Abstract
We sought to determine whether thrombophilic defects increase recurrent venous thromboembolism (VTE) during warfarin therapy. Six hundred sixty-one patients with unprovoked VTE who were randomized to extended low-intensity (international normalized ratio [INR], 1.5-1.9) or conventional-intensity (INR, 2.0-3.0) anticoagulant therapy were tested for thrombophilia and followed for a mean of 2.3 years. One or more thrombophilic defects were present in 42% of patients. The overall rate of recurrent VTE was 0.9% per patient-year. Recurrent VTE was not increased in the presence of factor V Leiden (hazard ratio [HR], 0.7; 95% CI, 0.2-2.6); the 20210G>A prothrombin gene mutation (HR, 0); antithrombin deficiency (HR, 0); elevated factor VIII (HR, 0.7; 95% CI, 0.1-5.4); elevated factor XI (HR, 0.7; 95% CI, 0.1-5.0), or elevated homocysteine (HR, 0.7; 95% CI, 0.1-5.3), but showed a trend to an increase with an antiphospholipid antibody (HR, 2.9; 95% CI, 0.8-10.5). Compared with patients with no thrombophilic defects, the rate of recurrence was not increased in the presence of one (HR, 0.7; 95% CI, 0.2-2.3) or more than one (HR, 0.7; 95% CI, 0.2-3.4) defect. We conclude that single or multiple thrombophilic defects are not associated with a higher risk of recurrent VTE during warfarin therapy.
Introduction
More than a third of patients with unprovoked venous thromboembolism (VTE) have hereditary or acquired biochemical predispositions to thrombosis, often termed “thrombophilia.”1-3 Thrombophilia has 3 potentially important implications.4-6 First, thrombophilia might increase the risk of recurrent VTE during treatment, which would suggest the need for a more intense level of anticoagulant or an alternative anticoagulant. Second, thrombophilia might increase the risk of recurrent VTE after treatment is stopped, which would suggest the need for a longer duration of anticoagulant therapy. Third, because many thrombophilias are inherited in an autosomal dominant fashion, their presence may have implications for family counseling. Current evidence suggests that the presence of a thrombophilic defect is not a clinically important risk factor for recurrent VTE after anticoagulant therapy is stopped.1,7 Few studies have evaluated whether thrombophiliac defects are risk factors for recurrent VTE during anticoagulant therapy.
The Extended Low-intensity Anticoagulation for unprovoked Thromboembolism (ELATE) trial was a randomized comparison of low-intensity (target international normalized ratio [INR], 1.5-1.9) and conventional-intensity (target INR, 2.0-3.0) anticoagulation for prevention of recurrent VTE in patients with unprovoked VTE who had completed at least 3 months of conventional-intensity therapy.8 In this study, we found that low-intensity therapy was less effective at preventing recurrent VTE, providing us with an opportunity to determine whether hereditary or acquired thrombophilia is associated with an increased risk of recurrent VTE during warfarin therapy. The current substudy was prospectively planned and was separately funded by the Canadian Institute of Health Research (Ottawa, ON). Most episodes of recurrent VTE in the ELATE trial occurred in patients who were treated with low-intensity therapy. Because most episodes of recurrent VTE during warfarin therapy occur while the INR is subtherapeutic (ie, less than INR 2.0), and as the INR is subtherapeutic about 20% of the time during warfarin therapy, whether thrombophilia increases the risk of recurrent VTE when warfarin is subtherapeutic is clinically important.9
Methods
The design and main results of the ELATE trial have previously been described in detail.8 In brief, patients were eligible for ELATE if they had completed at least 3 months of conventional-intensity warfarin therapy for one or more episodes of unprovoked proximal deep vein thrombosis or pulmonary embolism and did not have either a requirement for, or a contraindication to, conventional-intensity anticoagulant therapy. Patients who were known to have an antiphospholipid antibody were ineligible; however, screening for an antiphospholipid antibody was not required to determine eligibility. Patients with other thrombophilic defects, as determined by genetic or coagulation testing, were eligible for ELATE. To be included in the current analysis, patients needed to have provided a blood specimen at the time of enrollment, and to have been receiving warfarin therapy during follow-up (patients were censored from the time that warfarin was stopped). The ELATE trail and the current analysis were approved by the institutional review boards of all participating clinical centers, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Treatment, follow-up, and clinical outcomes
Using a double-blind design, patients were randomized to receive either long-term low-intensity (target INR, 1.5-1.9) or conventional-intensity (target INR, 2.0-3.0) warfarin therapy.8 INR monitoring was performed by clinical center personnel and the interval between INR measurements left to their discretion. Patients were seen every 6 months and were told to report to the center immediately if they developed symptoms suggestive of venous thromboembolism or had evidence of bleeding. Recurrent venous thromboembolism was objectively confirmed and all suspected outcome events and deaths were classified by a central adjudication committee whose members were unaware of treatment group assignments and the results of thrombophilia testing.
Laboratory assays
All thrombophilia assays were performed in a central laboratory by technologists who were unaware of treatment-group assignment and clinical course. The results of laboratory testing were not made available to the clinical centers during the study. The following assays were performed (cutoff values that were used to define an abnormal or elevated level were selected before performing analyses).
Factor V Leiden.
Factor V Leiden was identified as described by Bertina et al.10 Positive results were subclassified as heterozygous or homozygous for the mutation.
Prothrombin gene variant.
The 20210G>A prothrombin gene was identified as described by Poort et al.11 Positive results were subclassified as heterozygous or homozygous for the mutation.
Antithrombin.
Antithrombin activity was measured using an amidolytic assay as described by Andersson et al.12 A value of less than 0.79 U/mL was considered indicative of antithrombin deficiency.
Lupus anticoagulant.
The presence of a lupus anticoagulant was identified using 2 assays, each combined with a confirmatory test if the initial screening test result was positive. The first assay uses a lupus anticoagulant–sensitive activated partial thromboplastin time reagent (PTT LA; Diagnostica Stago, Abbott, Mississauga, ON), an abnormal test being confirmed using a hexagonal phospholipid assay (Sta-clot LA; Diagnostica Stago, Abbott).13 The second assay measures the clotting time after addition of dilute Russell viper venom, an abnormal test being confirmed by demonstration of normalization of results after addition of a high concentration of phospholipid (American Diagnostica, Montreal, QC).14 If either of the assays was abnormal and the confirmatory test provided evidence of normalization of the test results, patients were considered to have a lupus anticoagulant.
Anticardiolipin antibody.
The presence of an anticardiolipin antibody, either IgG or IgM, was determined using enzyme immunoassays (Corogenix; Med-Ox Diagnostics, Nepean, ON) as described by Harris et al.15 An anticardiolipin antibody was considered present if either the IgG or IgM antibody titer was more than 5 standard deviations above the normal mean.
Homocysteine.
Total plasma homocysteine was measured using a fluorescence polarization immunoassay on an Imx analyzer (Abbott Laboratories Diagnostic Division, Abbott Park, IL) as described by Boushey et al.16 A homocysteine level above the 90th percentile for patients in this study (> 15.4 μM) was considered elevated.
Factor VIII and XI levels.
Levels of factors VIII and XI were measured using a one-stage, activated partial thromboplastin time based clotting assay (Thrombosil; Beckman Coulter, Mississauga, ON) adapted from Biggs.17 A level above the 90th percentile for patients in this study (> 2.59 U/mL for factor VIII and > 1.93 U/mL for factor XI) was considered elevated.
Statistical analysis
Kaplan-Meier curves were used to display the cumulative proportion of patients, with and without thrombophilia defects, who had recurrent VTE during follow up. All patients who were receiving warfarin (low- and conventional-intensity groups) were included in these analyses. Patients in both treatment groups were censored (ie, not included in the analysis) from the time that they stopped warfarin therapy. Cox proportional hazards modeling of VTE recurrence, without adjustment for potential covariates, was used to estimate hazard ratios and their 95% confidence intervals, and to assess the influence of thrombophilic abnormalities and treatment group, and interactions between these variables. Average recurrence rates were calculated by dividing the number of patients with recurrence by the patient-years at risk; confidence intervals for rates are based on the Poisson distribution. SAS (Cary, NC) version 9.1 was used for all calculations. All reported P values are 2-sided.
Results
A total of 738 patients from 16 clinical centers were enrolled in the ELATE trial.8 Relevant to the current analysis, of the 145 patients who met the inclusion criteria for the ELATE trial, 2.1% (31 patients) were excluded from participation because of the presence of a known antiphospholipid antibody (testing for antiphosphospholipid antibodies was not routine, and the proportion of patients that met the inclusion criteria who were tested is not known).8 Of the 738 patients who were enrolled in the ELATE trial, 661 patients (90%) are included in the current analysis (all thrombophilia tests could not be performed on all 661 patients, usually because of inadequate blood samples). A total of 77 patients in ELATE did not take part in this substudy because 58 did want to participate, 6 were unintentionally not asked to participate, and 4 had inadequate blood samples. The reasons for not including an additional 9 patients were not recorded. These 77 patients were similar to those included in this analysis (Table 1) in terms of age (57 vs 57; P = .8), duration of treatment before randomization (10.6 vs 12.4 months; P = .4), and treatment allocation (42% vs 51% allocated to low-intensity therapy; P = .2), but there was a higher proportion of females (56% vs 44%; P = .05). Two of the 77 patients who did not participate in this substudy had a recurrent episode of thrombosis. Both were patients who were allocated to low-intensity anticoagulant therapy and had stopped warfarin.
Baseline characteristics of participants
| Characteristic . | Low intensity, n = 337 . | Conventional intensity, n = 324 . | Total, N = 661 . |
|---|---|---|---|
| Mean age, y (SD) | 57 (15) | 57 (15) | 57 (15) |
| Female sex, n (%) | 140 (42) | 149 (46) | 289 (44) |
| Previous episodes of VTE, n (%) | |||
| 1 | 90 (27) | 86 (27) | 176 (27) |
| 2 | 172 (51) | 165 (51) | 337 (51) |
| 3 | 49 (15) | 57 (18) | 106 (16) |
| 4 or more | 26 (8) | 15 (5) | 41 (6) |
| Treated for 3-4 months before enrollment, n (%) | 133 (39) | 130 (40) | 263 (40) |
| Months of warfarin (INR 2.0-3.0) before enrollment, mean (SD) | 12.0 (16.5) | 12.8 (18.3) | 12.4 (17.4) |
| Most recent type of VTE, n (%) | |||
| Deep vein thrombosis only | 231 (69) | 207 (64) | 438 (66) |
| Pulmonary embolism | 106 (31) | 117 (36) | 223 (34) |
| Factor V Leiden, n/N (%) | 91/328 (27.7) | 80/318 (25.2) | 171/646 (26.5) |
| Heterozygous | 84 (25.6) | 77 (24.2) | 161 (24.9) |
| Homozygous | 7 (2.1) | 3 (1.0) | 10 (1.6) |
| Prothrombin gene mutation, n/N (%) | 32/327 (9.8) | 28/317 (8.8) | 60/644 (9.3) |
| Heterozygous | 32 (9.8) | 26 (8.2) | 58 (9.0) |
| Homozygous | 0 (0) | 2 (0.6) | 2 (0.3) |
| Antiphospholipid antibody, n/N (%) | 28/337 (8.3) | 26/324 (8.0) | 54/661 (8.2) |
| Anticardiolipin antibody | 20 (5.9) | 20 (6.2) | 40 (6.1) |
| Lupus anticoagulant | 9 (2.7) | 10 (3.1) | 19 (2.9) |
| Antithrombin deficiency, n/N (%) | 15/329 (4.6) | 8/316 (2.5) | 23/645 (3.6) |
| Elevated factor VIII, n/N (%) | 26/335 (7.8) | 41/324 (12.7) | 67/659 (10.2) |
| Elevated factor XI, n/N (%) | 28/331 (8.5) | 38/316 (12.0) | 66/647 (10.2) |
| Elevated homocysteine, n/N (%) | 27/325 (8.3) | 37/313 (11.8) | 64/638 (10.0) |
| No. of abnormalities, n (%) | |||
| None | 147 (43.6) | 133 (41.0) | 280 (42.4) |
| 1 | 137 (40.6) | 133 (41.0) | 270 (40.8) |
| 2 | 44 (13.1) | 47 (14.6) | 91 (13.8) |
| 3 | 7 (2.1) | 8 (2.5) | 15 (2.3) |
| 4 | 2 (0.6) | 3 (0.9) | 5 (0.8) |
| Characteristic . | Low intensity, n = 337 . | Conventional intensity, n = 324 . | Total, N = 661 . |
|---|---|---|---|
| Mean age, y (SD) | 57 (15) | 57 (15) | 57 (15) |
| Female sex, n (%) | 140 (42) | 149 (46) | 289 (44) |
| Previous episodes of VTE, n (%) | |||
| 1 | 90 (27) | 86 (27) | 176 (27) |
| 2 | 172 (51) | 165 (51) | 337 (51) |
| 3 | 49 (15) | 57 (18) | 106 (16) |
| 4 or more | 26 (8) | 15 (5) | 41 (6) |
| Treated for 3-4 months before enrollment, n (%) | 133 (39) | 130 (40) | 263 (40) |
| Months of warfarin (INR 2.0-3.0) before enrollment, mean (SD) | 12.0 (16.5) | 12.8 (18.3) | 12.4 (17.4) |
| Most recent type of VTE, n (%) | |||
| Deep vein thrombosis only | 231 (69) | 207 (64) | 438 (66) |
| Pulmonary embolism | 106 (31) | 117 (36) | 223 (34) |
| Factor V Leiden, n/N (%) | 91/328 (27.7) | 80/318 (25.2) | 171/646 (26.5) |
| Heterozygous | 84 (25.6) | 77 (24.2) | 161 (24.9) |
| Homozygous | 7 (2.1) | 3 (1.0) | 10 (1.6) |
| Prothrombin gene mutation, n/N (%) | 32/327 (9.8) | 28/317 (8.8) | 60/644 (9.3) |
| Heterozygous | 32 (9.8) | 26 (8.2) | 58 (9.0) |
| Homozygous | 0 (0) | 2 (0.6) | 2 (0.3) |
| Antiphospholipid antibody, n/N (%) | 28/337 (8.3) | 26/324 (8.0) | 54/661 (8.2) |
| Anticardiolipin antibody | 20 (5.9) | 20 (6.2) | 40 (6.1) |
| Lupus anticoagulant | 9 (2.7) | 10 (3.1) | 19 (2.9) |
| Antithrombin deficiency, n/N (%) | 15/329 (4.6) | 8/316 (2.5) | 23/645 (3.6) |
| Elevated factor VIII, n/N (%) | 26/335 (7.8) | 41/324 (12.7) | 67/659 (10.2) |
| Elevated factor XI, n/N (%) | 28/331 (8.5) | 38/316 (12.0) | 66/647 (10.2) |
| Elevated homocysteine, n/N (%) | 27/325 (8.3) | 37/313 (11.8) | 64/638 (10.0) |
| No. of abnormalities, n (%) | |||
| None | 147 (43.6) | 133 (41.0) | 280 (42.4) |
| 1 | 137 (40.6) | 133 (41.0) | 270 (40.8) |
| 2 | 44 (13.1) | 47 (14.6) | 91 (13.8) |
| 3 | 7 (2.1) | 8 (2.5) | 15 (2.3) |
| 4 | 2 (0.6) | 3 (0.9) | 5 (0.8) |
Factor V Leiden was the most common thrombophilic defect with a prevalence of 26.5%. The 20210G>A prothrombin gene was present in 9.3%, an antiphospholipid antibody was present in 8.2%, and antithrombin deficiency was present in 3.6% of patients (Table 1). By definition (ie, above the 90th percentile for study population), 10% of patients were categorized as having elevated levels of each of factor VIII (> 2.59 U/mL), factor XI (> 1.93 U/mL), and homocysteine (> 15.4 μM). Of the 661 patients, 42.4% had no abnormality, 40.8% had 1 abnormality, 13.8% had 2 abnormalities, 2.3% had 3 abnormalities, and 0.8% had 4 abnormalities (homozygous states were considered as 2 abnormalities).
Among the 661 patients who participated in the current substudy, 6 patients (3 low-intensity and 3 conventional-intensity patients) had a recurrent episode of venous thromboembolism after warfarin therapy had been stopped; these episodes of recurrence are not included in the current analysis because they did not occur while patients were receiving warfarin.
Recurrent venous thromboembolism while on warfarin according to the presence or absence of thrombophilia
Mean duration of follow-up was 2.3 years and the overall rate of recurrent VTE was 0.9% per patient-year while patients were on warfarin (Table 2). The rate of recurrence was 1.5% per patient-year (11 events) among the 337 patients allocated to low-intensity therapy and 0.4% per patient-year (3 events) among the 324 patients allocated to conventional-intensity therapy (HR, 3.7; 95% CI, 1.03-13.2).
Recurrent venous thromboembolism in patients on low-intensity or conventional-intensity warfarin according to the presence of one or more thrombophilias
| Thrombophilia . | Recurrent venous thromboembolism . | ||
|---|---|---|---|
| Events/n . | Percentage per patient-year (95% CI) . | Hazard ratio (95% CI) . | |
| Factor V Leiden | 3*/171 | 0.8 (0.2-2.2) | 0.7 (0.2-2.6) |
| Prothrombin gene mutation | 0/60 | 0.0 (0.0-2.9) | 0 (nc) |
| Antiphospholipid antibody | 3†/54 | 2.3 (0.4-6.7) | 2.9 (0.8-10.5) |
| Antithrombin deficiency | 0/23 | 0.0 (0.0-7.0) | 0 (nc) |
| Factor VIII elevation | 1/67 | 0.7 (0.0-4.0) | 0.7 (0.1-5.4) |
| Factor XI elevation | 1/66 | 0.6 (0.0-3.5) | 0.7 (0.1-5.0) |
| Homocysteine elevation | 1/64 | 0.7 (0.0-3.9) | 0.7 (0.1-5.3) |
| No. of abnormalities | |||
| None | 7‡/280 | 1.1 (0.5-2.3) | 1.0 (referent) |
| 1 | 5§/270 | 0.8 (0.3-1.9) | 0.7 (0.2-2.3) |
| 2 | 2‖/91 | 1.0 (0.1-3.5) | 0.8 (0.2-4.1) |
| 1 or more | 7/381 | 0.8 (0.3-1.7) | 0.7 (0.3-2.0) |
| 2 or more | 2/111 | 0.8 (0.1-2.9) | 0.7 (0.2-3.4) |
| All patients | 14/661 | 0.9 (0.6-1.5) | |
| Thrombophilia . | Recurrent venous thromboembolism . | ||
|---|---|---|---|
| Events/n . | Percentage per patient-year (95% CI) . | Hazard ratio (95% CI) . | |
| Factor V Leiden | 3*/171 | 0.8 (0.2-2.2) | 0.7 (0.2-2.6) |
| Prothrombin gene mutation | 0/60 | 0.0 (0.0-2.9) | 0 (nc) |
| Antiphospholipid antibody | 3†/54 | 2.3 (0.4-6.7) | 2.9 (0.8-10.5) |
| Antithrombin deficiency | 0/23 | 0.0 (0.0-7.0) | 0 (nc) |
| Factor VIII elevation | 1/67 | 0.7 (0.0-4.0) | 0.7 (0.1-5.4) |
| Factor XI elevation | 1/66 | 0.6 (0.0-3.5) | 0.7 (0.1-5.0) |
| Homocysteine elevation | 1/64 | 0.7 (0.0-3.9) | 0.7 (0.1-5.3) |
| No. of abnormalities | |||
| None | 7‡/280 | 1.1 (0.5-2.3) | 1.0 (referent) |
| 1 | 5§/270 | 0.8 (0.3-1.9) | 0.7 (0.2-2.3) |
| 2 | 2‖/91 | 1.0 (0.1-3.5) | 0.8 (0.2-4.1) |
| 1 or more | 7/381 | 0.8 (0.3-1.7) | 0.7 (0.3-2.0) |
| 2 or more | 2/111 | 0.8 (0.1-2.9) | 0.7 (0.2-3.4) |
| All patients | 14/661 | 0.9 (0.6-1.5) | |
nc indicates not calculable.
Heterozygous in all 3 patients.
Lupus anticoagulant in 2 and anticardiolipin antibody in 1 patient.
One conventional intensity; 6 low intensity.
Two conventional intensity; 3 low intensity.
Two low intensity.
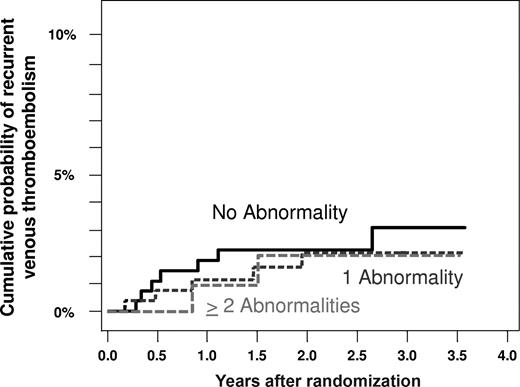
The absolute rates of recurrent VTE in patients with each of the 7 thrombophilic defects, and the associated hazard ratios compared with patients without each defect, are shown in Table 2 and Figure 1. The absolute rate of recurrent venous thromboembolism was less than 1% per patient-year (Figure 1), and the associated hazard ratio compared with patients without the same defect was less than 1.0 for patients with factor V Leiden, the 20210G>A prothrombin gene mutation, antithrombin deficiency, or elevated levels of factor VIII, factor XI, or homocysteine during warfarin therapy (Table 2). Based on 3 episodes of recurrence among 54 patients with an antiphospholipid antibody, the rate of recurrent VTE was 2.3% per patient-year (95% CI, 0.4-6.7) and the associated hazard ratio was 2.9 (95% CI, 0.8-10.5; Table 2, Figure 1). Comparison of all patients with any thrombophilic defect versus those without such defects yielded no evidence of a higher rate of recurrent VTE (HR, 0.7; 95% CI, 0.3-2.0). In addition, there was no evidence that patients with 2 or more thrombophilic defects had a higher rate of recurrent VTE compared with patients with no abnormality (HR, 0.7; 95% CI, 0.2-3.4), or that the presence of increasing numbers of thrombophilic defects was associated with a higher risk of recurrence (HR, 0.8 [95% CI, 0.4-1.6] per defect; Figure 2).
Absolute rates of recurrent venous thromboembolism in patients with unprovoked venous thrombosis receiving either low-intensity or conventional-intensity warfarin according to the type or number of thrombophilic defects.
Absolute rates of recurrent venous thromboembolism in patients with unprovoked venous thrombosis receiving either low-intensity or conventional-intensity warfarin according to the type or number of thrombophilic defects.
Cumulative probability of recurrent venous thromboembolism in patients with unprovoked venous thrombosis receiving either low-intensity or conventional-intensity warfarin according to the number of thrombophilic defects.
Cumulative probability of recurrent venous thromboembolism in patients with unprovoked venous thrombosis receiving either low-intensity or conventional-intensity warfarin according to the number of thrombophilic defects.
Efficacy of low-intensity compared with conventional-intensity anticoagulation in patients with and without a thrombophilic abnormality
The hazard ratio for recurrent VTE in patients allocated to low-intensity compared with conventional-intensity warfarin therapy was 5.6 (95% CI, 0.7-47) in the 280 patients with no thrombophilic defect and 2.7 (95% CI, 0.5-14.0) in the 381 patients with one or more defects (test of interaction for a difference in these HRs: P = .6).
Discussion
We found no evidence that the presence of thrombophilic defects, singly or in combination, was associated with an increased risk of recurrent VTE during extended-duration warfarin therapy. Other than factor V Leiden, the prevalence of individual abnormalities was low and, consequently, the precision of the estimates for relative risk and absolute risk of recurrent VTE for individual abnormalities was also low, as reflected by wide 95% confidence intervals for hazard ratios and absolute event rates. However, when the 7 thrombophilic defects are considered collectively, based on the upper border of the 95% confidence interval for the hazard ratio, we can reliably exclude a 2-fold increase in the risk of recurrent VTE in patients with one or more thrombophilic abnormalities compared with those with no abnormality. In addition, there was no suggestion that the presence of multiple thrombophilic abnormalities was associated with an increased risk of recurrence. Consistent with the overall findings of the ELATE trial, there was a very low absolute risk of recurrent VTE in patients with thrombophilia who were taking conventional-intensity warfarin therapy (0.4% per patient-year; 95% CI, 0.1-1.4). The relative risk of recurrent VTE with low-intensity compared with conventional-intensity warfarin was also similar in patients with and without thrombophilia, further suggesting that the presence of thrombophilia does not influence the efficacy of warfarin at preventing recurrent VTE. Because patients with known antiphospholipid antibodies were excluded from the study, and because there was a trend toward a higher rate of recurrent VTE among the 54 patients who had an antiphospholipid antibody, it is possible that the rates of recurrence during warfarin therapy are higher in patients with an antiphospholipid antibody than in those without such an abnormality.
Consistent with our findings, the PREVENT study found that, compared with placebo, low-intensity warfarin therapy reduced the risk of recurrent VTE to a similar extent in patients with factor V Leiden or the 20210G>A prothrombin gene as it did in patients with neither abnormality.18 Several other studies have also reported low rates of recurrent VTE during conventional-intensity anticoagulant therapy in patients with factor V Leiden,19 in patients with rarer hereditary thrombophilias,20 and in patients with a first episode of unprovoked VTE3 or a second episode of VTE,21 many of whom are likely to have thrombophilia.
Limitations of this study include the small number of patients with individual thrombophilic abnormalities, thereby precluding precise estimates of the risk of recurrence with each defect, that patients were not assessed for deficiencies of protein C or protein S because they were on warfarin therapy, which lowers levels of both proteins, and that recurrent VTE during initial therapy (ie, before enrollment in ELATE) was not assessed. Strengths of this study include that most patients enrolled in the ELATE trial participated, that the patient characteristics were similar in those who did and did not participate, that laboratory analyses were performed centrally by laboratory personnel blinded to clinical outcomes, that few patients were lost to follow up, and that outcomes were centrally adjudicated in a standardized way without knowledge of thrombophilia status.
In conclusion, with the possible exception of antiphospholipid antibodies, the presence of single or multiple thrombophilic abnormalities is not associated with a higher risk of recurrent VTE during warfarin therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Canadian Institutes of Health Research (Ottawa, ON) in the form of a Grant in Aid (MOP 37695) and a Team Grant in Venous Thromboembolism (FRN 79846). C.K. and M.R. were supported by the Heart and Stroke Foundation of Canada (Ottawa, ON). J.S.G. and J.I.W. were supported by the Heart and Stroke Foundation of Ontario (Toronto, ON). C.K., M.A.C., J.S.G., J.I.W., and P.W. were supported by the Canadian Institutes of Health Research. S.R.K. was supported by Fonds de la Recherche en Santé du Québec (Montréal, QC). M.J.K. was supported by the University of Western Ontario (London, ON). D.R.A. was supported by Dalhousie University (Halifax, NS).
Authorship
Contribution: C.K. was the principal investigator and wrote the first draft of the paper; C.K., J.A.J., M.J.K., D.R.A., P.W., B.M.K., M.G., and J.S.G. were the steering committee; C.K., M.J.K., D.R.A., P.W., J.S.G., J.I.W., M.A.C., S.D., A.G.T., W.G., S.S., P.N., C.D., S.R.K., J.K., M.R., and J.H. were involved with patient enrollment and care; J.A.J. performed the statistical analyses; and B.M.K. coordinated the study. All authors had access to all of the data and contributed to revision of the paper.
A list of the Extended Low-intensity Anticoagulation for Thromboembolism (ELATE) Investigators is available on the Blood website; see the Supplemental Appendix link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. Kearon, Hamilton Health Sciences, Henderson Division, 711 Concession Street, Hamilton, ON, L8V 1C3; e-mail: kearonc@mcmaster.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal