The most significant morbidity resulting from hemophilia A or B is the progressive destruction of joints due to repetitive intra-articular bleeding episodes. In this issue of Blood, Sun and colleagues now demonstrate that localized intra-articular FIX protein or gene delivery protects against hemophilic synovitis.
The current treatment of patients suffering from hemophilia with recombinant or plasma-derived clotting factors has greatly improved their quality of life and life expectancy. Nevertheless, patients are still at risk of developing uncontrolled bleeding episodes, particularly in the joints where 85% of all bleeding occurs. This results in hemophilic synovitis, a condition that is characterized by chronic inflammation and neoangiogenesis which, in turn, triggers recurrent bleeding, setting off a degenerative cycle. Ultimately, the joint degeneration in these so-called target joints progressively worsens and develops into end-stage hemophilic arthropathy. Unfortunately, factor VIII (FVIII) or factor IX (FIX) replacement in response to ongoing bleeds does not halt the progression of existing arthropathy.
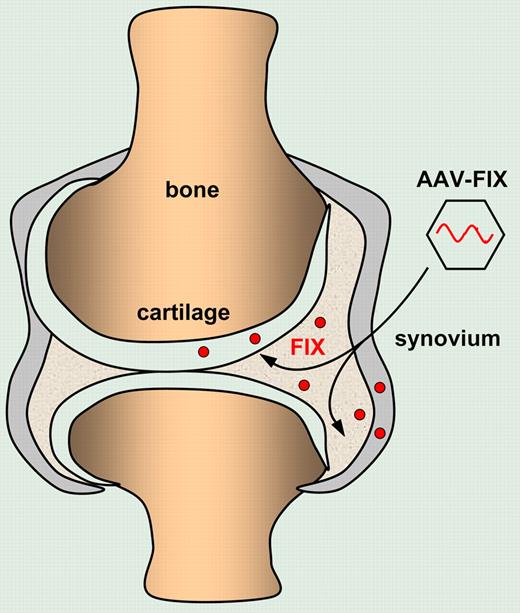
Intra-articular injection of AAV vectors encoding FIX results in local FIX production by transduced chondrocytes and synviocytes that protect against hemophilic synovitis.
Intra-articular injection of AAV vectors encoding FIX results in local FIX production by transduced chondrocytes and synviocytes that protect against hemophilic synovitis.
Sun et al now describe a novel approach whereby the clotting factors are administered locally by protein or gene delivery into affected hemophilic joints. In a series of elegant experiments, they first established a hemophilic synovitis model by needle puncture into the knee joint. Interestingly, the hemophilic mice were protected from synovitis when the FIX protein was administered intra-articularly, in the absence of any measurable circulating FIX. In contrast, intravenous administration of FIX, even at higher doses, failed to prevent the development of hemophilic synovitis. The authors subsequently demonstrated that intra-articular delivery of an adeno-associated viral vector (AAV) encoding FIX resulted in transduction of synoviocytes and chondrocytes. Most importantly, the ensuing localized production of FIX in the joint itself provided protection against hemophilic synovitis. Although the joint-targeted approach would not permit correction of some of the life-threatening sequelae of hemophilia, including the retroperitoneal and intracerebral hemorrhagic episodes, the present study suggests that joint-directed therapies may be useful adjuncts in hemophilia care in addition to intravascular replacement therapy. One of the particularly attractive aspects of this approach is that the high localized FIX levels in the joint after intra-articular AAV injection may augment or exceed joint protection afforded by a lower FIX level following systemic gene therapy. In addition, a joint-targeted approach would require a lower and thus potentially safer dose of gene delivery vectors to achieve such a localized therapeutic effect. Indeed, in a recent gene therapy trial for hemophilia B, based on hepatic FIX gene delivery using AAVs, therapeutic circulating FIX levels could only be detected in the highest dose cohorts.1 Unfortunately, FIX levels declined, probably due to a vector-dose dependent, AAV-specific T cell–mediated immune response that accounted for the immune rejection of the AAV-transduced hepatocytes.1,2 It is therefore possible that the use of lower vector doses for localized gene delivery into the joints may obviate some of these concerns associated with systemic vector delivery. Moreover, the localized clotting factor production following targeted joint delivery may result in a lower risk of developing neutralizing antibodies to FVIII or FIX (clinically referred to as “inhibitors”), which is a major complication of current treatment. It is particularly encouraging that no inhibitors to FIX were detected in the present model, since the strain of FIX-deficient mice used is prone to develop inhibitors to FIX. However, this may not necessarily be the case in the proinflammatory environment of an arthritic hemophilic target joint. Indeed, the synovitis model described here mimics a “prophylactic” approach whereby the FIX protein or vector is administered before the onset of joint degeneration. Whether this novel approach would also be effective to treat preexisting chronic arthropathy would need to be validated. This would more accurately mimic the inflammatory environment of target joints in patients suffering from hemophilia. The present study by Sun et al paves the way toward large animal studies in hemophilic dogs and provides a model to further dissect the mechanisms of hemophilic synovitis and arthropathy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal