The PI3Kδ isoform is required for both optimal growth of Abelson-transformed leukemia cells and antileukemic NK-cell effectors. Therefore, the simultaneous inhibition of PI3Kδ in tumor and host cells results in therapeutic failure.
Deregulation of the phosphoinositide 3-kinase (PI3K) pathway can occur by activating mutations in growth factor receptors or in the PIK3CA locus coding for PI3Kα, by loss of function of the lipid phosphatase PTEN, by up-regulation of protein kinase B (PKB/Akt), or by the impairment of the tuberous sclerosis complex (TSC1/2). All these events stimulate cancer growth and proliferation and have thus prompted a major interest in therapeutic inhibition of the PI3K pathway. One particular PI3K isoform, PI3Kδ, is selectively expressed in leukocytes and, hence, might constitute a pharmacologic focus for the “targeted” treatment of hematological malignancies.
In an elegant study published in the present issue of Blood, Zebedin and colleagues explore the effect of the PI3Kδ knockout on Abelson virus (cAbl)–induced leukemia in mice. For this, PI3Kδ was either removed from the transformed cells themselves, or from the host environment into which transformed cells were injected. These experiments reveal a formidable contradiction. PI3Kδ deficiency in leukemic cells retarded tumor progression while PI3Kδ deficiency in nonleukemic host cells accelerated the fatal course of leukemia. Intriguingly, the simultaneous removal of PI3Kδ from leukemic cells and the host had no effect on leukemic progression at all. This latter result suggests that complete and selective pharmacologic PI3Kδ inhibition (something that obviously would affect both tumor and host cells) would have no therapeutic benefit on PI3Kδ-overexpressing Bcr/abl-positive human leukemias.
Zebedin et al also show that PI3Kδ−/− mice exhibit an accelerated development of cancers that are usually controlled by natural killer (NK) cells, such as Abelson-transformed cells, Eμ-myc–induced B-cell lymphoma, EL4 thymoma, and B16 melanoma. These PI3Kδ effects were also found on a RAG2−/− background, indicating that they must involve other immune effectors besides B or T lymphocytes. Indeed, PI3Kδ−/− NK cells poorly lysed leukemic cells, correlating with a general defect in exocytosis that affects both degranulation and IFNγ secretion. Although formal proof that accelerated tumor progression must be attributed to this NK defect is elusive, these results make it highly plausible that PI3Kδ-dependent effectors of the innate immune system play a major role in tumor control.
The results by Zebedin et al contribute to a general debate on the mechanisms through which anticancer chemotherapeutics (fail to) act. Accumulating evidence indicates that radiotherapy and some chemotherapeutic agents, in particular anthracyclines, can trigger specific immune responses that result either from immuno-genic cancer cell death or from immuno-stimulatory off-target effects. This anticancer immune response then helps to eliminate residual cancer cells (that failed to be killed by chemotherapy) or maintain micrometastases (or perhaps cancer stem cells) in a stage of dormancy.1
Ideally, anticancer chemotherapeutics should induce a cellular stress response and/or immunogenic cancer cell death that trigger an effective immune response. Genotoxic agents induce NKG2D ligands through an ATM-dependent and Chk1- dependent DNA damage pathway.2 Such NKG2D ligands on the surface of tumor cells then permit their lysis by NK cell–expressing (and possibly CD8+T cell)–expressing NKG2D. Similarly, multiple chemotherapeutics stimulate the expression of death receptors (such as Fas/CD95 or TRAIL receptors) that facilitate the induction of apoptosis by immune cells.1 Another example is provided by calreticulin (and perhaps other chaperones) that is exposed on the surface of anthracycline-treated tumor cells before they undergo apoptosis, thereby facilitating their uptake by dentritic cells (DCs) which, in turn, present tumor antigen to cytotoxic T lymphocytes.3
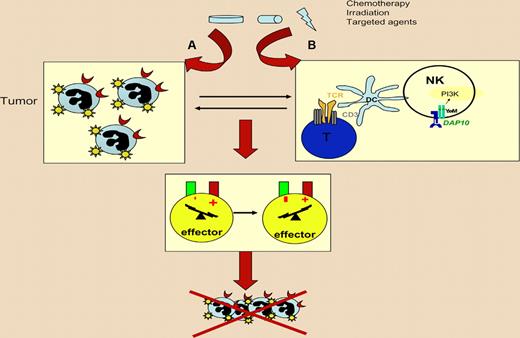
Best case scenario for anticancer therapy with respect to immune effectors. The cytostatic or cytotoxic agent should (A) stimulate a cell-autonomous response that facilitates recognition (and ultimately lysis) of tumor cells by immune effectors and (B) have immunostimulatory “side effects” on the host that boost the innate and/or cognate immune anticancer response. The incapacity to mediate such effects or worse, immunosuppressive side effects, can entail therapeutic failure.
Best case scenario for anticancer therapy with respect to immune effectors. The cytostatic or cytotoxic agent should (A) stimulate a cell-autonomous response that facilitates recognition (and ultimately lysis) of tumor cells by immune effectors and (B) have immunostimulatory “side effects” on the host that boost the innate and/or cognate immune anticancer response. The incapacity to mediate such effects or worse, immunosuppressive side effects, can entail therapeutic failure.
In addition, the ideal anticancer agent should exert direct immunostimulatory effects on immune effectors. Nontargeted genotoxic anticancer agents often impair the immune system through the induction of myelosuppression and through the elimination of proliferating lymphocytes or other immune effectors. However, some targeted agents, such as the tyrosine kinase inhibitor imatinib mesylate, can activate NK cells4 and expand specific NK-cell subpopulations with potent antitumor effects.5 Thus, in vivo administration of imatinib can trigger the NK cell–dependent control of cancers that do not respond to imatinib in vitro.4 Conversely, another tyrosine kinase inhibitor, sorafenib, strongly inhibits T-cell activation by DCs and, hence, might subvert the anticancer immune response.6 Moreover, specific inhibitors of the lipid kinase PI3Kδ — which have been initially developed as immunosuppressive agents for the treatment of autoimmune disease7 — can compromise multiple immune effectors including NK cells, as demonstrated by Zebedin et al, as well with disastrous consequences for anticancer therapies.
Altogether, this scenario should stimulate careful monitoring of positive or negative immunologic effects exerted by targeted anticancer agents, both in animal models and in clinical trials. Therapeutic success of cancer therapies definitively depends on their direct or indirect effects on DC, T, and NK cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal