Abstract
Background: Treatment options for patients with AML are limited. Poor outcomes are associated with increasing age, decreasing performance status, unfavorable cytogenetics, and the presence of comorbid conditions. Elderly patients ³ 65 years with AML have a short survival (median 2.4 months) (Lang 2005), and for older patients who receive treatment, 1-year survival is 5–25% (Menzin 2002, Kantarjian 2006, Burnett 2007). CR/CRp is a desired outcome from induction treatment and a requisite for meaningful survival. Laromustine (Cloretazine) is a novel sulfonylhydrazine alkylating agent which preferentially targets the O6 position of guanine resulting in DNA cross-links. Results of two sequential Phase II multi-center studies of single agent laromustine (CLI-033 and CLI-043) conducted in elderly untreated AML patients have been reported (Giles 2007, Schiller 2008). The purpose of this analysis is to assess survival for a combined group of patients from the two studies who achieved a response to laromustine (CR/CRp by IWG criteria [Cheson 2003]).
Methods: Eighty-five patients in study CLI-043 and 55 patients from a retrospectively identified de novo subset of CLI-033 who reasonably met the eligibility criteria for CLI- 043, received laromustine 600 mg/m2 as induction therapy (N=140). Poor risk was defined as the presence of at least one risk factor: age ³ 70 yrs, unfavorable cytogenetics, PS2, or pulmonary, cardiac, or hepatic comorbidity. 86% (120/140) pts had 2 or more risk factors. 16% (22/140) pts received a second induction of laromustine 600 mg/m2. 24% (34/140) patients received a consolidation cycle; per individual protocol, 15 pts received consolidation with laromustine 400 mg/m2 and 19 pts received cytarabine 400 mg/ m2. Kaplan-Meier analysis of survival was used to better understand the clinical benefit of CR/CRp.
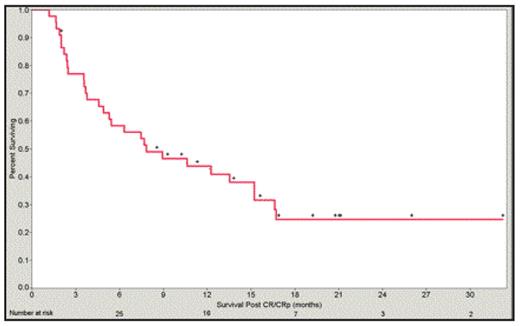
Results: 52/140 (37%) pts achieved a CR/CRp; 44/140 (31%) pts survived ³ 30 days following CR/CRp. All but one patient achieved CR/CRp following first induction. Estimated Kaplan-Meier survival from time of response for these 44 patients is shown below. Six and 12 month survival for this subset is 58% and 44%, respectively. The 30-day mortality for the entire group is 14% (20/140).
Conclusions: Early mortality, progressive disease, and short CR/CRp duration account for short median survival rates in leukemia studies. The 30-day mortality for this group compares favorably with published data of early death rates in older patients with multiple risk factors who receive induction chemotherapy (Appelbaum 2006, Kantarjian 2006). Relatively long survival in a subset of older patients with multiple risk factors who achieve a response to laromustine implies clinical benefit, and confirms the concept that obtaining a CR/CRp is in itself a benefit.
Disclosures: O’Brien:Vion Pharmaceuticals, Inc.: Research Funding. Schiller:Vion Pharmaceuticals, Inc.: Honoraria, Research Funding. Stuart:The Vaccine Co.: Research Funding; Genzyme: Research Funding; Sunesis: Research Funding; Cephalon: Research Funding; Vion Pharmaceuticals, Inc.: Honoraria, Research Funding; Antisoma: Consultancy, Research Funding; Seattle Genetics: Research Funding; Isiris: Research Funding. Hudak:Vion Pharmaceuticals, Inc.: Employment. Giles:Vion Pharmaceuticals, Inc.: Consultancy, Research Funding. Off Label Use: Cloretazine® for the use of AML.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal