memory CD8 T cells. During the contraction phase, IL-15 begins to become important by mediating survival of Ag-specific CD8 T cells, as these cells are not proliferating.31 Specifically, a subpopulation of short-lived effectors (SLECs), defined as KLRG-1+, are predominantly affected by the lack of IL-15.32 In our model, IL-15 transpresentation by DCs limited the contraction of the Ag-specific memory CD8 T cells by increasing the proportion of SLEC-like cells. Considering all memory CD8 T cells use IL-15, our findings yield 2 possible scenarios: SLECs may be more responsive or more accessible to DCs providing IL-15.
The limited requirements for DCs in transpresenting IL-15 to naive and differentiating memory CD8 T cells highlights the idea that cells other than DCs are providing IL-15 to CD8 T cells during these earlier phases. This is not surprising, as previous studies using BM chimeras indicated that IL-15Rα expression by parenchymal cells is important for generation of naive and memory CD8 T cells.7,12 Our current studies provide further evidence for this, as IL-15 transpresentation by DCs cannot assume this role of the parenchyma. When IL-15Rα expression was restricted to DCs, the contraction of the Ag-specific CD8 T cells was more exaggerated, resulting in less memory CD8 T cells generated; however, when IL-15Rα expression was present on DCs along with other cell types, the contraction and generation of memory CD8 T cells was normal. Therefore, our study provides evidence that cells other than DCs, which could include CD11c− hematopoietic cells and parenchymal cells, can also participate in the generation of memory CD8 T cells in vivo.
The incomplete abilities of DCs to transpresent IL-15 to CD8 T cells was not only present during the generation of memory CD8 T cells, but could also be observed to a small degree during the homeostasis of memory CD8 T cells. While parenchymal cells can have a minor contribution in homeostatic proliferation,7 the combination of IL-15Rα on both DCs and parenchyma did not completely restore normal levels of homeostatic proliferation, providing evidence that hematopoietic cells other than DCs transpresent IL-15 to memory CD8 T cells during the memory phase.
In our model, the sheer effectiveness of DCs in transpresenting IL-15 to CD8 T cells could indicate that DCs are superior at transpresentation; however, this could be due to the imprecise specificity of the transgenic promoter. Whereas high expression of CD11c is specific to DCs, low levels of CD11c are expressed on non-DCs, such as activated T cells, NK cells, and IELs.33 In our Tg models, even when some IL-15Rα is expressed on these cells, it was not accompanied by IL-15 expression. As IL-15 is not transferred from other transpresenting cells even under inflammatory circumstances,11 this IL-15Rα does not appear to be transpresenting IL-15. Furthermore, since previous studies have shown that IL-15Rα expression by T cells is dispensable for generation and maintenance of memory CD8 T cells and the IL-15–dependent development of IELs,6,7,12 the IL-15Rα expression that is expressed by T cells is likely irrelevant.
Another caveat of our model are the supraphysiologic levels of IL-15Rα present on DCs that could make the DCs more efficient. Indeed, recent reports demonstrate that the presence of IL-15Rα can stabilize IL-15 protein and increase bioactivity34 ; however, our studies do not support the idea that higher IL-15Rα translates to more IL-15 transpresentation, as IL-15Rαhi DCs did not have higher levels of IL-15 than IL-15Rαlo DCs. Altogether, our observations suggest that IL-15 expression appears to be endogenously controlled and is the limiting factor in transpresentation.
In summary, we have developed an in vivo model whereby DCs are the only cell able to transpresent IL-15. Using this model, DCs are shown to effectively mediate the generation and maintenance of memory CD8 T cells via IL-15 transpresentation, thus identifying a novel function of DCs. Lastly, the inability of IL-15 transpresented by DCs to recover all of IL-15 functions in CD8 T cells is a sterling example of how cell specificity dictates responsiveness to transpresented IL-15. In general, these findings will enable future studies to better focus on mechanisms regulating T-cell homeostasis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Bhavin Shah and Luis Acero for technical assistance, the Genetically Engineered Mouse Facility at the University of Texas M. D. Anderson Cancer Center, Thomas Brocker (Institute for Immunology, Ludwig-Maximilian University, Munich, Germany) for generously providing the CD11c promoter construct, and the NIH tetramer facility for providing tetramer. We also thank Willem Overwijk and Loredana Frasca (M. D. Anderson Cancer Center) for critical reading of the manuscript.
This research was supported by National Institutes of Health grant AI070910 and the M.D. Anderson Trust Fellowship (K.S.).
National Institutes of Health
Authorship
Contribution: S.W.S. performed a majority of the experiments, analyzed and interpreted data, and cowrote the manuscript; L.J.M. and E.F.C. performed some experiments and analyzed data; and K.S.S. designed, analyzed, and interpreted data and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kimberly S. Schluns, Department of Immunology, M/C 901, UT MDACC, PO Box 301429, Houston, TX 77030; e-mail: kschluns@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal