Abstract
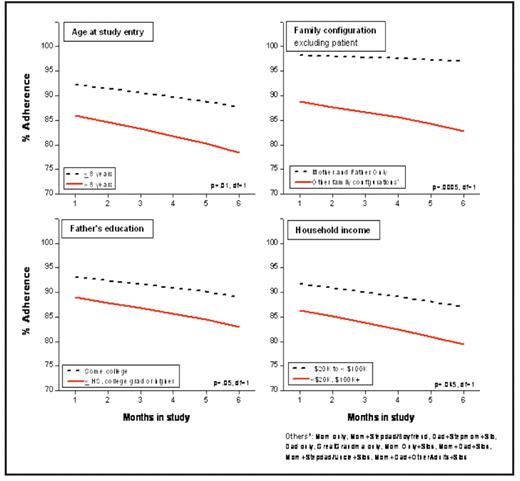
Use of contemporary risk-based therapy in children with ALL has resulted in five-year survival rates exceeding 80%. Achievement of durable remissions requires a maintenance phase composed of oral administration of antimetabolites (6-mercaptopurine and methotrexate) for approximately two years. Previous studies have shown that low systemic exposure to oral 6MP adversely affects prognosis, thus emphasizing the critical need for therapeutic levels throughout maintenance. However, significant inter-patient variability in red cell thioguanine nucleotide (6TGN – a major metabolite of 6MP) concentrations exists, and could in part be related to failure to adhere to prescribed therapy. Non-adherence in pediatric ALL patients has been reported – however, small sample sizes and varying methods of assessment make it difficult to understand the magnitude of this problem. The purpose of our study was to describe adherence to oral 6MP in a large multi-ethnic cohort of children with ALL. Patients were eligible to participate if they were diagnosed with ALL at age less than 22 years, belonged to one of four ethnic/racial groups (Asian, African-American, Caucasian, or Hispanic), and had completed at least 24 weeks of maintenance therapy. We have restricted the current report to Caucasians, where we have completed our target accrual. To measure 6MP adherence, we used the Medication Event Management System (MEMS) and supplied each patient with a MEMS TrackCap. This electronic cap allowed the collection of real-time data by recording the date and time(s) when the 6MP bottle was opened over a 6-month period. The MEMS data was downloaded at the end of the 6-month study period. Patients/parents also completed a self-administered sociodemographic questionnaire. Longitudinal analysis was performed using the Generalized Estimating Equations. A total of 173 Caucasian patients provided 26,424 person-days of observation for 6MP adherence. The median age at diagnosis was 5 years (1 to 19), and at study participation was 6 years (range, 2 to 20); median time from diagnosis was 18.8 months, and from start of maintenance, 8.1 months; 67% were males. NCI criteria for high-risk disease were present in 42% of the patients. The median annual household income was between $50K and $75K; 79% of the mothers and 72% of the fathers had received education beyond high school. The median number of household members (including patient) was 4 (range, 2 to 10). Adherence was defined as the ratio of 6MP bottle openings to actual 6MP doses prescribed, calculated as a percentage (“percent adherence”). Prescribed doses for the entire 6-month period were reviewed for each patient, and instances when 6MP was withheld by the prescriber due to toxicity or illness were taken into account for purposes of calculating adherence. The mean percent adherence over the 6-month study period was 85% (range 11% to 100%). The mean monthly percent adherence declined significantly over the 6-month study period (p=0.002). Multivariate analysis identified certain subgroups that were at increased risk of lower percent adherence (Figure): age >8 years at study entry (p=0.01); households that included members other than the mother, father, and patient (<0.001); father’s education ≤ high school or ≥ college degree (p=0.05), and annual household income <$20k or ≥ $100K (p=.045). In this study, 19% of the study participants were <80% adherent at the end of the first study month; this increased to 30% by the end of the 6-month study period. Over 6% of patients were <50% adherent at the end of the first month, and this increased to 11% at month 6 – demonstrating that over 10% of the patients were taking less than 50% of their prescribed doses of 6MP. This study demonstrates that non-adherence to 6MP is prevalent in children undergoing treatment for ALL and increases with time on maintenance. It further delineates certain sociodemographic variables that define those at highest risk for non-adherence. Patients from this study will be followed long-term to understand the impact of non-adherence on outcome. Future research needs to focus on developing targeted, multidisciplinary interventions to reduce non-adherence to therapy.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal