Abstract
Sirolimus is an effective agent used in graft-versus-host disease (GVHD) prophylaxis after allogeneic transplantation. It also has antiproliferative effects on vascular endothelium when used to coat coronary artery stents. We noted an excess of veno-occlusive disease (VOD) in a clinical trial, and retrospectively reviewed the records of 488 patients to determine the association between sirolimus and VOD. When used with cyclophosphamide/total body irradiation (Cy/TBI) conditioning, sirolimus is associated with an increased incidence of VOD (OR 2.35, P = .005). The concomitant use of methotrexate further increased this rate (OR 3.23, P < .001), while sirolimus without methotrexate was not associated with an increased risk of VOD (OR 1.55, P = .33). Mortality after VOD diagnosis was unaffected, and overall treatment-related mortality was lowest when sirolimus was used without methotrexate. Similar findings were noted in matched, related, and unrelated as well as mismatched donor subgroups. When used with busulfan-based conditioning, sirolimus use was associated with an even higher rate of VOD (OR 8.8, P = .008). Our findings suggest that sirolimus use is associated with VOD after TBI-based transplantation when used with methotrexate after transplantation. Sirolimus-based GVHD prophylaxis without methotrexate is associated with the greatest overall survival. Myeloablative doses of busulfan should not be used with sirolimus-based immunosuppression.
Introduction
Sirolimus is the first available inhibitor of the mammalian Target of Rapamycin (mTOR), a central molecule in eukaryotic homeostasis. As such, the drug has numerous effects on diverse tissues; however, cells of the immune system are its primary target. Sirolimus has been widely used as an immunosuppressant in solid organ transplantation to prevent immune-mediated graft rejection.1-4 More recently, sirolimus has been used in hematopoietic stem cell transplantation (HSCT).5,6 In phase II trials in matched related and unrelated donor transplantation, the substitution of sirolimus for methotrexate was associated with 100-day rates of grade II-IV acute graft-versus-host disease (GVHD) of 20.5% with 100-day TRM of 4.8%.6 In addition, the use of sirolimus in these and other studies was associated with a reduction of cytomegalovirus (CMV) viremia,7 and a reduction in the incidence and severity of oral mucositis.8
Sirolimus is also commonly used to coat endovascular stents used after balloon dilation of an occluded coronary artery.9 The mechanism of action of sirolimus in this setting is to block the proliferative responses of vascular smooth muscle cells, fibroblasts, and endothelial cells to cytokines such as basic fibroblast growth factor (bFGF), platelet-derived growth factor, vascular endothelial cell growth factor (VEGF), hypoxia, and transforming growth factor-β after mechanical injury.10-12 Some of the endovascular side effects of sirolimus are encountered in clinical transplantation. We previously reported an increased incidence of thrombotic microangiopathy after sirolimus-based GVHD prophylaxis in HSCT,13 and there are numerous reports of sirolimus-mediated vascular side effects in solid organ transplantation as well. For example, a perceived increased incidence of hepatic artery and portal vein thrombosis caused the temporary interruption of an international liver transplantation trial,14 although subsequent studies did not confirm this increased risk.15 In addition, there are numerous reports of delayed wound healing16,17 or increased rates of infection18 in other solid organ transplantation settings. Some of these complications may have been related to serum drug concentrations of sirolimus.15
Veno-occlusive disease (VOD, also referred to as sinusoidal obstruction syndrome [SOS]) of the liver is a common complication of myeloablative conditioning therapy and HSCT. It occurs with a frequency of 5% to 15%, and its known risk factors include the use of an unrelated donor stem cell source, prior hepatic injury, and advanced recipient age. The use of the alkylating agent, busulfan, has also been demonstrated to be a risk for VOD.19-22 The primary mechanism of injury in hepatic VOD is thought to be damage to hepatic sinusoidal endothelial cells and hepatocytes caused by the conditioning regimen.23 Due to a suggestion of an increase in VOD incidence in patients receiving sirolimus for GVHD prophylaxis, we conducted a retrospective analysis of our experience with sirolimus in patients undergoing myeloablative allogeneic HSCT to determine the association between sirolimus use and the risk of VOD.
Methods
This is a retrospective review of VOD incidence at the Dana-Farber Cancer Institute (DFCI) between January 1, 2000, and May 31, 2007. Patients included in this analysis underwent myeloablative transplantation using cyclophosphamide (Cy; 1800 mg/m2 × 2 days) in combination with total body irradiation (14 Gy in 7 fractions at a dose rate of 10 cGy/min with partial lung shielding; Cy/TBI). Patients who received peripheral blood stem cells or bone marrow from human leukocyte antigen (HLA)–matched siblings or matched and mismatched unrelated donors are included in this analysis. Patients with nonmalignant disorders and recipients of umbilical cord blood, autologous and syngeneic stem cells were excluded. All patients signed informed consent in accordance with the Declaration of Helsinki for research analyses pertaining to their transplantation outcomes. The Office for the Protection of Research Subjects at the Dana-Farber/Harvard Cancer Center provided Institutional Review Board approval for these studies.
A second analysis was performed to examine the smaller subset of patients who received busulfan at our institute during the same time period. Patients prospectively enrolled in the Blood and Marrow Transplant Clinical Trial Network (BMT CTN) Protocol 0402 (protocol document available at https://web.emmes.com/study/bmt) were combined with the DFCI cohort for this analysis. Patients from the BMT CTN trial all received busulfan (≥ 14 mg/kg oral busulfan or ≥ 11.2 mg/kg busulfan intravenously, or a targeted busulfan dosing strategy aimed at a serum concentration ≥ 600 ng/mL at steady state) with cyclophosphamide (Bu/Cy). DFCI patients were selected with the same algorithm as above; however, in this analysis all subjects received intravenous busulfan (12.8 mg/kg total with or without pharmacokinetic monitoring) in combination with cyclophosphamide (1800 mg/m2 × 2 days).
VOD definition
VOD was diagnosed using a combination of clinical, radiological, and pathological criteria. VOD was defined clinically according to the Baltimore criteria,24 which include a serum bilirubin more than or equal to 2 mg/dL and at least 2 of the following 3 clinical findings: (1) ascites, (2) weight gain more than or equal to 5% above baseline, and (3) new hepatomegaly. Ultrasound of the liver with Doppler flow analysis of the portal circulation was used in all cases to document reversal of portal flow. Where ambiguous, transjugular liver biopsy with ascertainment of transhepatic wedge pressures was performed. All cases of suspected or confirmed VOD were confirmed by chart review, and there was no upper time limit from transplantation for the diagnosis of VOD.
Statistical analysis
Two-sided Fisher exact test was performed to compare categorical variables. Continuous variables were compared by the 2-sided Wilcoxon rank-sum test. Multivariable exact logistic regression models were constructed to explore potential risk factors for VOD. Overall survival (OS) was defined as the time from transplantation to death from any cause. The method of Kaplan and Meier25 and the log-rank test26 were used to compare survival curves. Cox proportional hazards regression analysis was performed to estimate the risks while adjusting for differences in baseline transplantation characteristics. Transplantation risk was defined as “low” for patients with acute leukemia in first or subsequent remission, chronic myeloid leukemia in chronic phase, or refractory anemia with or without ringed sideroblasts. All others were considered “high” risk. Cumulative incidence curves were constructed for treatment-related mortality (TRM) with relapse (with or without death due to disease) as a competing risk and compared using the Gray test.27 TRM was defined as death due to any posttransplantation event or complication other than relapse. Patients without TRM or relapse were censored at the date last known alive. Competing risk regression analysis28 was performed to compare the risk of TRM and relapse between groups while adjusting for baseline transplantation characteristics. The interaction terms were tested in both Cox and competing risks regression analyses.
Results
DFCI Cy/TBI retrospective review
Between January 1, 2000, and May 31, 2007, 535 patients underwent myeloablative allogeneic stem cell transplantation using the Cy/TBI regimen at the DFCI. All patients received a calcineurin inhibitor, and 272 (51%) received sirolimus as part of their GVHD prophylaxis regimen. Initially, sirolimus patients were treated on clinical trials, and in later years, sirolimus was given at the discretion of the treating physician, according to locally defined treatment plans. Both clinical trial and treatment plan patients were required to have adequate measures of hepatic, renal, pulmonary, and cardiac function prior to transplantation. In all patients, sirolimus administration was uniform, with a 12-mg loading dose administered on day −3 and a daily dose thereafter of 4 mg, adjusted to attain a serum trough level of 3 to 12 ng/mL. Among sirolimus patients, 100% received tacrolimus (serum trough level 5-10 ng/mL), and 136 (50%) also received short-course methotrexate (5 mg/m2, days 1, 3, 6 after transplantation, total 15 mg/m2), while all non-sirolimus patients were scheduled to receive full-dose methotrexate (15 mg/m2 day 1, 10 mg/m2 on days 3 and 6 after transplantation, 45 mg/m2 total). Among non-sirolimus patients, 29 patients who received cyclosporine (11%) rather than tacrolimus, and an additional 18 patients who received other GVHD prophylactic regimens were excluded from further analysis. The final sample size for analysis was 488 patients.
The patients were divided into 3 groups based on GVHD prophylaxis. GVHD prophylaxis regimens were tacrolimus with methotrexate (Tac/Mtx), tacrolimus, sirolimus, and methotrexate (Tac/Sir/Mtx) and tacrolimus with sirolimus (Tac/Sir). Transplantation characteristics for the 3 groups are shown in Table 1. Significant differences were noted in the use of peripheral blood stem cells and the use of unrelated and mismatched donors. Patients in the Tac/Sir group all underwent transplantation after 2002. Grade II-IV acute GVHD was less common among sirolimus patients, and lowest in the Tac/Sir group (40% vs 32% vs 21%, P = .001). Similarly, grade III-IV acute GVHD was lowest in the Tac/Sir group (18% vs 17% vs 6%, P = .002). There were no differences in the rates of chronic GVHD (P = .83). The median follow-up time for all patients was 26 months.
Baseline characteristics of the cyclophosphamide/total body irradiation cohort
| . | Tac/Mtx . | Tac/Sir/Mtx . | Tac/Sir . | P . |
|---|---|---|---|---|
| Sample size | 216 | 136 | 136 | |
| Median age, y (range) | 41 (18, 60) | 43 (19, 63) | 43 (19, 60) | .44 |
| Sex, female | 96 (44) | 51 (38) | 65 (48) | .21 |
| Donor-recipient sex match | ||||
| M-M | 72 (33) | 56 (41) | 45 (33) | |
| M-F | 48 (22) | 29 (21) | 26 (19) | |
| F-M | 44 (20) | 25 (18) | 34 (25) | |
| F-F | 52 (24) | 26 (19) | 31 (23) | |
| Stem cell source | ||||
| Peripheral blood stem cells | 130 (60) | 76 (56) | 133 (98) | <.001 |
| Bone marrow | 86 (40) | 60 (44) | 3 (2) | |
| HLA match | ||||
| Matched, unrelated | 77 (35) | 88 (65) | 59 (43) | <.001 |
| Matched, related | 118 (55) | 10 (7) | 73 (54) | |
| Mismatched | 21 (10) | 38 (28) | 4 (3) | |
| Second or subsequent transplantation | 12 (6) | 4 (3) | 4 (3) | .38 |
| Year of transplantation ≥ 2002 | 146 (68) | 94 (69) | 136 (100) | <.001 |
| Disease | ||||
| AML | 86 (40) | 49 (36) | 57 (42) | — |
| ALL | 36 (17) | 25 (18) | 9 (7) | |
| CML | 37 (17) | 25 (18) | 27 (20) | |
| MDS | 23 (11) | 16 (12) | 14 (10) | |
| NHL/HL | 22 (10) | 16 (12) | 21 (16) | |
| Other | 12 (6) | 5 (4) | 8 (6) | |
| Transplantation risk–low | 54 (25) | 25 (18) | 37 (27) | .19 |
| Acute GVHD | ||||
| Grade II-IV | 81 (40) | 41 (32) | 28 (21) | .001 |
| Grade III-IV | 37 (18) | 22 (17) | 8 (6) | .002 |
| Chronic GVHD | 63 (55) | 33 (57) | 60 (59) | .83 |
| . | Tac/Mtx . | Tac/Sir/Mtx . | Tac/Sir . | P . |
|---|---|---|---|---|
| Sample size | 216 | 136 | 136 | |
| Median age, y (range) | 41 (18, 60) | 43 (19, 63) | 43 (19, 60) | .44 |
| Sex, female | 96 (44) | 51 (38) | 65 (48) | .21 |
| Donor-recipient sex match | ||||
| M-M | 72 (33) | 56 (41) | 45 (33) | |
| M-F | 48 (22) | 29 (21) | 26 (19) | |
| F-M | 44 (20) | 25 (18) | 34 (25) | |
| F-F | 52 (24) | 26 (19) | 31 (23) | |
| Stem cell source | ||||
| Peripheral blood stem cells | 130 (60) | 76 (56) | 133 (98) | <.001 |
| Bone marrow | 86 (40) | 60 (44) | 3 (2) | |
| HLA match | ||||
| Matched, unrelated | 77 (35) | 88 (65) | 59 (43) | <.001 |
| Matched, related | 118 (55) | 10 (7) | 73 (54) | |
| Mismatched | 21 (10) | 38 (28) | 4 (3) | |
| Second or subsequent transplantation | 12 (6) | 4 (3) | 4 (3) | .38 |
| Year of transplantation ≥ 2002 | 146 (68) | 94 (69) | 136 (100) | <.001 |
| Disease | ||||
| AML | 86 (40) | 49 (36) | 57 (42) | — |
| ALL | 36 (17) | 25 (18) | 9 (7) | |
| CML | 37 (17) | 25 (18) | 27 (20) | |
| MDS | 23 (11) | 16 (12) | 14 (10) | |
| NHL/HL | 22 (10) | 16 (12) | 21 (16) | |
| Other | 12 (6) | 5 (4) | 8 (6) | |
| Transplantation risk–low | 54 (25) | 25 (18) | 37 (27) | .19 |
| Acute GVHD | ||||
| Grade II-IV | 81 (40) | 41 (32) | 28 (21) | .001 |
| Grade III-IV | 37 (18) | 22 (17) | 8 (6) | .002 |
| Chronic GVHD | 63 (55) | 33 (57) | 60 (59) | .83 |
M indicates male; F, female; AML, acute myeloid leukemia; ALL, acute lymphobastic leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic disorder; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; and GVHD, graft-versus-host disease. (The rate of acute GVHD is crude and does not include patients who died prior to day 30.)
Fifty-nine patients (12.1%) developed VOD. Of these, 29 (49%) cases were confirmed histologically with liver biopsy. The remaining patients had a clinical diagnosis of VOD according to the Baltimore criteria with radiologic evidence of portal blood flow reversal. When stratified by sirolimus use, the incidence of VOD was 15.8% among sirolimus patients and 7.4% among non-sirolimus patients (OR 2.35, 95% CI 1.28-4.30, P = .005). When sirolimus users were further stratified by concomitant methotrexate use, heterogeneity was noted, therefore the patients were then categorized by GVHD prophylaxis regimen for all further comparisons. The crude incidences of VOD are shown in Table 2. Among Tac/Sir patients, the incidence was 11%, while it was 21% in Tac/Sir/Mtx patients. In the reference Tac/Mtx group, the crude incidence was 7%. VOD was diagnosed later among sirolimus patients than in non-sirolimus patients (21 vs 14 days from transplantation, P = .05) although this did not affect the clinical outcome of VOD. Among the 59 patients with VOD, there were 23 deaths. Mortality after the diagnosis of VOD among the 3 groups was similar (Tac/Mtx 44% vs Tac/Sir/Mtx 39% vs Tac/Sir 33%, P = .89), and there was no difference noted in the distribution of Defibrotide use among the 3 groups (64% overall, P = .1).
Comparison of VOD rates between prophylaxis groups
| . | Overall (n=488) . | MRD (n=202) . | MUD/MM (n=286) . | |||
|---|---|---|---|---|---|---|
| VOD incidence (n, %)* | Odds ratio to reference | VOD incidence (n, %)† | Odds ratio to reference | VOD incidence (n, %)‡ | Odds ratio to reference | |
| Tac/Mtx | 16 (7) | 5 (4) | 11 (11) | |||
| Tac/Sir/Mtx | 28 (21) | 3.23 (1.61-6.69, P < .001) | 2 (20) | 5.51 (0.46-41.2, P = .19) | 26 (21) | 2.05 (0.92-4.88, P = .09) |
| Tac/Sir | 15 (11) | 1.55 (0.69-3.48, P = .33) | 8 (11) | 2.72 (0.75-11.0, P = .15) | 7 (16) | 1.01 (0.31-3.05, P > .99) |
| . | Overall (n=488) . | MRD (n=202) . | MUD/MM (n=286) . | |||
|---|---|---|---|---|---|---|
| VOD incidence (n, %)* | Odds ratio to reference | VOD incidence (n, %)† | Odds ratio to reference | VOD incidence (n, %)‡ | Odds ratio to reference | |
| Tac/Mtx | 16 (7) | 5 (4) | 11 (11) | |||
| Tac/Sir/Mtx | 28 (21) | 3.23 (1.61-6.69, P < .001) | 2 (20) | 5.51 (0.46-41.2, P = .19) | 26 (21) | 2.05 (0.92-4.88, P = .09) |
| Tac/Sir | 15 (11) | 1.55 (0.69-3.48, P = .33) | 8 (11) | 2.72 (0.75-11.0, P = .15) | 7 (16) | 1.01 (0.31-3.05, P > .99) |
MRD indicates matched related donor; and MUD/MM, matched unrelated donor or mismatched donor.
P = .001
P = .06
P = .11
Using the Tac/Mtx group as a reference, the incidence of VOD was significantly increased in the Tac/Sir/Mtx group (OR 3.23, 95% CI 1.61-6.69, P < .001), but not the Tac/Sir group (OR 1.55, 95% CI 0.69-3.48, P = .33; Table 2). The only other factor associated with VOD in univariate analysis was the use of an unrelated or mismatched donor. In multivariable exact logistic regression analysis, the association between an increased incidence of VOD and the use of Tac/Sir/Mtx GVHD prophylaxis persisted (adjusted OR 2.76, 95% CI 1.34-5.72, P = .006), but the use of Tac/Sir as GVHD prophylaxis did not attain statistical significance (adjusted OR 1.53, 95% CI 0.69-3.40, P = .29). Transplantation risk status approached statistical significance (adjusted OR 1.77, 95% CI 0.96-3.27, P = .068; Table 3).
Multivariable exact logistic regression model for VOD outcome
| Variable . | OR . | 95% CI . | P . |
|---|---|---|---|
| Age, ≤ 50 vs > 50 y | 0.65 | 0.35-1.20 | .176 |
| Transplantation risk, low vs high | 1.77 | 0.96-3.27 | .068 |
| MRD vs MUD/MM | 0.59 | 0.29-1.20 | .145 |
| PBSC vs BM | 1.33 | 0.56-3.15 | .525 |
| Donor-recipient sex: MF or FM vs MM or FF | 0.85 | 0.47-1.51 | .567 |
| Second/subsequent vs first transplantation | 1.35 | 0.35-5.25 | .669 |
| Year of transplantation ≥ 2002 vs < 2002 | 0.71 | 0.29-1.77 | .461 |
| Tac/Sir/Mtx vs Tac/Mtx | 2.76 | 1.34-5.72 | .006 |
| Tac/Sir vs Tac/Mtx | 1.53 | 0.69-3.40 | .294 |
| Variable . | OR . | 95% CI . | P . |
|---|---|---|---|
| Age, ≤ 50 vs > 50 y | 0.65 | 0.35-1.20 | .176 |
| Transplantation risk, low vs high | 1.77 | 0.96-3.27 | .068 |
| MRD vs MUD/MM | 0.59 | 0.29-1.20 | .145 |
| PBSC vs BM | 1.33 | 0.56-3.15 | .525 |
| Donor-recipient sex: MF or FM vs MM or FF | 0.85 | 0.47-1.51 | .567 |
| Second/subsequent vs first transplantation | 1.35 | 0.35-5.25 | .669 |
| Year of transplantation ≥ 2002 vs < 2002 | 0.71 | 0.29-1.77 | .461 |
| Tac/Sir/Mtx vs Tac/Mtx | 2.76 | 1.34-5.72 | .006 |
| Tac/Sir vs Tac/Mtx | 1.53 | 0.69-3.40 | .294 |
MRD indicates matched, related donor; MUD/MM, matched, unrelated donor or mismatched donor; PBSC, peripheral blood stem cell; and BM, bone marrow.
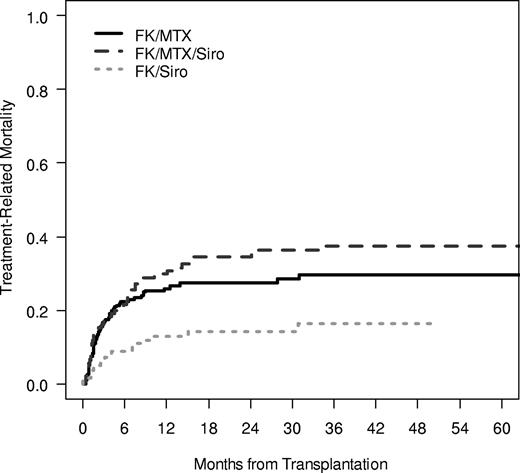
The cumulative incidence of TRM was significantly influenced by GVHD prophylaxis regimen, with the lowest rate of TRM noted in the Tac/Sir group (100-day TRM 17% vs 16% vs 7%; 1-year TRM 26% vs 30% vs 13%, P < .001; Figure 1). The causes of death were split evenly among GVHD prophylaxis groups, with 58%, 60%, and 51% of all deaths attributable to TRM in the 3 GVHD prophylaxis group and the remaining deaths related to disease relapse. Overall survival was significantly different between groups, with the highest overall survival noted in the Tac/Sir group (3-year OS 47% vs 37% vs 68%, P < .001; Figure 2). Multivariable Cox regression analysis demonstrated a significant association between overall survival and patient age, the occurrence of VOD, the year of transplantation and the use of Tac/Sir as GVHD prophylaxis. Examining only pretransplantation variables, overall survival was favorably influenced by age less than or equal to 50 (HR 0.58, P < .001), matched related donor (MRD; HR 0.70, P = .03), year of transplantation 2002 or later (HR 0.58, P = .006), and Tac/Sir GVHD prophylaxis (HR 0.54, P = .003; Table 4).
Treatment-related mortality. Treatment-related mortality was examined in a competing risk fashion, with relapse as the competing risk. The 1-year cumulative incidence of TRM in the Tac/Sir group was 13% compared with 30% in the Tac/Sir/Mtx group and 26% in the Tac/Mtx group (P < .001).
Treatment-related mortality. Treatment-related mortality was examined in a competing risk fashion, with relapse as the competing risk. The 1-year cumulative incidence of TRM in the Tac/Sir group was 13% compared with 30% in the Tac/Sir/Mtx group and 26% in the Tac/Mtx group (P < .001).
Overall survival of Cy/TBI-treated patients. Three-year survival in the Tac/Sir group is 68% in comparison to 37% in the Tac/Sir/Mtx group and 47% in the Tac/Mtx group (P < .001).
Overall survival of Cy/TBI-treated patients. Three-year survival in the Tac/Sir group is 68% in comparison to 37% in the Tac/Sir/Mtx group and 47% in the Tac/Mtx group (P < .001).
Cox model for overall survival using transplantation baseline characteristics
| Variable . | Overall (n = 488)* . | MRD (n = 202)* . | MUD/MM (n = 286)* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age, 50 y or younger vs older than 50 y | 0.58 | 0.43-0.78 | < .001 | 0.48 | 0.30-0.78 | .003 | 0.64 | 0.43-0.94 | .03 |
| Transplant risk, low vs high | 0.79 | 0.56-1.11 | .18 | 1.01 | 0.60-1.71 | .97 | 0.69 | 0.44-1.09 | .11 |
| MRD vs MUD/MM | 0.70 | 0.50-0.97 | .03 | ||||||
| PBSC vs BM | 1.38 | 0.93-2.04 | .11 | 1.53 | 0.76-3.08 | .23 | 1.33 | 0.83-2.15 | .23 |
| Donor-recipient sex: MF or FM vs MM or FF | 1.10 | 0.84-1.45 | .50 | 0.73 | 0.46-1.17 | .19 | 1.37 | 0.98-1.93 | .07 |
| Second/subsequent vs first transplantation | 1.01 | 0.54-1.87 | .98 | 0.78 | 0.31-2.00 | .60 | 1.21 | 0.52-2.81 | .65 |
| Year of transplantation, 2002 or later vs before 2002 | 0.58 | 0.39-0.85 | .006 | 0.43 | 0.22-0.84 | .01 | 0.65 | 0.39-1.10 | .11 |
| Tac/Sir/Mtx vs Tac/Mtx | 1.11 | 0.80-1.55 | .54 | 1.68 | 0.63-4.49 | .30 | 1.07 | 0.74-1.55 | .72 |
| Tac/Sir vs Tac/Mtx | 0.54 | 0.36-0.81 | .003 | 0.73 | 0.40-1.33 | .30 | 0.45 | 0.24-0.82 | .009 |
| Variable . | Overall (n = 488)* . | MRD (n = 202)* . | MUD/MM (n = 286)* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age, 50 y or younger vs older than 50 y | 0.58 | 0.43-0.78 | < .001 | 0.48 | 0.30-0.78 | .003 | 0.64 | 0.43-0.94 | .03 |
| Transplant risk, low vs high | 0.79 | 0.56-1.11 | .18 | 1.01 | 0.60-1.71 | .97 | 0.69 | 0.44-1.09 | .11 |
| MRD vs MUD/MM | 0.70 | 0.50-0.97 | .03 | ||||||
| PBSC vs BM | 1.38 | 0.93-2.04 | .11 | 1.53 | 0.76-3.08 | .23 | 1.33 | 0.83-2.15 | .23 |
| Donor-recipient sex: MF or FM vs MM or FF | 1.10 | 0.84-1.45 | .50 | 0.73 | 0.46-1.17 | .19 | 1.37 | 0.98-1.93 | .07 |
| Second/subsequent vs first transplantation | 1.01 | 0.54-1.87 | .98 | 0.78 | 0.31-2.00 | .60 | 1.21 | 0.52-2.81 | .65 |
| Year of transplantation, 2002 or later vs before 2002 | 0.58 | 0.39-0.85 | .006 | 0.43 | 0.22-0.84 | .01 | 0.65 | 0.39-1.10 | .11 |
| Tac/Sir/Mtx vs Tac/Mtx | 1.11 | 0.80-1.55 | .54 | 1.68 | 0.63-4.49 | .30 | 1.07 | 0.74-1.55 | .72 |
| Tac/Sir vs Tac/Mtx | 0.54 | 0.36-0.81 | .003 | 0.73 | 0.40-1.33 | .30 | 0.45 | 0.24-0.82 | .009 |
Wald test P value for the coefficient.
MRD indicates matched, related donor; MUD/MM, matched, unrelated donor or mismatched donor; PBSC, peripheral blood stem cell; BM, bone marrow.
Subset analyses
Subjects were stratified into 2 groups by donor type to determine if similar associations with sirolimus and VOD persisted. Among the MRDs (n = 202) and matched, unrelated, or mismatched donor group (MUD/MM, n = 286), similar trends for the increase in VOD incidence among sirolimus users was noted (MRD OR 3.05, 95%CI 1.00-9.29, P = .06; MUD/MM OR 1.68, 95% CI 0.81-3.50, P = .17). When stratified by the 3 GVHD prophylaxis regimens, these trends did not attain statistical significance (P = .06 for MRD and P = .11 for MUD/MM; Table 2). However, in both the MRD group and the MUD/MM group, 3-year overall survival was superior in the Tac/Sir group (MRD OS 50% vs 44% vs 68%, P = .05; MUD/MM OS 43% vs 36% vs 72%, P = .005; Figure 3A,B). Multivariable modeling for predictors of overall survival demonstrated similar factors as in the unstratified analysis, with the exception that Tac/Sir was not a predictive factor for MRD survival, and year of transplantation was not predictive of survival in the MUD/MM model (Table 4). These differences might be due to the fact that no MRD patients in the Tac/Sir group received transplants before year 2002, whereas MUD/MM patients were balanced in year of transplantation and GVHD regimen. All interaction terms were tested, but none of them was significant, thus not included in the final model. Smaller sample sizes in the subset analyses may also account for these differences, since the hazard ratios were similar across groups.
Overall survival of MRD and MUD/MM CyTBI patients. (A) Overall survival of MRD (A) and MUD/MM (B) Cy/TBI patients. Overall survival was significantly better in the Tac/Sir group in comparison with the Tac/Sir/Mtx and Tac/Mtx in both the MRD (P = .05) and MUD/MM groups (P = .005).
Overall survival of MRD and MUD/MM CyTBI patients. (A) Overall survival of MRD (A) and MUD/MM (B) Cy/TBI patients. Overall survival was significantly better in the Tac/Sir group in comparison with the Tac/Sir/Mtx and Tac/Mtx in both the MRD (P = .05) and MUD/MM groups (P = .005).
Combined DFCI and BMT CTN Bu/Cy analysis
The recognized risk of busulfan on VOD incidence noted in BMT CTN trial prompted a second analysis examining a smaller number of patients who received transplants at our institute. Since this cohort was small, we combined it with a prospectively enrolled group of patients participating in the BMT CTN Protocol 0402 who also received busulfan conditioning. All 10 subjects from BMT CTN Protocol 0402 who received Bu/Cy conditioning prior to transplantation from HLA-matched sibling donors were combined with 29 DFCI patients who received Bu/Cy (Methods) between January 1, 2000, and May 31, 2007. Clinical characteristics of these patients are presented in Table 5. Among these patients, 6 were randomized (BMT CTN) and 7 were assigned (DFCI) to receive sirolimus as part of their GVHD prophylaxis regimen. Eight of 13 sirolimus patients (4 CTN, 4 DFCI; 62%) developed VOD in comparison with 4 of 26 (0 CTN, 4 DFCI; 15%) non-sirolimus patients (OR 8.8, 95% CI 1.88-41.21, P = .008). Small sample size precluded further statistical analysis or multivariable modeling.
Baseline characteristics of the busulfan/cyclophosphamide cohort
| Characteristics . | Sirolimus . | No sirolimus . | P . |
|---|---|---|---|
| N | 13 | 26 | |
| Median age, y (range) | 50 (11-57) | 45 (23-56) | .28 |
| Sex, female | 7 (54) | 13 (50) | >.99 |
| Cell source | |||
| Peripheral blood stem cells | 11 (85) | 22 (82) | >.99 |
| Bone marrow | 2 (15) | 4 (18) | |
| HLA matching | |||
| Matched-related | 9 (69) | 15 (58) | .89 |
| Matched-unrelated | 3 (23) | 7 (27) | |
| Mismatched | 1 (8) | 4 (15) | |
| Multiple transplantations | 0 (0) | 9 (41) | .02 |
| Year of transplantation 2002 or later | 13 (100) | 21 (81) | .15 |
| Disease | |||
| AML | 8 (62) | 15 (58) | |
| ALL | 0 (0) | 4 (15) | |
| CLL/SLL/PLL | 1 (8) | 0 (0) | |
| MDS | 1 (8) | 3 (12) | |
| NHL | 1 (8) | 4 (15) | |
| Aplastic anemia | 2 (15) | 0 (0) | |
| Transplantation risk, low | 11 (85) | 18 (69) | .45 |
| Acute GVHD (grade II-IV) | 3 (23) | 7 (27) | >.99 |
| Characteristics . | Sirolimus . | No sirolimus . | P . |
|---|---|---|---|
| N | 13 | 26 | |
| Median age, y (range) | 50 (11-57) | 45 (23-56) | .28 |
| Sex, female | 7 (54) | 13 (50) | >.99 |
| Cell source | |||
| Peripheral blood stem cells | 11 (85) | 22 (82) | >.99 |
| Bone marrow | 2 (15) | 4 (18) | |
| HLA matching | |||
| Matched-related | 9 (69) | 15 (58) | .89 |
| Matched-unrelated | 3 (23) | 7 (27) | |
| Mismatched | 1 (8) | 4 (15) | |
| Multiple transplantations | 0 (0) | 9 (41) | .02 |
| Year of transplantation 2002 or later | 13 (100) | 21 (81) | .15 |
| Disease | |||
| AML | 8 (62) | 15 (58) | |
| ALL | 0 (0) | 4 (15) | |
| CLL/SLL/PLL | 1 (8) | 0 (0) | |
| MDS | 1 (8) | 3 (12) | |
| NHL | 1 (8) | 4 (15) | |
| Aplastic anemia | 2 (15) | 0 (0) | |
| Transplantation risk, low | 11 (85) | 18 (69) | .45 |
| Acute GVHD (grade II-IV) | 3 (23) | 7 (27) | >.99 |
AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; PLL, prolymphocytic leukemia; MDS, myelodysplastic disorder; and NHL, non-Hodgkin lymphoma.
Discussion
These analyses demonstrate a higher than expected rate of VOD after myeloablative transplantation when sirolimus is included in the GVHD prophylaxis regimen. This increase was most significant when methotrexate was given concomitantly with sirolimus. Despite this increase in VOD, after Cy/TBI-based myeloablative conditioning, overall survival was superior in sirolimus-treated patients who were not also treated with methotrexate. This is in contrast to Bu/Cy conditioning, where the rate of VOD and VOD-related mortality was excessive.
The precise biologic mechanism involving the interaction of sirolimus, methotrexate, and myeloablative conditioning to cause VOD is unclear. Higher conditioning intensity and the use of methotrexate (as compared with immune suppression–free T cell–depleted myeloablative transplantation) are associated with VOD.23 The hepatic sinusoidal endothelial cell is thought to be the primary target of conditioning and methotrexate-related injury in VOD. One plausible explanation for the increase in VOD incidence noted here is that sirolimus may inhibit endothelial cell function, as demonstrated in its use as coating for coronary artery stents.29 In addition, sirolimus may accelerate the senescence of the hepatic endothelial cell in response to injury.30 Other hepatic targets may be implicated in the pathophysiology of VOD as well. For instance, sirolimus can inhibit the platelet-derived growth factor–mediated proliferation of hepatic stellate cells in animal models.31 In addition, sirolimus may inhibit vascular smooth muscle proliferation in response to injury32 and may reduce endogenous levels of VEGF that may be required for endothelial recovery.33 Thus, sirolimus may not only be a direct toxic agent causally linked to the occurrence of VOD, but may prevent healing of the hepatic sinusoid complex once endothelial injury has occurred. We did not examine serum levels in this analysis to determine if there was dose-dependent injury to the endothelium, but previously noted no relationship between serum drug concentrations and thrombotic microangiopathy.13
Without a clear, single biologic mechanism to explain the increased risk of VOD, we can only postulate that the etiology of sirolimus-associated injury is likely similar to that which we proposed for thrombotic microangiopathy.13 In thrombotic microangiopathy, sirolimus can only potentiate the effects of other agents, such as calcineurin inhibitors, but is insufficient alone to cause microangiopathy. This relationship is maintained here, where sirolimus in combination with methotrexate, but not alone, was able to increase the incidence of VOD.
Busulfan is one of the most commonly used agents as conditioning in nonmyeloablative transplantation, and we have used this agent at nonmyeloablative doses ranging from 3.2 to 6.4 mg/kg in more than 100 patients in combination with sirolimus-based GVHD prophylaxis without any documented cases of VOD.34,35 In contrast, when given in myeloablative doses as in this retrospective review, the incidence was more than 30%. In this series, busulfan was always administered with cyclophosphamide, which also has been causally linked with VOD,36 but busulfan can independently increase the risk of VOD when given with sirolimus. There are documented cases of VOD occurring after myeloablative doses of busulfan administered with fludarabine (E. Broun, personal communication), as well as cases of VOD when myeloablative doses of busulfan and fludarabine are given with the sirolimus analog, everolimus.37 Other factors may influence the risk of VOD after busulfan exposure, such as the presence of glutathione metabolism polymorphisms,38 which by altering busulfan metabolism may independently influence VOD. In animal models, sirolimus exposure decreases hepatic glutathione production.39 The use of targeted oral dosing of busulfan or the use of the intravenous preparation of busulfan, with more reliable pharmacokinetics, may be associated with a lower incidence of VOD.40,41
With an increased rate of VOD in the Cy/TBI cohort without a decrement in overall survival, there must be a compensatory decrease in non-VOD TRM or relapse rates when sirolimus is used, since the rate of mortality after VOD diagnosis was no different between groups. In fact, several groups are now investigating the role of sirolimus as an antineoplastic agent, and we have demonstrated a significant decrease in the relapse rate of patients with lymphoid malignancies undergoing sirolimus-based transplantation procedures.42 In this analysis, nonsignificant decreases in the rates of relapse were demonstrated as well (data not shown). In addition, in the non-methotrexate Tac/Sir group, TRM was lower, contributing to the improved overall survival rate. This is almost certainly attributable to the omission of methotrexate. In previous phase II studies of sirolimus without methotrexate, we demonstrated the complete disappearance of idiopathic pneumonia syndrome.6 With earlier engraftment due to the omission of methotrexate, the rate of life-threatening infections may also be lower, and the rate of certain viral infections, most notably CMV, has decreased as well.7 Finally, grades II-IV acute GVHD were notably lower in the Tac/Sir group, and in recent studies at our center, GVHD-attributable mortality has been minimal.6
Since non-VOD outcomes appear to be improved when sirolimus is incorporated into the GVHD prophylaxis regimen, strategies to mitigate the risk of VOD in sirolimus-treated patients may further improve transplantation outcomes. Several possible strategies can be considered. First, based on the results of this analysis, sirolimus should not be given with methotrexate for GVHD prophylaxis when high-dose conditioning regimens are used. Alternatively, another approach is to begin sirolimus only after conditioning related injury at the level of the hepatic sinusoid has resolved, or at a time when sufficient healing has occurred such that the added endothelial injury associated with sirolimus would not be detrimental. In liver transplantation, sirolimus is often not introduced until major wound healing has occurred, around 30 days from transplantation. Currently, we administer a sirolimus loading dose on day − 3 prior to transplantation, immediately after completing cyclophosphamide and on the same day that TBI begins. Thus, sirolimus is present in therapeutic concentrations through the majority of the period of conditioning. It is unclear if waiting until the day of stem cell infusion (as is commonly performed with agents such as mycophenolate mofetil) or even waiting until several days after transplantation to administer sirolimus would be of any detriment with respect to the incidence of GVHD. Studies comparing different administration schedules of sirolimus can be performed but will be too small to determine if this strategy will have a significant impact on the occurrence of VOD. Determining the optimal time to administer sirolimus based on markers of endothelial injury, such as plasminogen activator inhibitor-1,43 thrombomodulin,44 von Willebrand factor, or E-selectin may help guide this decision. However, the expression of some of these markers, such as PAI-1, may specifically be altered with exposure to sirolimus,45 making the interpretation of these tests difficult.
A third approach to preventing the VOD associated with sirolimus is to use another agent as VOD prophylaxis. Defibrotide is a polydisperse mixture of single-stranded DNA oligoncleotides. This compound targets vascular endothelium and appears to have local antithrombotic, anti-ischemic, and anti-inflammatory activity, and has been used as a therapeutic agent in VOD in a number of phase II trials, demonstrating promising results.46,47 While the precise mechanism of action of defibrotide in VOD is unknown, it appears that defibrotide can bind to vascular endothelium via the A1 and A2 adenosine receptors and have local antiapoptotic effects. In addition, it can modulate platelet activity. In addition, it may have profibrinolytic activity as well.23 Nonrandomized clinical trials evaluating the role of defibrotide as a prophylactic agent against VOD have now been reported, with positive results in both adults and pediatric populations.48,49 Neither of these trials enrolled patients receiving sirolimus, however. Therefore, a trial evaluating the role of defibrotide prophylaxis in patients receiving sirolimus at our institution is now being planned.
This retrospective analysis was unable to include pretransplantation variables that may be associated with an increased risk of VOD, such as prior liver function test abnormalities and preclinical cirrhosis,23 or the use of gemtuzumab ozogamycin prior to transplantation.50 In future analyses involving sirolimus, these factors certainly need to be considered.
In summary, the use of sirolimus as GVHD prophylaxis is associated with an increased risk of VOD after myeloablative transplantation. After Cy/TBI-based transplantation, the incidence is highest when sirolimus is given with methotrexate. However, despite this increase in VOD, overall outcomes in Cy/TBI-based transplantation were unchanged or improved, particularly when methotrexate was omitted. This is in contrast to patients who received Bu/Cy conditioning prior to transplantation, in which there was an unacceptable excess in the VOD rate and early mortality associated with VOD. With these important differences in mind, it is our opinion that sirolimus-based GVHD prophylaxis can continue to be used in Cy/TBI-based transplantation, as long as methotrexate is not used concomitantly, and further studies should occur to assess transplantation outcomes associated with sirolimus use. At the present time, we must also recommend that sirolimus not be used in conjunction with myeloablative doses of busulfan until there is a greater understanding of the etiology of VOD associated with sirolimus and busulfan use. In fact, the Blood and Marrow Transplant Clinical Trial Network trial has been amended to exclude patients receiving busulfan-based conditioning. It is hoped that the implications of VOD on the long-term outcome of sirolimus use in HSCT using non-busulfan regimens will be ascertained with the completion of this prospective, phase III trial.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute grants PO1 HL070149 and U01 HL69249. C.C. is supported by the Stem Cell Cyclists of the Pan-Mass Challenge.
National Institutes of Health
Authorship
Contribution: C.C. designed the research, cared for research subjects, collected data, analyzed data, and reviewed the manuscript. K.S. and H.T.K. analyzed data and reviewed the manuscript. P.R. and V.T.H. cared for research subjects, collected data, and reviewed the manuscript. E.L., C.R., R.F., D.W., and S.C. collected data. S.C., J.K., P.A., E.A. cared for research subjects and reviewed the manuscript. M.H. reviewed the manuscript. J.H.A. and R.S. designed the research, cared for research subjects, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Corey Cutler, MD, MPH, FRCP(C), Department of Medical Oncology, Dana-Farber Cancer Institute, 44 Binney Street, D1B13 Boston, MA 02115; e-mail: corey_cutler@dfci.harvard.edu.